Abstract
The diagnosis of hepatic cystic echinococcosis is based on ultrasonography and confirmed by serology. However, no biological marker of cyst viability is currently available implying years-long patient follow-up, which is not always feasible in endemic areas. We characterized the performance of an immunoblotting test based on human hydatid cyst fluid with particular regard to its ability to distinguish between cyst stages. Sera from patients with cysts in different stages showed distinctive band pattern recognition. Most importantly, the test discriminated in 80% of cases CE3a from CE3b transitional cysts, known to have different viability profiles. Interestingly, we observed a rapid change in band pattern recognition of sera from one patient at time points when his cyst passed from active to transitional to inactive stages. Further identification of different antigens expressed by different cyst stages will support the development of diagnostic tools that could early define cyst viability, to guide clinical decision making, and shorten patient follow-up.
Introduction
Cystic echinococcosis (CE) is a zoonosis caused by infection with the larval stage of Echinococcus granulosus, a dog tapeworm of high medical and veterinary relevance.1 Humans and livestock become infected after ingestion of food contaminated by parasite eggs shed with dog feces. After ingestion, eggs release embryos (oncospheres) that penetrate the gut wall, and by hematogenous spread reach liver, lungs, and other sites where the larval development starts in the form of fluid-filled cysts (hydatid cysts).2
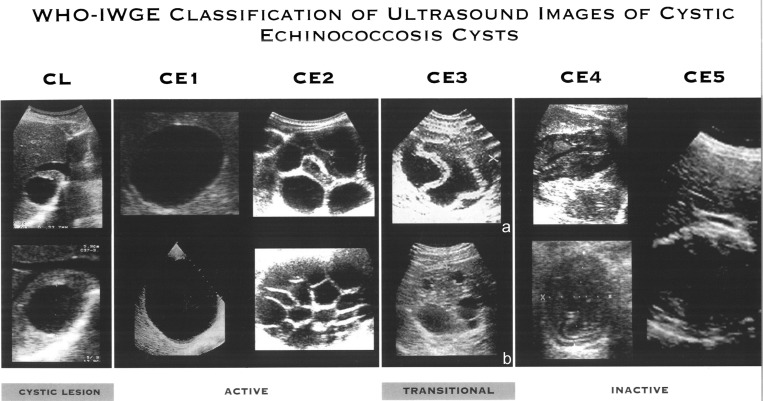
Although the natural history of cyst development is not completely known, they appear to pass through different stages, from active to inactive forms, as classified by the World Health Organization (WHO; Figure 1),3 and supported by functional studies4; this is of clinical relevance for clinical management and prognosis of infected patients.5
Figure 1.
World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) Classification of Echinococcus granulosus cyst stages on ultrasound3 with specification of CE3 cyst sub-classification in CE3a and CE3b, according to their different activity in functional studies.4
The diagnosis of CE is currently based on imaging techniques, with ultrasound (US) as the main modality for hepatic CE.6 Diagnostic confirmation is then achieved using a combination of immunodiagnostic tests such as indirect hemagglutination (IHA), indirect immunofluorescence (IFAT), enzyme-linked immunosorbent assay (ELISA), and immunoblotting (IB).7 However, serological tests have a number of drawbacks, such as 1) lack of standardization of antigenic preparations, and technique; 2) performance dependent on the type of antigen used (native or recombinant, cyst fluid or crude parasite homogenates); 3) low sensitivity (up to 25% false negatives) caused by several factors including location, stage, and size of the cyst; and 4) cross-reactivity with other helminths, mainly Echinococcus multilocularis and Taenia solium.7,8 In addition, no serological marker is currently available that can predict the evolution of a cyst, therefore years-long follow-up is required to ascertain a cure after treatment.
