Abstract
We conducted a longitudinal analysis of 117 lymphedema patients in a filariasis-endemic area of Haiti during 1995–2008. No difference in lymphedema progression between those who received or did not receive mass drug administration (MDA) was found on measures of foot (P = 0.24), ankle (P = 0.87), or leg (P = 0.46) circumference; leg volume displacement (P = 0.09), lymphedema stage (P = 0.93), or frequency of adenolymphangitis (ADL) episodes (P = 0.57). Rates of ADL per year were greater after initiation of MDA among both groups (P < 0.01). Nevertheless, patients who received MDA reported improvement in four areas of lymphedema-related quality of life (P ≤ 0.01). Decreases in foot and ankle circumference and ADL episodes were observed during the 1995-1998 lymphedema management study (P ≤ 0.01). This study represents the first longitudinal, quantitative, leg-specific analysis examining the clinical effect of diethylcarbamazine on lymphedema progression and ADL episodes.
Introduction
Lymphatic filariasis (LF) is a chronic disabling and debilitating parasitic infection that is one of the major causes of long-term disability worldwide.1 After acquisition of infection, there is evidence of subclinical changes that later progress to overt clinical disease, including lymphedema and elephantiasis, hydrocele in males, episodes of filarial adenolymphangitis, and chyluria.2,3 Persons with lymphatic filariasis often have acute bacterial dermatolymphangioadenitis (ADL) episodes, which are characterized by swelling, fever, pain, and inflammation of the affected extremity.4,5 Skin lesions, including interdigital lesions, serve as an entry point for bacteria believed to initiate the ADL episode.6 Episodes of ADL can increase the pace with which lymphedema progresses to elephantiasis.4 Repeated episodes of ADL accelerate damage to superficial lymphatic vessels in the skin, which results in worsened lymphatic dysfunction, fibrosis, and increased risk for future episodes of ADL.5,7–10
The Global Programme to Eliminate Lymphatic Filariasis has two components: primary prevention, which uses mass drug administration (MDA) with diethylcarbamazine (DEC) and albendazole or ivermectin and albendazole to interrupt LF transmission and secondary or tertiary prevention, which focuses on preventing and managing disability for affected persons.11 Disability prevention for patients with filarial morbidity includes basic lymphedema management for those with lymphedema and hydrocelectomy for men with hydrocele. Lymphedema management involves leg hygiene, early treatment of bacterial and fungal infections, elevation, and exercises.12 Clinical and histopathologic studies suggest that lymphedema management can decrease the number of ADL episodes9,13–19 and halt or, in some cases, partially reverse disease progression.14,16–18,20
Although there are several studies demonstrating improvement in lymphedema in patients who adhere to a lymphedema management regimen, the literature exploring the effect of mass drug administration with DEC, either alone or in combination with albendazole, on filarial morbidity is inconclusive. Recent clinical research using ultrasonography and lymphoscintigraphy has documented the reversal of early lymph-vessel damage in Brugia malayi–infected children after MDA with DEC in India.21 However, earlier studies in Brazil have failed to find a decrease in lymphatic vessel dilation after treatment with DEC in adults infected with Wuchereria bancrofti.22,23 Of the 13 published studies assessing the effect of DEC on lymphedema-specific filarial morbidity, nine found a beneficial effect of DEC on lymphedema20,24–31 and the other studies demonstrated no effect.32–35 In addition, seven of eight studies demonstrated that DEC decreased the incidence of ADL episodes in patients with lymphedema14,20,24–26,32,36 and one study showed no effect.33 It is important to note that these studies differ with regard to study design, evaluation criteria, frequency and dosage of drug treatment, case definitions, and clinical follow-up, thus making direct comparisons difficult. For most of these studies, the primary outcome was microfilaremia as opposed to clinical morbidity (i.e., lymphedema and ADL episodes), and many had small sample sizes. In addition, none of the studies were conducted in a filariasis-endemic area in the Western Hemisphere.
Léogane, Haiti has long been endemic for W. bancrofti infection with antigen prevalence documented as high as 50% in some communities.37 Yearly MDA with DEC began in Léogane in October 2000 for persons more than two years of age. Because of concerns of toxicity, women of childbearing age were not administered albendazole until 2002. Drugs have been distributed yearly, with the exception of 2006, in which MDA was suspended because of a gap in funding.38,39 The major health facility for Léogane Commune is Sainte Croix Hospital.40 In 1995, a lymphedema management study was initiated at the outpatient clinic at Sainte Croix Hospital to help lymphedema patients manage their symptoms and prevent further acceleration of the disease.
The objective of this study was to assess the impact of mass drug administration with DEC on clinical measures of filarial morbidity, including lymphedema progression and the number of ADL episodes per year, as well as on quality of life indicators in a cohort of Haitian lymphedema patients living in a filariasis-endemic area. A secondary objective involved assessing the impact of lymphedema management on these same clinical measures of filarial morbidity.
Methods
Study population.
A cohort of 175 lymphedema patients was enrolled in a prospective lymphedema management study that was conducted during 1995–1998 at the outpatient clinic of Sainte Croix Hospital in the Léogane Commune. Patients visited the clinic regularly during 1995–1998, and some persons from this original cohort were also involved with other clinical studies conducted at Sainte Croix Hospital outpatient lymphedema clinic in 2000, 2001, and 2002. The original cohort of 175 patients was subsequently targeted for long-term follow-up in 2008. Databases for the 1995–1998 prospective cohort study and the long-term follow-up in 2008 were combined with additional data from a cross-sectional study conducted in 2000 and a prospective cohort study conducted during 2001–2002 that contained pertinent data on this study cohort.41,42 The study protocols and consent forms were approved by the Ethics Committee at Sainte Croix Hospital and the Institutional Review Board at the Centers for Disease Control and Prevention. Written informed consent was obtained from patients for their involvement in all studies.
Clinical history and physical examination.
During study years spanning 1995 to 2008, patients were evaluated at the clinic or were seen at home if they were either unwilling or unable to come to the clinic. Patients underwent physical examinations specific to their lymphedema, which included measurements of lower limb volume and circumference, lymphedema stage, and an assessment of the presence of interdigital lesions.
Circumference measurements were taken (in cm) at three fixed points: the foot 10 cm proximal to the tip of the first toe, the ankle 10 cm from the floor, and the leg 25 cm from the floor. The volume of each leg (in mL) was determined by measuring the displacement of a standard volume of water into a calibrated cylinder.43 Severity of lymphedema was staged on a seven-stage classification system developed by Dreyer and others.12 Questionnaires were administered at enrollment of each study with information about patient demographics, history of lymphedema, quality of life, compliance with lymphedema management, and ADL episode history in the previous 12 months. The EuroQol-5D questionnaire was used for quality of life questions. For the purposes of this study, an ADL episode was defined as a combination of two or more of the following symptoms: swelling, redness, and pain in a lower extremity with or without systemic manifestations of fever, and chills. Data were obtained on both legs for all patients, regardless of whether lymphedema was visualized.
Study exposure.
Mass drug administration is conducted through distribution posts in Haiti where the drugs are provided with water to help participants swallow the pills. The drug distributors insist on direct observation of treatment. The main exposure evaluated was a self-reported dichotomous variable collected in 2008, which indicated whether a person had ever ingested DEC during MDA. If a patient reported ever having taken DEC during MDA, they were considered as having received MDA. If a patient reported never receiving DEC during MDA, they were considered as never having received MDA. Pre-intervention refers to the period before initiation of MDA (before 2000), whereas post-intervention refers to the year 2000 and beyond, which was after the initiation of MDA. A secondary exposure evaluated was the impact of the lymphedema management study that was conducted during 1995–1998.
Study outcomes.
We investigated six primary outcomes of interest: foot circumference, ankle circumference, leg circumference, leg volume (assessed by water displacement), stage of lymphedema, and number of ADL episodes per year. For measurement outcomes (i.e., foot, ankle, and leg circumference, and volume displacement of leg) for prospective cohort studies, the median values were calculated for each year to mirror the data available in the cross-sectional studies. The number of ADL episodes in the previous 12-month period was self-reported upon enrollment in cross-sectional and prospective cohort studies. For prospective cohort studies after enrollment, the number of ADL episodes per year was calculated by summing the number of ADL episodes reported since the last visit during a 12-month period. Stage of lymphedema was recorded after a thorough physical examination of the patient's legs.
In addition to the six quantitative outcomes, a qualitative outcome, quality of life, was investigated. During the 2008 study, patients who received MDA retrospectively self-reported perceptions of quality of life before taking DEC and reported current perspectives on quality of life. Differences in four areas of quality of life directly related to the patient's lymphedema were compared pre- and post-intervention: experience of pain or discomfort, suffering from anxiety or depression, problems with mobility, and difficulties performing usual activities.
Study covariates.
Demographic variables collected across all studies included sex, age, education level, literacy, and wealth quintile (Table 1). A principal components analysis was applied to assets, which were included in the Haitian Demographic and Health Survey, to create a household socioeconomic index. Ownership of a bicycle, phone, car, refrigerator, radio, and television were included in the principal components analysis. Patients were then assigned to wealth quintiles based on the value of the index. The LF transmission level was estimated for each patient by the section of the Léogane Commune in which the patient's home was located and rated as low, intermediate, or high level transmission based on antigenemia data obtained from sentinel site surveys during 1995–2008.37,39,44 Lymphedema-specific covariates included self-reported number of years with lymphedema and physical examination findings of number of interdigital lesions at each visit. Compliance with lymphedema management was assessed by using a hygiene composite score, which was created for each person at every time point where information was available. The composite score was comprised of three components: washing, elevation, and exercise. Patients were grouped into three categories: highly compliant (participating in washing, elevation, and exercise activities several times per week to every day), moderately compliant (participating in these activities two times per month to once a week), and rarely or never compliant (participating in these activities never to once per month). Similarly, the extent to which a person self-bandaged his or her leg was categorized as highly compliant for those who bandaged several days a week to everyday, moderately compliant for those who bandaged two or more times a month to once a week, and rarely or never compliant for those who never bandaged or bandaged once per month.
Table 1.
Demographic and clinical characteristics of lymphedema cohort at enrollment by mass drug administration (MDA) exposure status, Léogane, Haiti*
| Characteristic | Received MDA (n = 102) | Did not receive MDA (n = 15) | P |
|---|---|---|---|
| Female sex | 88 (86.3%) | 13 (86.7%) | 1.00† |
| Mean age, years (range) | 35.8 (10–75) | 38.8 (13–62) | 0.15‡ |
| Education | |||
| None | 37 (36.3%) | 7 (46.7%) | 0.11‡ |
| Primary school | 54 (52.9%) | 8 (53.3%) | |
| Secondary school | 10 (9.8%) | 0 (0.0%) | |
| Post-secondary school | 1 (1.0%) | 0 (0.0%) | |
| Literate | 52 (51.0%) | 7 (46.7%) | 0.79† |
| Transmission | |||
| Low | 0 (0.0%) | 1 (6.7%) | < 0.01‡ |
| Medium | 3 (2.9%) | 2 (13.3%) | |
| High | 99 (97.1%) | 12 (80.0%) | |
| Wealth quintile | n = 14 | ||
| Lowest | 34 (33.3%) | 4/14 (28.6%) | 0.80‡ |
| Second | 35 (34.3%) | 7/14 (50.0%) | |
| Middle | 10 (9.8%) | 0 (0.0%) | |
| Fourth | 11 (10.8%) | 2/14 (14.3%) | |
| Highest | 12 (11.8%) | 1/14 (7.1%) | |
| Home bandaging (managed by patient) | |||
| Infrequent | 57 (55.9%) | 8 (53.3%) | 0.34‡ |
| Moderate | 22 (21.6%) | 1 (6.7%) | |
| Frequent | 23 (22.5%) | 6 (40.0%) | |
| Hygiene composite | n = 78 | n = 14 | |
| Rarely/never compliant | 15/78 (19.2%) | 6/14 (42.9%) | 0.28‡ |
| Moderately compliant | 24/78 (30.8%) | 1/14 (7.1%) | |
| Highly compliant | 39/78 (50.0%) | 7/14 (50.0%) | |
| Lymphedema duration, years§ | n = 204 | n = 30 | |
| < 5 | 133/204 (65.2%) | 16/30 (53.3%) | 0.12‡ |
| 5–14 | 44/204 (21.6%) | 6/30 (20.0%) | |
| ≥ 15 | 27/204 (13.2%) | 8/30 (26.7%) | |
| Measurements | |||
| Mean foot circumference, cm (range) | 23.74 (18.50–32.70) | 24.51 (19.60–28.90) | 0.07‡ |
| Mean ankle circumference, cm (range) | 25.20 (18.15–45.10) | 26.59 (17.35–37.55) | 0.06‡ |
| Mean leg circumference, cm (range) | 30.55 (22.90–50.50) | 33.23 (21.90–52.00) | 0.02‡ |
| Mean volume displacement of leg, cm (range) | 1,847.7 (940.0–3,425.0) | 1,957.3 (1,055.0–2,730.0) | 0.18‡ |
| Mean ADL episodes in past 12 months (range) | 1.15 (0–12) | 1.3 (0–12) | 0.99‡ |
| Stage of leg lymphedema§ | n = 204 | n = 30 | |
| 0 | 19/204 (9.3%) | 4/30 (13.3%) | 0.01‡ |
| 1–3 | 168/204 (82.4%) | 15/30 (50.0%) | |
| ≥ 4 | 17/204 (8.3%) | 11/30 (36.7%) | |
Except where indicated, values are no. (%). ADL = adenolymphangitis.
Significance of difference by Pearson's chi-square test.