Standardization of techniques, antigenic preparations, characterization of new antigens, and development of recombinant antigen-based assays are required to improve the performance of CE immunodiagnosis and to specifically address these issues.8 Here, we present preliminary results of the diagnostic performance of an IB test based on human hydatid cyst fluid (h-HCF) with the aim to assess 1) the usefulness of an IB using HCF from human cysts as an antigen; and 2) its sensitivity, specificity, and, most importantly, its ability to distinguish between cyst stages.
Materials and Methods
Sera from CE patients.
Fifty serum samples from 50 CE patients with a single hepatic cyst were included in the study. All patients were diagnosed by US. Cysts were classified according to the WHO standardized classification,3 and diagnosis was confirmed by serology using routine ELISA (RIDASCREEN Echinococcus IgG, R-Biopharm, Darmstadt, Germany; cutoff OD 1.1), and IHA (Cellognost Echinococcosis IHA, Siemens Healthcare Diagnostics, Marburg, Germany; cutoff titer 1:64) tests in our diagnostic parasitology laboratory. Sera were collected in our clinic, and stored at −20°C until use. Samples were divided into three groups. Group 1: 10 sera from patients with an active cyst (CE1 and CE2); group 2: 20 sera from patients with a transitional cyst (CE3a and CE3b); group 3: 20 sera from patients with an inactive cyst (CE4 and CE5), which were all serologically negative in routine tests except for one.
In a preliminary attempt to assess whether h-HCF-IB could detect changes in cyst viability, we evaluated its performance on serial serum samples obtained from one patient when the cyst stage changed from active (CE1) to transitional (CE3a) to inactive (CE4) in a short period of time (24 months), as the result of albendazole treatment.
Control sera.
Sera from 20 healthy volunteers and from 15 patients with other helminthic infections (Strongyloides, Entamoeba, Toxocara, Trichinella, Schistosoma, Cysticercus, Fasciola) were included as a control group.
Parasitic material.
Echinococcal cyst fluid was collected from a patient who underwent percutaneous aspiration of a liver CE cyst for therapeutic purposes in our clinic. The patient had never received treatment with albendazole before aspiration. The cyst was classified as type CE1 according to the standardized WHO classification, and identified as fertile (presence of protoscoleces) upon light microscopy examination. The fluid was clarified by centrifugation at 5000 × g for 15 min at 4°C, and stored at −20°C until used.
The patient gave his written informed consent for both the percutaneous aspiration, and the use of the fluid for research purposes, and the use of this biological sample was approved by the Ethical Committee of San Matteo Hospital Foundation, Pavia, Italy.
Immunoblotting.
Parasite antigens were separated electrophoretically in 12% sodium dodecyl sulfate (SDS) polyacrylamide gels under reducing conditions, along with a molecular weight ladder (Prestained SDS PAGE standards broad range, Bio-Rad, Hercules, CA). Briefly, protein concentrations were determined by Spectrophotometer (NanoDrop ND-1000, Wilmington, DE). Proteins were loaded at a concentration of 1 μg/well, and then electrophoretically transferred onto nitrocellulose membranes (0.45 μm, Bio-Rad). Membranes with blotted antigens were blocked for 40 min at room temperature in block buffer (4% skimmed milk-0.2% Tween20-PBS1X). After washing three times with wash buffer (0.2% Tween20-PBS1X), membranes were incubated for 1 h at room temperature with sera diluted 1:1500 in block buffer. After an additional three washes, membranes were incubated for 1 h with HRP-conjugated goat anti-human IgG (A0170, Sigma-Aldrich, St. Louis, MO) diluted 1:5000 in block buffer. Bound antibodies were detected using Chemiluminescent HRP Substrate (Immobilon Western, Millipore, Billerica, MA), and visualized on x-ray films.
Statistical analysis.
Sensitivity and specificity of the h-HCF-IB test were calculated according to recognition of at least one specific (AgB and/or Ag5) band. For the purpose of this study, which addressed the ability of the h-HCF-IB test to assess cyst viability, positivity of the test on sera from patients with inactive CE4 and CE5 cysts was considered a false positive result. Gold standard was the US classification of CE cysts. In the absence of biological data from material obtained by invasive procedures regarding the true viability status of single CE3a cysts, these were considered viable for the purpose of the calculation of test performance.