Significance of difference by Wilcoxon rank-sum test.
Leg-specific variable.
Statistical methods.
The effects of MDA with DEC on the six quantitative measures of lymphedema-specific morbidity mentioned above, as well as on four quality of life measures were assessed. In addition, the effects of the lymphedema management study on the same six measures of lymphedema-specific morbidity were examined. The sign test was used to determine the effect of MDA on perceptions of quality of life. For quantitative measures of morbidity, models using outcome measures from both legs were fit using generalized estimating equations to control for correlated observations on both legs of the same person over time.45 Significant covariates and those determined necessary to include based on previous studies were controlled for in all of these models.4,46 Any missing covariates were multiply imputed.47
For leg measurement models with continuous outcomes such as foot, ankle, and leg circumference, as well as volume displacement of the leg, multivariable linear models were fit, and the response for each model was the actual measurement. Poisson regression was used to model the log number of ADL episodes per year. For the lymphedema stage outcome, a proportional odds model was fit using three stage categories: no lymphedema (stage 0), early lymphedema (stages 1–3), and advanced lymphedema (stages 4–7). The two cumulative logits were based on comparing higher to lower stage categories, and the resulting odds ratios are for advanced lymphedema versus early and no lymphedema and for advanced and early lymphedema versus no lymphedema.
All models assessing the effects of MDA enabled the same four comparisons. Two comparisons assessed differences in the rates of change for measures of lymphedema progression and for the number of ADL episodes between the persons who received MDA pre- and post-intervention. The second two comparisons examined the rates of change pre- and post-intervention for persons who received MDA and those who did not receive MDA. The lymphedema management models ignored MDA exposure groups and allowed for examination of rates of change both during the lymphedema management study years (1995–1998) and after the conclusion of the study (1998–2008).
All analyses were performed by using SAS® version 9.2 (SAS Institute Inc., Cary, NC).The sign test for the quality of life assessment was performed by using PROC UNIVARIATE. The generalized estimating equations models for the quantitative measures of morbidity were fit by using PROC GENMOD with either the independent correlation structure (proportional odds model for stage category outcome) or the autoregressive correlation structure (all other models).
Results
Study population.
Of the 175 patients in the original cohort, 117 (67%) patients were enrolled in the 2008 follow-up study (23 died, 10 moved out of the country, 24 were unable to be located, and 1 refused participation). This cohort of 117 lymphedema patients and the 58 patients from the original cohort who were lost to follow-up did not differ significantly with regard to sex (P = 0.09, by chi-square test), age category (P = 0.11, by Wilcoxon rank-sum test), literacy (P = 0.87, by chi-square test), duration of lymphedema (P = 0.15, by Wilcoxon rank-sum test), or stage of lymphedema (P = 0.10, by Wilcoxon rank-sum test).
These analyses were limited to the 117 patients who participated in the 2008 long-term follow-up study. Of the 117 patients, 15 (12.8%) did not receive MDA and 102 (87.2%) received MDA; 53 patients reported receiving MDA seven times, 27 patients reported receiving MDA five or six times, 10 patients reported receiving MDA three or four times, 11 patients reported receiving MDA one or two times, and one patient reported receiving MDA but could not recall the number of times. Patients had a median of six time points of observation included in the analysis (range = 3–8 time points of observations for all participants).
Demographic characteristics.
Patients who received MDA and those who did not receive MDA in this cohort were similar for demographic and clinical characteristics at enrollment (Table 1). Of the 117 lymphedema patients, the mean age was 36.2 years (range = 10–75 years) and 101 (86.3%) were female. A total of 59 (50.4%) patients were literate and 80 (69.0%) were in one of the two lowest wealth quintiles. Most (63.7%) reported lymphedema for less than five years and most (78.2%) had lymphedema stages 1–3. There were differences between the two groups with regard to LF transmission level, stage of lymphedema, and leg circumference. The group that did not receive MDA had a slightly greater proportion of patients with higher stages, a slightly larger mean leg circumference, and a greater proportion of patients who had lived in lower transmission areas.
Comparison between patients who received MDA and those who did not receive MDA.
When we compared rates of change between persons who received MDA and those who did not receive MDA during the pre-intervention phase, no differences in rates of change were found for any of the lymphedema-specific morbidity outcomes between those who later received MDA and those who did not (see below for model details). Therefore, post-intervention comparisons were made and are shown in Tables 2 and 3. All covariates controlled for when comparing persons who received MDA and those who did not were similarly controlled for in the within group comparisons noted below.
Table 2.
Effect of mass drug administration (MDA) on quantitative lower extremity measurements pre- and post-intervention for 117 lymphedema patients, Léogane, Haiti, 1995–2008*
| Outcome | Rate of change per year among those who received MDA | Rate of change per year among those who did not receive MDA | Difference in rate (received MDA – did not receive MDA) | 95% confidence interval for difference in rate | P |
|---|---|---|---|---|---|
| Foot circumference† | |||||
| Pre-intervention | –0.22 | –0.34 | 0.12 | –0.13 to 0.37 | 0.35 |
| Post-intervention | –0.16 | –0.06 | –0.10 | –0.25 to 0.06 | 0.24 |
| Ankle circumference‡ | |||||
| Pre-intervention | –0.34 | –0.79 | 0.45 | –0.25 to 1.15 | 0.21 |
| Post-intervention | –0.21 | –0.24 | 0.03 | –0.26 to 0.31 | 0.86 |
| Leg circumference§ | |||||
| Pre-intervention | –0.18 | –0.51 | 0.33 | –0.39 to 1.04 | 0.37 |
| Post-intervention | –0.17 | –0.04 | –0.13 | –0.46 to 0.21 | 0.46 |
| Volume displacement¶ | |||||
| Pre-intervention | 34.84 | 45.56 | –10.72 | –136.98 to 115.53 | 0.87 |
| Post-intervention | 37.68 | 103.93 | –66.25 | –143.37 to 10.87 | 0.09 |
Each outcome was adjusted for model-specific variables by using a generalized estimating equations (GEEs) repeated measures linear regression. Additional outcome specific variables controlled for beyond those listed in the text are reported below.
Circumference (in cm) of the foot 10 cm from the tip of the first toe. Additionally adjusted for sex and wealth quintile.
Circumference (in cm) of the ankle 10 cm from the floor. Additionally adjusted for sex.
Circumference (in cm) of the ankle 25 cm from the floor. Additionally adjusted for age.
¶Amount of water (in mL) displaced from a standard volume of water. Additionally adjusted for sex and lymphedema duration.
Table 3.
Effect of mass drug administration (MDA) on ADL episodes and stage of lymphedema pre- and post-intervention for 117 lymphedema patients, Léogane, Haiti, 1995–2008
| Outcome | Ratio for year increase among those who received MDA | Ratio for year increase among those who did not receive MDA | Comparison of ratios (received MDA/did not receive MDA) | 95% confidence interval for comparison of ratios | P |
|---|---|---|---|---|---|
| ADL episodes* | |||||
| Pre-intervention | 0.64 | 0.56 | 1.14 | 0.81–1.64 | 0.44 |
| Post-intervention | 1.23 | 1.29 | 0.95 | 0.80–1.13 | 0.57 |
| Stage of lymphedema† | |||||
| Pre-intervention | 0.95 | 0.99 | 0.96 | 0.68–1.35 | 0.82 |
| Post-intervention | 0.94 | 0.94 | 1.00 | 0.84–1.21 | 0.93 |
Ratio reported is rate ratio. Adjusted for model-specific variables by using generalized estimating equations (GEEs) repeated measures Poisson regression. Defined as the number of adenolymphangitis (ADL) episodes per year.
Ratio reported is odds ratio for both stage category 3 vs. categories 2 and 1 and for stage categories 3 and 2 vs. category 1. Adjusted for model-specific variables by using a GEE repeated measures cumulative logistic regression.
Effect of MDA on quantitative leg measurements.
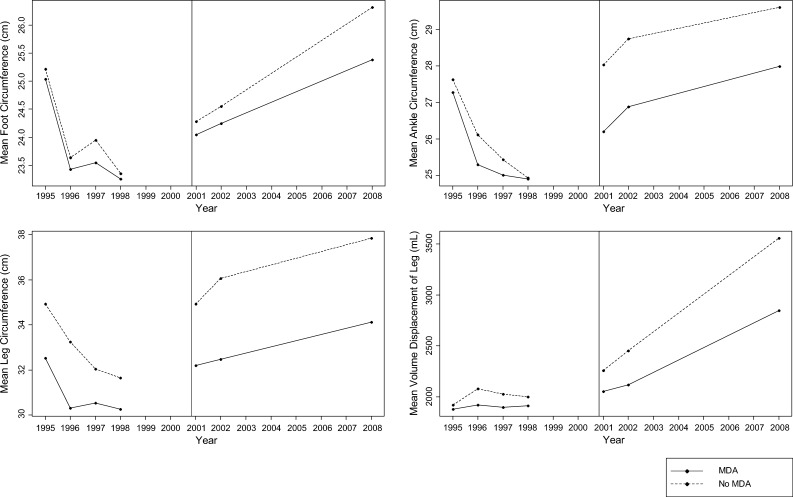
During the post-intervention period (2000–2008), there was no difference in the rate of change between those who received MDA and those who did not for any of the measurement outcomes (i.e., foot, ankle, and leg circumference, and volume displacement of leg) (Table 2 and Figure 1). These models all controlled for compliance with lymphedema management, level of transmission, bandage use, the number of ADL episodes per year, presence of interdigital lesions, and stage of lymphedema. In addition, other significant covariates were controlled for in these models (Table 2).
Figure 1.
Quantitative lower extremity measurements for 1995–2008 among 117 lymphedema patients who received and did not receive mass drug administration (MDA) in Léogane, Haiti. The vertical line represents implementation of the intervention, MDA, in October 2000. All graphs represent the raw mean of the quantitative measurements and do not reflect the actual slope of the line after controlling for other covariates.
Effect of MDA on ADL episodes.
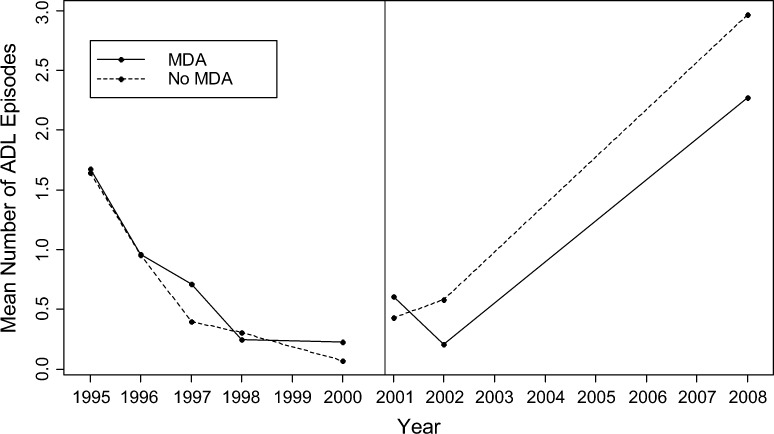
Post-intervention, no difference in the changes in rates of ADL episodes per year were found when comparing persons who received MDA and those who did not (P = 0.57) (Table 3 and Figure 2). This model adjusted for literacy, transmission level, compliance with lymphedema management, wealth quintile, presence of interdigital lesions, and stage of lymphedema.
Figure 2.
Mean number of adenolymphangitis (ADL) episodes during 1995–2008 among 117 lymphedema patients who received and did not receive mass drug administration (MDA) in Léogane, Haiti. The vertical line represents implementation of the intervention, MDA, in October 2000. The data represent the raw mean number of ADL episodes per year and do not reflect the actual slope of the line after controlling for other covariates.
Effect of MDA on lymphedema stage.
The change in odds of a higher stage category for persons who did not receive MDA did not differ from the change for those who received MDA post-intervention (P = 0.93) (Table 3). Compliance with lymphedema management, level of transmission, bandage use, number of ADL episodes in the past year, compressive bandaging, presence of interdigital lesions, and lymphedema duration were controlled for in the model.
Over the 13-year period, there was no difference between persons who received MDA and those who did not in terms of the percentage of persons transitioning between stage categories (regressing, staying in the same, or advancing a stage category) (P = 0.67, by Wilcoxon rank-sum test). Similarly, no difference was found between the percentage of persons who progressed to an advanced lymphedema stage from either early or no lymphedema between MDA groups (P = 0.71, by chi-square test). Among the entire cohort of 234 legs from 117 participants, 23 (9.8%) of 234 regressed a stage category, 171 (73.1%) of 234 remained in the same stage category, and 40 (17.1%) of 234 advanced a stage category between 1995 and 2008.
Within group comparisons (received or did not receive MDA) pre- and post- intervention.
Effect of MDA on quantitative leg measurements, lymphedema stage, and ADL episodes.
No significant difference in the rate of change was found pre- and post-intervention within either persons who received MDA or those who did not with respect to foot, ankle, and leg circumference measurements and volume displacement of leg (P > 0.06) (Figure 1). Similarly, the change in odds of having a higher stage category did not differ pre- or post-intervention within either MDA group (P > 0.80). In contrast, the rate of increase in the log number of ADL episodes was significantly greater post-intervention than pre-intervention among both MDA groups (P < 0.01) (Figure 2).
Quality of life.
Patients who received MDA reported a significant improvement post-intervention compared with pre-intervention in all areas of quality of life assessed. Of 101 patients, 34 (34.7%) reported an improvement in their experience of pain or discomfort (P < 0.01), 27 (27.0%) of 100 documented an improvement in their suffering from anxiety or depression (P = 0.01), 26 (25.7%) indicated fewer problems with mobility (P < 0.01), and 37 (36.6%) reported a decrease in difficulties performing usual activities (P < 0.01). These differences were not seen for patients who did not receive MDA.