Results
The h-HCF-IB test had a sensitivity of 83%, and a specificity of 98%. Among sera from patients with active and transitional echinococcal cysts, five classified as CE1 tested negative with the h-HCF-IB test, and three of them were also negative on routine serology. All sera from control patients tested negative with the test, although only one patient with a CE4 cyst was positive, as well as with routine serology.
The IB profiles of the sera were qualitatively and quantitatively homogeneous within each cyst stage group. Antigens with molecular weights (MW) of 8, 16, 24, 34–50, 80–90 kDa were identified.
Group 1: active CE1 and CE2 cysts.
As shown in Figure 2, serum samples from group 1 responded differently to h-HCF depending on the cyst stage: sera of patients with CE2 cysts recognized bands of 8, 16, and 24 kDa, which can be attributed to AgB subunits,9 whereas sera from subjects with CE1 cysts did not recognize or recognized very weakly these bands.
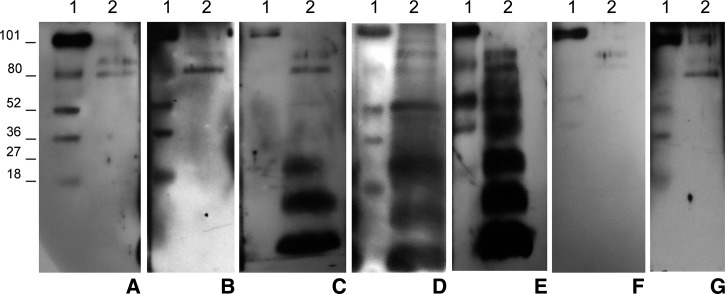
Figure 2.
Representative images of bands pattern recognized by sera from patients with hepatic cystic echinococcosis (CE) in different stages, and control subject using the human hydatid cyst fluid immunoblotting (h-HCF-IB) test based on h-HCF as a source of antigen. Lane 1: molecular weight ladder. Lane 2: h-HCF bands pattern recognized by sera from healthy subjects (A); patients with CE1 (B), CE2 (C), CE3a (D), CE3b (E), and CE4 (F) cysts; other parasitic infections (G); in the case shown Entamoeba histolitica). Note the constant recognition of two unspecific bands of high molecular weight (80–90 kDa) by all samples. The AgB bands of 8, 16, and 24 kDa are recognized by sera from patients with CE2, CE3a, and CE3b cysts, whereas bands belonging to Ag5 (34 and 50 kDa) only by sera of patients with CE3a (weakly or only the band of 50 kDa), and CE3b cysts.
Group 2: transitional CE3 cysts.
Sera in group 2 recognized all the subunits of AgB, and bands between 34–50 kDa, which can be attributed to Ag5 subunits.10 In 8 of 10 cases, the test allowed discrimination between CE3a, and CE3b cysts as sera from patients with CE3a cysts always recognized AgB bands but only one subunit (50 kDa) of Ag5 bands, whereas sera from subjects with CE3b cysts always recognized all bands of the two antigens. In the other two CE3a cases, sera recognized both Ag5 bands, but with weaker intensity.
Group 3: inactive CE4 and CE5 cysts.
Only one serum from group 3 recognized all the subunits of AgB and Ag5; this patient also had positive results on serological routine tests. All other sera from group 3 did not recognize any specific bands.
Control group.
All sera from the control group recognized non-specific bands with a MW between 80–90 kDa independently of presence (and etiology) or absence of other parasitic conditions. These bands were also recognized by sera from all CE groups and were therefore considered unspecific.
Evaluation of longitudinal sera.
Regarding the longitudinal study carried out on the patient whose cyst changed from active (CE1) to transitional (CE3a) to inactive (CE4), we found that the rapid change in cyst viability seen on US was paralleled by a clear change in the band recognition pattern on h-HCF-IB, whereas routine serology continued to be positive (Figure 3). This result is likely due to a reduction of Echinococcus antigens production, and consequently to a lower production of anti-Echinococcus-specific IgG by the immune system.
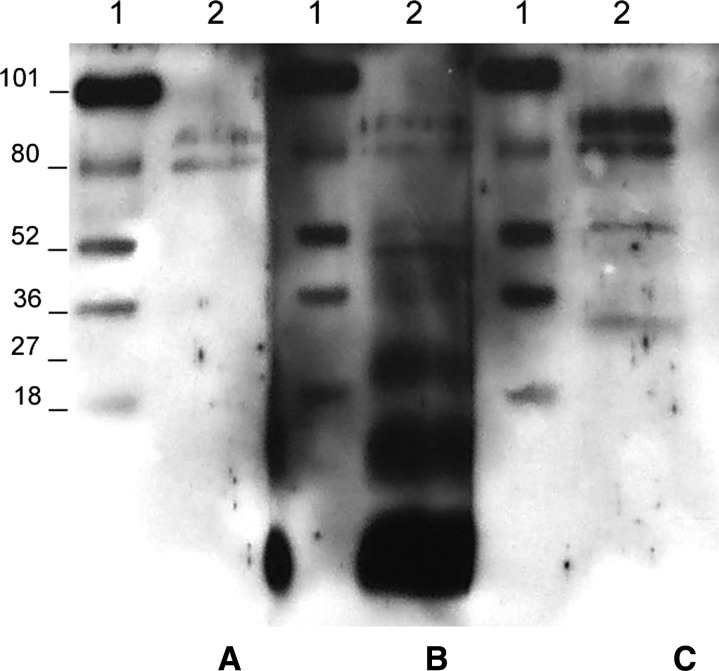
Figure 3.
Human hydatid cyst fluid immunoblotting (h-HCF-IB) of sera from a cystic echinococcosis (CE) patient whose cyst passed from being active (CE1) to transitional (CE3a) to inactive (CE4) as the result of albendazole treatment. Lane 1: molecular weight ladder; lane 2: h-HCF band pattern recognized by serum obtained at CE1 (A, December 2010), CE3a (B, May 2011), and CE4 (C, December 2012) cyst stage.
Discussion
Immunological tests are a valuable aid in the diagnosis of CE, which still relies primarily on imaging methods. Despite the constant search for new molecular tools,11,12 however, current serological tests lack sensitivity and cannot predict the evolution of cysts either after treatment or spontaneously.7,11
IB is one of the most sensitive and specific techniques, and it is generally used as a confirmatory test when routinely used assays such as IHA and ELISA are inconclusive. Sheep HCF, and native or recombinant AgB, and Ag5 are the main antigens used in IB.7
HCF is a complex mixture of parasite excretory–secretory products, and host-derived molecules.13 However, the known presence of host-derived molecules did not appear to negatively influence the specificity of our test. Indeed, only bands of high MW, which could be attributed to immunoglobulin heavy chains, were unspecifically detected in all samples.
The principal molecules present in large amounts in the cyst fluid are AgB and Ag5.9,14,15 AgB is an immunogenic lipoprotein encoded by several genes16 involved in several host–parasite interaction mechanisms, promoting parasite establishment and survival in the intermediate host.17–20 There is also evidence that AgB genes are differentially expressed in the different stages of the parasite life cycle, and parasite tissues during development in the host.9,16,21,22 Ag5 is an immunogenic glycoprotein expressed in all stages of the parasite life cycle, and is secreted from the surface of the worm.9,23–25 Ag5 is present in elevated concentration in HCF, and it may have a relevant function in the development of the metacestode.10
HCF for diagnostic purposes is obtained from sheep cysts, but its use is hampered by cross-reactivity of sera from patients with other parasitic infections, and by difficulties in its standardization.26–28
In this study, we developed a new IB test using h-HCF with high specificity and sensitivity, and evaluated the antibody response of patients infected with hepatic CE cysts in different stages. Although it would be difficult to include HCF from human cysts in standardized serological tests, molecular characterization of bands recognized by sera from infected patients could represent a useful tool to identify antigens, which can be subsequently used in tests with improved diagnostic performances.