Effect of lymphedema management.
The ADL episodes and foot and ankle circumferences significantly decreased during the lymphedema management study (1995–1998) (P ≤ 0.01). This period was followed by one of significant increase in ADL episodes (P < 0.01) and no significant change in foot or ankle circumference in the years after conclusion of the study (P ≥ 0.23). Although leg circumference did not reach significance, there was a trend toward a higher rate of regression during the lymphedema management study compared with after the study. Volume displacement of the leg failed to show a significant decrease during the lymphedema management study (P = 0.07), but the change in volume displacement of the leg significantly increased after the lymphedema management study ended (P = 0.02) (Table 4).
Table 4.
Rate of change per year on lower extremity measurements, ADL episodes, and stage of lymphedema during and post-lymphedema management study of 117 lymphedema patients, Léogane, Haiti, 1995–2008*
| Outcome | Rate of change per year during lymphedema management study (1995–1998) | P | Rate of change per year post-lymphedema management study (2000–2008) | P |
|---|---|---|---|---|
| Foot circumference† | –0.23 | < 0.01 | –0.12 | 0.10 |
| Ankle circumference‡ | –0.37 | 0.01 | –0.18 | 0.23 |
| Leg circumference§ | –0.20 | 0.18 | –0.13 | 0.46 |
| Volume displacement¶ | 45.01 | 0.07 | 56.69 | 0.02 |
| ADL episodes# | 0.59 | < 0.01 | 1.28 | < 0.01 |
Adjusted for the same model specific variables as the mass drug administration models by using a generalized estimating equations (GEEs) repeated measures linear regression unless noted otherwise below. ADL = adenolymphangitis.
Circumference (in cm) of the foot 10 cm from the tip of the first toe.
Circumference (in cm) of the ankle 10 cm from the floor.
Circumference (in cm) of the ankle 25 cm from the floor.
Amount of water (in mL) displaced from a standard volume of water.
Rate of change reported is a rate ratio using a GEE repeated measures Poisson regression model. Rate defined as the number of ADL episodes per year.
Discussion
This study represents the first longitudinal, quantitative, leg-specific analysis examining the clinical effect of DEC on lymphedema progression and ADL episodes over a 13-year period. The results of this analysis did not find a difference in the progression of lymphedema between those who received DEC during MDA and those who did not on measures of foot circumference, ankle circumference, leg circumference, volume displacement of leg, and stage of lymphedema. In addition, there was no difference found in the frequency of ADL episodes between patients who had received DEC and those who had not. Unlike prior studies that examined the prevalence of lymphedema within a population before and after MDA began, the strength of this analysis lies in its ability to examine the change in lymphedema and ADL episodes by accounting for correlation over time in a cohort of persons who were seen repeatedly over a 13-year period (median number of time points of observation = 6).
Despite finding no difference in physical measures of lymphedema progression or ADL episodes, patients who received MDA reported improvement in four areas of quality of life (experience of pain or discomfort, suffering from anxiety or depression, problems with mobility, and difficulties performing usual activities). These results indicate that despite the lack of demonstrable physical improvement in lymphedema and ADL episodes, lymphedema patients can experience psychological benefits after receiving MDA.
The significant increase in the number of ADL episodes after 2000 among persons who received MDA and those who did not indicates that the rate of ADL episodes among this lymphedema cohort increased despite the administration of MDA. These results could indicate that even after administration of MDA in the commune, LF transmission still remained high, thus precipitating ADL episodes.48 Data showed that after six consecutive years of decreasing antigenemia caused by administration of MDA, the suspension of MDA in 2006 resulted in a significant increase in antigenemia.39 Before initiation of MDA, antigen prevalence in Léogane was 50.1%.37 Antigen prevalence steadily decreased from 29.8% in 200337 to 23.2% in 2005, but increased to 31.2% in 2007 after suspension of MDA.39 In addition to the missed MDA, systematic noncompliance, which has been reported in Léogane, could also contribute to the recrudescence of infection in the area.1,39,49
As an alternative hypothesis, significant increase in ADL episodes in this cohort since 2000 could reflect the status of the hospital's lymphedema management program and patient compliance with lymphedema management techniques. Despite controlling for compliance with lymphedema management in this analysis by using a composite score, the components of the hygiene composite score were based on self-reported compliance, which may not accurately reflect what patients were actually practicing at home. Furthermore, for this analysis, we were unable to control for the intensive clinic-based lymphedema management study during 1995–1998. In all other years, there was no active clinic-based lymphedema management program in which patients were regularly attending the clinic. Instead, patients were practicing self-treatment at home and encouraged to seek care as needed for their lymphedema from the outpatient clinic. However, because of the history of political unrest in Haiti, including political turbulence in 2004, the resources available at the clinic have varied over time, specifically regarding availability of healthcare professionals, support groups, and home visit options for patients. This variability in clinic-based care, and specifically the cessation of a lymphedema management study after 1998, could have contributed to the significant increase in ADL episodes observed among persons who received MDA and those who did not after 2000.
The significant improvements detected in foot and ankle circumferences and ADL episodes, along with the lack of increase in leg volume during the lymphedema management study during 1995–1998, suggest that lymphedema management programs can greatly impact quantitative measures of lymphedema.41 Data from this cohort also suggest qualitative improvements in quality of life measures attributable to the lymphedema management program. In 2008, among the lymphedema cohort, 94.0% (110 of 117) reported that hygiene had improved their leg somewhat or greatly and 89.7% (105 of 117) reported that their health in general had improved since they started going to the lymphedema clinic. The teaching, supervision, and reinforcement of lymphedema management techniques offered at the clinic during the intensive lymphedema management study could have greatly contributed to quantitative and qualitative improvements in lymphedema. In addition, the community awareness campaigns and frequent support groups for LF patients during 1995–1998 could have contributed to sustaining patient motivation for self-care at home, further adding to an improvement in lymphedema.
This analysis has several limitations, including the small number of patients in the cohort. In addition, it was unknown in which years DEC was or was not taken by any person. The small number of persons who reported not receiving MDA may have decreased the power to detect a difference between the persons who received MDA and those who did not post-intervention. Despite use of quantitative measures of lymphedema progression, use of leg circumference as an indicator for LF progression is somewhat controversial because it varies depending on time of day, amount of time a person spends on one's feet, and the female monthly hormonal cycle.12 In addition, investigators have reported inconsistencies with inter-observer reliability of measurements (Squire D, unpublished data, 2009). However, leg circumference is preferable to the outcomes other studies have used, including lymphedema stage, because lymphedema progression can be extremely variable within stages. The retrospective nature of this analysis required combining prospective cohort and cross-sectional studies for which data were collected differently. In addition, these data are subject to recall bias given that patients were asked to self-report numerous activities or experiences, particularly over a 12-month period. Lastly, because of the nature of data collection for the qualitative outcomes, we were unable to control for potential cofounders, which may have limited our results. Furthermore, we were only able to examine the perceptions of quality of life pre- and post-intervention among persons who received MDA.