The h-HCF-IB test had a sensitivity of 83% and a specificity of 98%, calculated considering as a true negative result that of sera from patients with inactive CE4-CE5 cysts. The relatively low sensitivity was caused by the high percentage (71%) of sera from patients with active CE1 cysts that tested negative with h-HCF-IB. On the other hand, the specificity obtained, lower than 100%, was not caused by cross-reactivity of the test but by the positivity of one serum from a patient with an inactive CE4 cyst, which was also positive on routine serology. Overall, these results are comparable with those reported in the literature using sheep hydatid fluid fractions,28 although our test showed lower sensitivity compared with a commercial IB test routinely used for diagnostic purposes (Echinococcus Western Blot IgG, LDBIO, Lyon, France—Se 83% h-HCF-IB versus Se 98% commercial kit29).
The bands recognized by sera from CE patients (8, 16, 24, 34–50 kDa) belonged to the two main antigens present in the HCF, AgB (8, 16, and 24 kDa), and Ag5 (34–50 kDa). Patients with cysts in active stages (CE1 and CE2) responded differently to h-HCF: sera of patients with CE1 cysts recognized inconsistently the AgB subunits, whereas sera of patients with cysts in the CE2 stage recognized all three subunits of AgB. Patients with inactive cysts (CE4 and CE5) neither recognized Echinococcus-specific bands in the h-HCF-IB test nor in the routine assays, with the exception of one subject who was positive on both tests. Patients with transitional cysts (CE3a and CE3b) recognized all subunits of AgB and Ag5, and, most interestingly, h-HCF-IB allowed, in 80% of cases, to discriminate between CE3a and CE3b on the basis of Ag5 band recognition. To our knowledge, this is the first report of such a performance using serological tests.
To assess whether h-HCF-IB could be useful in detecting early changes in band pattern recognition after treatment, and therefore potentially able to indicate therapy outcome at an early phase, we tested its performance on serial sera obtained from one patient at the time when its cyst changed from active (CE1) to transitional (CE3a) to inactive (CE4) in a short period of time (24 months), as the result of albendazole treatment. Interestingly, our preliminary results support the potential ability of this IB to evaluate early changes in cyst viability.
Taken together, although preliminary, and on a small cohort of patients, these results strongly support the hypothesis that different antigens are expressed by different cyst stages of the larval phase of E. granulosus, as suggested by functional studies,4 and not just by different phases of the parasite life cycle.30 This warrants the evaluation of the transcriptome/proteome from different cyst stages. These differences might be useful in clinical practice to correctly define cyst stages and their viability. Our preliminary results show a clear and rapid change in band pattern recognition when a cyst from a single patient changed from active to inactive, and this opens new perspectives in the clinical management of CE patients and their follow-up. On this account, it would be of interest to evaluate the behavior of band pattern recognition in a larger cohort of patients, and in longitudinal studies, to assess whether early changes in band recognition could predict the evolution of a cyst either spontaneously or after treatment, and the timing of such changes.
In our study, failure to recognize specific bands by sera from patients with CE4-CE5 and CE1 cysts is consistent with a negative serology characteristic of patients with cysts in these stages.31,32 However, further studies are needed to investigate whether the h-HCF-IB test could detect early changes in cyst viability upon reactivation of CE4 cysts. For example, it would be of interest to assess whether the only patient with a CE4 cyst who tested positive on the h-HCF-IB test will see his cyst reactivating in the future, in comparison with the fate of those with inactive cysts who tested negative. Moreover, the mechanisms underlying the difference in band recognition between CE2 and CE3b cysts, both biologically active,4 remain to be clarified. A possible explanation is that only AgB is exposed to the host immune system by intact, young active cysts (CE2), whereas Ag5 elicits the production of specific antibodies after the cyst has been damaged (CE3b). CE3a cysts are known to be active in about half of the cases,4 and sera from patients with this cyst stage interestingly showed two patterns of band recognition, one similar to that of patients with CE2, and one similar to patients with CE3b cysts. It would be extremely useful to assess whether this behavior reflects the biological activity of the single CE3a cysts.