The failure to find an impact of DEC on ADL episodes is consistent with the findings of Beye and others,33 but is inconsistent with other studies assessing DEC.14,20,24–26,28,32,36 Similarly, the failure to find an improvement in lymphedema after receiving DEC is consistent with some studies,32–34 but inconsistent with several reports examining this question; although these studies used varied DEC dosages over varied treatment schedules.20,24–30,50 However, because of numerous differences in methods between studies, comparison with this study is difficult. There is only one other study that has examined the effect of DEC on leg circumference.20 The authors found a significant decrease in leg circumference after exposure to DEC, but only examined the effect over one year and failed to account for the correlation among persons. Although our research contributes to the literature examining the effect of DEC administered through MDA on lymphedema from a methods standpoint, it is clear that there is a need to better understand the effect of drugs used in the MDA (DEC and ivermectin), particularly in relation to albendazole, which is co-administered with these drugs, on filarial morbidity.
Prospective cohort studies are necessary to improve our understanding of this relationship. It is important to examine the consistency with which persons take MDA because the consecutive nature of ingestion may play a role in the rate of change of lymphedema over time. In addition, the effect of MDA on lymphedema-specific morbidity should be examined across a variety of ages because the reversibility of filarial damage observed by some researchers21 and not others22,23 could be dependent on age or duration of infection. New modalities for assessment of filarial lymphedema, such as bioimpedance spectroscopy and tissue tonometry, should also be considered. These modalities measure subclinical changes and could provide a more sensitive and accurate representation of the severity of lymphatic damage than the physical measurements and staging previously available.51
The improvement of lymphedema observed in this cohort during this 13-year period clearly coincided with results of the lymphedema management study during 1995–1998. Consistent with other literature is the significant decrease in ADL episodes14,16,17,20 and the decrease in foot and ankle circumference observed during the lymphedema management program.20 In addition, the improvement in quality of life associated with lymphedema management programs is consistent with previous findings.18,52 These results highlight the positive impact of morbidity control programs on quantitative and qualitative measures of lymphedema-specific morbidity. Therefore, LF elimination programs should prioritize integration of lymphedema management programs along with MDA to achieve their goal of reducing LF-related disability.
ACKNOWLEDGMENTS
We thank the lymphedema patients in Léogane, Haiti for generously devoting their time to participating in these studies throughout the years; the Notre Dame Haiti Program and their staff in Léogane, including Jacky Louis Charles, Dr. Luccène Desir, Dardith Desire, Shiller Emile, Wilnide Guerrier, Jean Makatu Innocent, Muracile Joseph, Wesly Pierre, Marie Michele Saint Fort, Mirna Simon, and Montilus Wilman for their dedication of time and assistance in collecting the data; Ben Dahl, and Natasha Hochberg for generously lending their datasets to be compiled for this work; Jacquelin Roberts for statistical support; Elisabeth Honorat for database assistance and help with implementation of the 2008 study; and Pat Lammie for continued support and insight.
Footnotes
Financial support: This study was supported by The University of Notre Dame Haiti Program with funding by the Bill and Melinda Gates Foundation; the Framework in Global Health (grant 5 R25 TW7733) to Emory University from the Fogarty International Center, National Institutes of Health; and the Centers for Disease Control and Prevention.
Authors' addresses: Brittany A. Eddy, c/o Partners in Health, Boston, MA, E-mail: beddy@pih.org, Anna J. Blackstock and LeAnne M. Fox, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: ablackstock@cdc.gov and lfox@cdc.gov. John M. Williamson, Center for Global Health Research, Centers for Disease Control and Prevention, Kenya Medical Research Institute, Kisian Kisumu, Kenya, E-mail: jwilliamson@ke.cdc.gov. David G. Addiss, Children Without Worms, Task Force for Global Health, Decatur, GA, E-mail: daddiss@taskforce.org. Thomas G. Streit, Center for Tropical Disease Research and Training, University of Notre Dame, Notre Dame, IN, E-mail: streit1@nd.edu. Valery M. Beau de Rochars, Department of Health Services Research, Management and Policy, University of Florida, Gainesville, FL, E-mail: madsenbeau@phhp.ufl.edu.
References
- 1.Talbot JT, Viall A, Direny A, de Rochars MB, Addiss D, Streit T, Mathieu E, Lammie PJ. Predictors of compliance in mass drug administration for the treatment and prevention of lymphatic filariasis in Leogane, Haiti. Am J Trop Med Hyg. 2008;78:283–288. [PubMed] [Google Scholar]
- 2.Shenoy RK, Suma TK, Kumaraswami V, Rahmah N, Dhananjayan G, Padma S, Abhilash G, Ramesh C. Preliminary findings from a cross-sectional study on lymphatic filariasis in children, in an area of India endemic for Brugia malayi infection. Ann Trop Med Parasitol. 2007;101:205–213. doi: 10.1179/136485907X154548. [DOI] [PubMed] [Google Scholar]
- 3.Bernhard P, Magnussen P, Lemnge MM. A randomized, double-blind, placebo-controlled study with diethylcarbamazine for the treatment of hydrocoele in an area of Tanzania endemic for lymphatic filariasis. Trans R Soc Trop Med Hyg. 2001;95:534–536. doi: 10.1016/s0035-9203(01)90031-8. [DOI] [PubMed] [Google Scholar]
- 4.Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: a review of the scientific literature. Filaria J. 2007;6:2. doi: 10.1186/1475-2883-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreyer G, Medeiros Z, Netto MJ, Leal NC, de Castro LG, Piessens WF. Acute attacks in the extremities of persons living in an area endemic for bancroftian filariasis: differentiation of two syndromes. Trans R Soc Trop Med Hyg. 1999;93:413–417. doi: 10.1016/s0035-9203(99)90140-2. [DOI] [PubMed] [Google Scholar]