Whatever the biological mechanism underlying these results, the ability of our h-HCF-IB test to recognize different band patterns by sera from patients with cysts in different stages, opens new opportunities to develop methods that would aid in the clinical management of CE infection and in shortening follow-up.
ACKNOWLEDGMENTS
We thank Professor Claudio Bandi, University of Milan, for the critical review of the manuscript and the provision of technical equipment.
Footnotes
Financial support: This work was partially funded by a grant “Ricerca Corrente” from the Italian Ministry of Health through the “San Matteo” Hospital Foundation (to E.B.).
Authors' addresses: Mara Mariconti, University of Milan, Milan, Italy, Policlinico San Matteo Hospital Foundation, Pavia, Italy, and WHO Collaborating Centre for Clinical Management of Cystic Echinococcosis Pavia, Italy, E-mail: maramariconti@libero.it. Chiara Bazzocchi, University of Milan, Milan, Italy, E-mail: chiara.bazzocchi@unimi.it. Francesca Tamarozzi, WHO Collaborating Centre for Clinical Management of Cystic Echinococcosis Pavia, Italy, and University of Pavia, Pavia, Italy, E-mail: f_tamarozzi@yahoo.com. Valeria Meroni, Francesca Genco, and Roberta Maserati, Policlinico San Matteo Hospital Foundation, Pavia, Italy, E-mails: v.meroni@smatteo.pv.it, gencofrancesca@gmail.com, and r.maserati@smatteo.pv.it. Enrico Brunetti, Policlinico San Matteo Hospital Foundation, Pavia, Italy, WHO Collaborating Centre for Clinical Management of Cystic Echinococcosis Pavia, Italy, and University of Pavia, Pavia, Italy, E-mail: enrico.brunetti@unipv.it.
References
- 1.Budke CM, Deplazes P, Torgerson PR. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis. 2006;12:296–303. doi: 10.3201/eid1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McManus DP. Echinococcosis with particular reference to Southeast Asia. Adv Parasitol. 2010;72:267–303. doi: 10.1016/S0065-308X(10)72010-8. [DOI] [PubMed] [Google Scholar]
- 3.WHO Informal Working Group International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003;85:253–261. doi: 10.1016/s0001-706x(02)00223-1. [DOI] [PubMed] [Google Scholar]
- 4.Hosch W, Junghanss T, Stojkovic M, Brunetti E, Heye T, Kauffmann GW, Hull WE. Metabolic viability assessment of cystic echinococcosis using high-field 1H MRS of cyst contents. NMR Biomed. 2008;21:734–754. doi: 10.1002/nbm.1252. [DOI] [PubMed] [Google Scholar]
- 5.Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Stojkovic M, Rosenberger K, Kauczor HU, Junghanss T, Hosch W. Diagnosing and staging of cystic echinococcosis: how do CT and MRI perform in comparison to ultrasound? PLoS Negl Trop Dis. 2012;6:e1880. doi: 10.1371/journal.pntd.0001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmena D, Benito A, Eraso E. Antigens for the immunodiagnosis of Echinococcus granulosus infection: an update. Acta Trop. 2006;98:74–86. doi: 10.1016/j.actatropica.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, McManus DP. Recent advances in the immunology and diagnosis of echinococcosis. FEMS Immunol Med Microbiol. 2006;47:24–41. doi: 10.1111/j.1574-695X.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 9.Lightowlers MW, Liu DY, Haralambous A, Rickard MD. Subunit composition and specificity of the major cyst fluid antigens of Echinococcus granulosus. Mol Biochem Parasitol. 1989;37:171–182. doi: 10.1016/0166-6851(89)90149-7. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzo C, Salinas G, Brugnini A, Wernstedt C, Hellman U, Gonzalez-Sapienza G. Echinococcus granulosus antigen 5 is closely related to proteases of the trypsin family. Biochem J. 2003;369:191–198. doi: 10.1042/BJ20021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben Nouir N, Gianinazzi C, Gorcii M, Muller N, Nouri A, Babba H, Gottstein B. Isolation and molecular characterization of recombinant Echinococcus granulosus P29 protein (recP29) and its assessment for the post-surgical serological follow-up of human cystic echinococcosis in young patients. Trans R Soc Trop Med Hyg. 2009;103:355–364. doi: 10.1016/j.trstmh.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Gonzalez A, Santivanez S, Garcia HH, Rodriguez S, Munoz S, Ramos G, Orduna A, Siles-Lucas M. Improved serodiagnosis of cystic echinococcosis using the new recombinant 2B2t antigen. PLoS Negl Trop Dis. 2012;6:e1714. doi: 10.1371/journal.pntd.0001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McManus DP. The Biology of Echinococcus and Hydatid Disease. London, UK: RCA Thompson; 1986. [Google Scholar]
- 14.Oriol C, Oriol R. Physiocochemical properties of a lipoprotein antigen of Echinococcus granulosus. Am J Trop Med Hyg. 1975;24:96–100. doi: 10.4269/ajtmh.1975.24.96. [DOI] [PubMed] [Google Scholar]
- 15.Oriol R, Williams JF, Perez Esandi MV, Oriol C. Purification of lipoprotein antigens of Echinococcus granulosus from sheep hydatid fluid. Am J Trop Med Hyg. 1971;20:569–574. doi: 10.4269/ajtmh.1971.20.569. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Li J, Jones MK, Zhang Z, Zhao L, Blair D, McManus DP. The Echinococcus granulosus antigen B gene family comprises at least 10 unique genes in five subclasses which are differentially expressed. PLoS Negl Trop Dis. 2010;4:e784. doi: 10.1371/journal.pntd.0000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chemale G, Ferreira HB, Barrett J, Brophy PM, Zaha A. Echinococcus granulosus antigen B hydrophobic ligand binding properties. Biochim Biophys Acta. 2005;1747:189–194. doi: 10.1016/j.bbapap.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Rigano R, Buttari B, Profumo E, Ortona E, Delunardo F, Margutti P, Mattei V, Teggi A, Sorice M, Siracusano A. Echinococcus granulosus antigen B impairs human dendritic cell differentiation and polarizes immature dendritic cell maturation towards a Th2 cell response. Infect Immun. 2007;75:1667–1678. doi: 10.1128/IAI.01156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rigano R, Profumo E, Bruschi F, Carulli G, Azzara A, Ioppolo S, Buttari B, Ortona E, Margutti P, Teggi A, Siracusano A. Modulation of human immune response by Echinococcus granulosus antigen B and its possible role in evading host defenses. Infect Immun. 2001;69:288–296. doi: 10.1128/IAI.69.1.288-296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siracusano A, Margutti P, Delunardo F, Profumo E, Rigano R, Buttari B, Teggi A, Ortona E. Molecular cross-talk in host-parasite relationships: the intriguing immunomodulatory role of Echinococcus antigen B in cystic echinococcosis. Int J Parasitol. 2008;38:1371–1376. doi: 10.1016/j.ijpara.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez G, Nieto A, Fernandez C, Orn A, Wernstedt C, Hellman U. Two different 8 kDa monomers are involved in the oligomeric organization of the native Echinococcus granulosus antigen B. Parasite Immunol. 1996;18:587–596. doi: 10.1046/j.1365-3024.1996.d01-38.x. [DOI] [PubMed] [Google Scholar]