- 6.McPherson T, Persaud S, Singh S, Fay MP, Addiss D, Nutman TB, Hay R. Interdigital lesions and frequency of acute dermatolymphangioadenitis in lymphoedema in a filariasis-endemic area. Br J Dermatol. 2006;154:933–941. doi: 10.1111/j.1365-2133.2005.07081.x. [DOI] [PubMed] [Google Scholar]
- 7.Pani SP, Srividya A. Clinical manifestations of bancroftian filariasis with special reference to lymphoedema grading. Indian J Med Res. 1995;102:114–118. [PubMed] [Google Scholar]
- 8.Pani SP, Yuvaraj J, Vanamail P, Dhanda V, Michael E, Grenfell BT, Bundy DA. Episodic adenolymphangitis and lymphoedema in patients with bancroftian filariasis. Trans R Soc Trop Med Hyg. 1995;89:72–74. doi: 10.1016/0035-9203(95)90666-5. [DOI] [PubMed] [Google Scholar]
- 9.Shenoy RK, Sandhya K, Suma TK, Kumaraswami V. A preliminary study of filariasis related acute adenolymphangitis with special reference to precipitating factors and treatment modalities. Southeast Asian J Trop Med Public Health. 1995;26:301–305. [PubMed] [Google Scholar]
- 10.Babu BV, Nayak AN, Dhal K. Epidemiology of episodic adenolymphangitis: a longitudinal prospective surveillance among a rural community endemic for bancroftian filariasis in coastal Orissa, India. BMC Public Health. 2005;5:50. doi: 10.1186/1471-2458-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ottesen EA. The global programme to eliminate lymphatic filariasis. Trop Med Int Health. 2000;5:591–594. doi: 10.1046/j.1365-3156.2000.00620.x. [DOI] [PubMed] [Google Scholar]
- 12.Dreyer G, Addiss DG, Dreyer P, Norões J. Basic Lymphoedema Management: Treatment and Prevention of Problems Associated with Lymphatic Filariasis. Hollis, NJ: Hollis Publishing Co; 2002. [Google Scholar]
- 13.Debrah AY, Mand S, Specht S, Marfo-Debrekyei Y, Batsa L, Pfarr K, Larbi J, Lawson B, Taylor M, Adjei O, Hoerauf A. Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis. PLoS Pathog. 2006;2:e92. doi: 10.1371/journal.ppat.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph A, Mony P, Prasad M, John S, Srikanth, Mathai D. The efficacies of affected-limb care with penicillin diethylcarbamazine, the combination of both drugs or antibiotic ointment, in the prevention of acute adenolymphangitis during bancroftian filariasis. Ann Trop Med Parasitol. 2004;98:685–696. doi: 10.1179/000349804225021451. [DOI] [PubMed] [Google Scholar]
- 15.Shenoy RK, Suma TK, Rajan K, Kumaraswami V. Prevention of acute adenolymphangitis in brugian filariasis: comparison of the efficacy of ivermectin and diethylcarbamazine, each combined with local treatment of the affected limb. Ann Trop Med Parasitol. 1998;92:587–594. doi: 10.1080/00034989859285. [DOI] [PubMed] [Google Scholar]
- 16.Shenoy RK, Kumaraswami V, Suma TK, Rajan K, Radhakuttyamma G. A double-blind, placebo-controlled study of the efficacy of oral penicillin, diethylcarbamazine or local treatment of the affected limb in preventing acute adenolymphangitis in lymphoedema caused by brugian filariasis. Ann Trop Med Parasitol. 1999;93:367–377. doi: 10.1080/00034989958366. [DOI] [PubMed] [Google Scholar]
- 17.Suma TK, Shenoy RK, Kumaraswami V. Efficacy and sustainability of a footcare programme in preventing acute attacks of adenolymphangitis in Brugian filariasis. Trop Med Int Health. 2002;7:763–766. doi: 10.1046/j.1365-3156.2002.00914.x. [DOI] [PubMed] [Google Scholar]
- 18.McPherson T. Impact on the quality of life of lymphoedema patients following introduction of a hygiene and skin care regimen in a Guyanese community endemic for lymphatic filariasis: a preliminary clinical intervention study. Filaria J. 2003;2:1. doi: 10.1186/1475-2883-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wijesinghe RS, Wickremasinghe AR, Ekanayake S, Perera MS. Efficacy of a limb-care regime in preventing acute adenolymphangitis in patients with lymphoedema caused by bancroftian filariasis, in Colombo, Sri Lanka. Ann Trop Med Parasitol. 2007;101:487–497. doi: 10.1179/136485907X193806. [DOI] [PubMed] [Google Scholar]
- 20.Kerketta AS, Babu BV, Rath K, Jangid PK, Nayak AN, Kar SK. A randomized clinical trial to compare the efficacy of three treatment regimens along with footcare in the morbidity management of filarial lymphoedema. Trop Med Int Health. 2005;10:698–705. doi: 10.1111/j.1365-3156.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- 21.Shenoy RK, Suma TK, Kumaraswami V, Rahmah N, Dhananjayan G, Padma S. Antifilarial drugs, in the doses employed in mass drug administrations by the Global Programme to Eliminate Lymphatic Filariasis, reverse lymphatic pathology in children with Brugia malayi infection. Ann Trop Med Parasitol. 2009;103:235–247. doi: 10.1179/136485909X398249. [DOI] [PubMed] [Google Scholar]
- 22.Dreyer G, Addiss D, Roberts J, Noroes J. Progression of lymphatic vessel dilatation in the presence of living adult Wuchereria bancrofti. Trans R Soc Trop Med Hyg. 2002;96:157–161. doi: 10.1016/s0035-9203(02)90288-9. [DOI] [PubMed] [Google Scholar]
- 23.Freedman DO, Bui T, De Almeida Filho PJ, Braga C, Maia e Silva MC, Maciel A, Furtado AF. Lymphoscintigraphic assessment of the effect of diethylcarbamazine treatment on lymphatic damage in human bancroftian filariasis. Am J Trop Med Hyg. 1995;52:258–261. doi: 10.4269/ajtmh.1995.52.258. [DOI] [PubMed] [Google Scholar]
- 24.March HN, Laigret J, Kessel JF, Bambridge B. Reduction in the prevalence of clinical filariasis in Tahiti following adoption of a control program. Am J Trop Med Hyg. 1960;9:180–184. doi: 10.4269/ajtmh.1960.9.180. [DOI] [PubMed] [Google Scholar]
- 25.Partono F, Maizels RM, Purnomo Towards a filariasis-free community: evaluation of filariasis control over an eleven year period in Flores, Indonesia. Trans R Soc Trop Med Hyg. 1989;83:821–826. doi: 10.1016/0035-9203(89)90343-x. [DOI] [PubMed] [Google Scholar]
- 26.Hewitt R, Kenney M, Chan A, Mohamed H. Follow-up observations on the treatment of bancroftian filariasis with hetrazan in British Guiana. Am J Trop Med Hyg. 1950;30:217–237. doi: 10.4269/ajtmh.1950.s1-30.217. [DOI] [PubMed] [Google Scholar]
- 27.Kenney M, Hewitt R. Treatment of bancroftian filariasis with hetrazan in British Guiana. Am J Trop Med Hyg. 1949;29:89–114. doi: 10.4269/ajtmh.1949.s1-29.89. [DOI] [PubMed] [Google Scholar]
- 28.Pani SP, Krishnamoorthy K, Prathibha J, Rao AS. Diethylcarbamazine and supportive measures for the treatment of brugian filariasis. Natl Med J India. 1989;2:260–263. [Google Scholar]
- 29.Moore TA, Reynolds JC, Kenney RT, Johnston W, Nutman TB. Diethylcarbamazine-induced reversal of early lymphatic dysfunction in a patient with bancroftian filariasis: assessment with use of lymphoscintigraphy. Clin Infect Dis. 1996;23:1007–1011. doi: 10.1093/clinids/23.5.1007. [DOI] [PubMed] [Google Scholar]
- 30.Bockarie MJ, Tisch DJ, Kastens W, Alexander ND, Dimber Z, Bockarie F, Ibam E, Alpers MP, Kazura JW. Mass treatment to eliminate filariasis in Papua New Guinea. N Engl J Med. 2002;347:1841–1848. doi: 10.1056/NEJMoa021309. [DOI] [PubMed] [Google Scholar]
- 31.Meyrowitsch DW, Simonsen PE, Makunde WH. Mass diethylcarbamazine chemotherapy for control of bancroftian filariasis through community participation: comparative efficacy of a low monthly dose and medicated salt. Trans R Soc Trop Med Hyg. 1996;90:74–79. doi: 10.1016/s0035-9203(96)90485-x. [DOI] [PubMed] [Google Scholar]
- 32.Ciferri F, Siliga N, Long G, Kessel JF. A filariasis-control program in American Samoa. Am J Trop Med Hyg. 1969;18:369–378. doi: 10.4269/ajtmh.1969.18.369. [DOI] [PubMed] [Google Scholar]
- 33.Beye HK, Edgar SA, Mille R, Kessel JF, Bambridge B. Preliminary observations on the prevalence, clinical manifestations and control of filariasis in the Society Islands. Am J Trop Med Hyg. 1952;1:637–661. doi: 10.4269/ajtmh.1952.1.637. [DOI] [PubMed] [Google Scholar]
- 34.Fan PC, Peng HW, Chen CC. Follow-up investigations on clinical manifestations after filariasis eradication by diethylcarbamazine medicated common salt on Kinmen (Quemoy) Islands, Republic of China. J Trop Med Hyg. 1995;98:461–464. [PubMed] [Google Scholar]
- 35.Das L, Subramanyam Reddy G, Pani S. Some observations on the effect of Daflon (micronized purified flavonoid fraction of Rutaceae aurantiae) in bancroftian filarial lymphoedema. Filaria J. 2003;2:5. doi: 10.1186/1475-2883-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kessel JF. An effective programme for the control of filariasis in Tahiti. Bull World Health Organ. 1957;16:633–664. [PMC free article] [PubMed] [Google Scholar]
- 37.Beau de Rochars MB, Kanjilal S, Direny AN, Radday J, Lafontant JG, Mathieu E, Rheingans RD, Haddix AC, Streit TG, Beach MJ, Addiss DG, Lammie PJ. The Leogane, Haiti demonstration project: decreased microfilaremia and program costs after three years of mass drug administration. Am J Trop Med Hyg. 2005;73:888–894. [PubMed] [Google Scholar]
- 38.World Health Organization Lymphatic filariasis: progress report on mass drug administration in 2007. Wkly Epidemiol Rec. 2008;83:333–348. [Google Scholar]
- 39.Won KY, Beau de Rochars M, Kyelem D, Streit TG, Lammie PJ. Assessing the impact of a missed mass drug administration in Haiti. PLoS Negl Trop Dis. 2009;3:e443. doi: 10.1371/journal.pntd.0000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyd HA, Waller LA, Flanders WD, Beach MJ, Sivilus JS, Lovince R, Lammie PJ, Addiss DG. Community- and individual-level determinants of Wuchereria bancrofti infection in Leogane Commune, Haiti. Am J Trop Med Hyg. 2004;70:266–272. [PubMed] [Google Scholar]
- 41.Addiss DG, Louis-Charles J, Roberts J, Leconte F, Wendt JM, Milord MD, Lammie PJ, Dreyer G. Feasibility and effectiveness of basic lymphedema management in Leogane, Haiti, an area endemic for bancroftian filariasis. PLoS Negl Trop Dis. 2010;4:e668. doi: 10.1371/journal.pntd.0000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Addiss DG, Michel MC, Michelus A, Radday J, Billhimer W, Louis-Charles J, Roberts JM, Kramp K, Dahl BA, Keswick B. Evaluation of antibacterial soap in the management of lymphoedema in Leogane, Haiti. Trans R Soc Trop Med Hyg. 2011;105:58–60. doi: 10.1016/j.trstmh.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Pani SP, Vanamail P, Yuvaraj J. Limb circumference measurement for recording edema volume in patients with filarial lymphedema. Lymphology. 1995;28:57–63. [PubMed] [Google Scholar]
- 44.Boyd A, Won KY, McClintock SK, Donovan CV, Laney SJ, Williams SA, Pilotte N, Streit TG, Beau de Rochars MV, Lammie PJ. A community-based study of factors associated with continuing transmission of lymphatic filariasis in Leogane, Haiti. PLoS Negl Trop Dis. 2010;4:e640. doi: 10.1371/journal.pntd.0000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 46.Kazura JW, Bockarie M, Alexander N, Perry R, Bockarie F, Dagoro H, Dimber Z, Hyun P, Alpers MP. Transmission intensity and its relationship to infection and disease due to Wuchereria bancrofti in Papua New Guinea. J Infect Dis. 1997;176:242–246. doi: 10.1086/514030. [DOI] [PubMed] [Google Scholar]
- 47.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- 48.Shi ZJ, Xie JZ, Hu XL, Li ZX, Ren YF, Sun DJ, Xu SR, Yuan YZ, Shen BG. Studies on the recurrent attacks of acute adenolymphangitis due to Malayan filariasis [in Chinese] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2000;18:79–83. [PubMed] [Google Scholar]
- 49.Mathieu E, Direny AN, de Rochars MB, Streit TG, Addiss DG, Lammie PJ. Participation in three consecutive mass drug administrations in Leogane, Haiti. Trop Med Int Health. 2006;11:862–868. doi: 10.1111/j.1365-3156.2006.01626.x. [DOI] [PubMed] [Google Scholar]
- 50.Meyrowitsch DW, Simonsen PE, Makunde WH. Mass diethylcarbamazine chemotherapy for control of bancroftian filariasis: comparative efficacy of standard treatment and two semi-annual single-dose treatments. Trans R Soc Trop Med Hyg. 1996;90:69–73. doi: 10.1016/s0035-9203(96)90484-8. [DOI] [PubMed] [Google Scholar]
- 51.Ward LC. Bioelectrical impedance analysis: proven utility in lymphedema risk assessment and therapeutic monitoring. Lymphat Res Biol. 2006;4:51–56. doi: 10.1089/lrb.2006.4.51. [DOI] [PubMed] [Google Scholar]
- 52.Shenoy RK, Suma TK, Kumaraswami V. A qualitative study on the feasibility and benefits of foot hygiene measures practiced by patients with brugian filariasis. J Commun Dis. 2003;35:9–16. [PubMed] [Google Scholar]