- 22.Monteiro KM, Cardoso MB, Follmer C, da Silveira NP, Vargas DM, Kitajima EW, Zaha A, Ferreira HB. Echinococcus granulosus antigen B structure: subunit composition and oligomeric states. PLoS Negl Trop Dis. 2012;6:e1551. doi: 10.1371/journal.pntd.0001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Felice G, Pini C, Afferni C, Vicari G. Purification and partial characterization of the major antigen of Echinococcus granulosus (antigen 5) with monoclonal antibodies. Mol Biochem Parasitol. 1986;20:133–142. doi: 10.1016/0166-6851(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 24.Jones MK, Zhang LH, Leggatt GR, Stenzel DJ, McManus DP. The ultrastructural localization of Echinococcus granulosus antigen 5. Parasitology. 1996;113:213–222. doi: 10.1017/s0031182000081993. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Xu H, Chen J, Gan W, Wu W, Hu X. Gene cloning, expression, and localization of antigen 5 in the life cycle of Echinococcus granulosus. Parasitol Res. 2012;110:2315–2323. doi: 10.1007/s00436-011-2766-9. [DOI] [PubMed] [Google Scholar]
- 26.Mohammadzadeh T, Sako Y, Sadjjadi SM, Sarkari B, Ito A. Comparison of the usefulness of hydatid cyst fluid, native antigen B and recombinant antigen B8/1 for serological diagnosis of cystic echinococcosis. Trans R Soc Trop Med Hyg. 2012;106:371–375. doi: 10.1016/j.trstmh.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Tawfeek GM, Elwakil HS, El-Hoseiny L, Thabet HS, Sarhan RM, Awad NS, Anwar WA. Comparative analysis of the diagnostic performance of crude sheep hydatid cyst fluid, purified antigen B and its subunit (12 Kda), assessed by ELISA, in the diagnosis of human cystic echinococcosis. Parasitol Res. 2011;108:371–376. doi: 10.1007/s00436-010-2074-9. [DOI] [PubMed] [Google Scholar]
- 28.Ortona E, Rigano R, Margutti P, Notargiacomo S, Ioppolo S, Vaccari S, Barca S, Buttari B, Profumo E, Teggi A, Siracusano A. Native and recombinant antigens in the immunodiagnosis of human cystic echinococcosis. Parasite Immunol. 2000;22:553–559. doi: 10.1046/j.1365-3024.2000.00336.x. [DOI] [PubMed] [Google Scholar]
- 29.Liance M, Janin V, Bresson-Hadni S, Vuitton DA, Houin R, Piarroux R. Immunodiagnosis of Echinococcus infections: confirmatory testing and species differentiation by a new commercial Western Blot. J Clin Microbiol. 2000;38:3718–3721. doi: 10.1128/jcm.38.10.3718-3721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkinson J, Wasmuth JD, Salinas G, Bizarro CV, Sanford C, Berriman M, Ferreira HB, Zaha A, Blaxter ML, Maizels RM, Fernandez C. A transcriptomic analysis of Echinococcus granulosus larval stages: implications for parasite biology and host adaptation. PLoS Negl Trop Dis. 2012;6:e1897. doi: 10.1371/journal.pntd.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celik T, Akarsu GA, Gungor C, Colak C, Ormeci N. Evaluation of antibodies against hydatid cyst fluid antigens in the post-treatment follow-up of cystic echinococcosis patients. Med Sci Monit. 2009;15:CR170–CR176. [PubMed] [Google Scholar]
- 32.Doiz O, Benito R, Sbihi Y, Osuna A, Clavel A, Gomez-Lus R. Western blot applied to the diagnosis and post-treatment monitoring of human hydatidosis. Diagn Microbiol Infect Dis. 2001;41:139–142. doi: 10.1016/s0732-8893(01)00293-0. [DOI] [PubMed] [Google Scholar]