Abstract
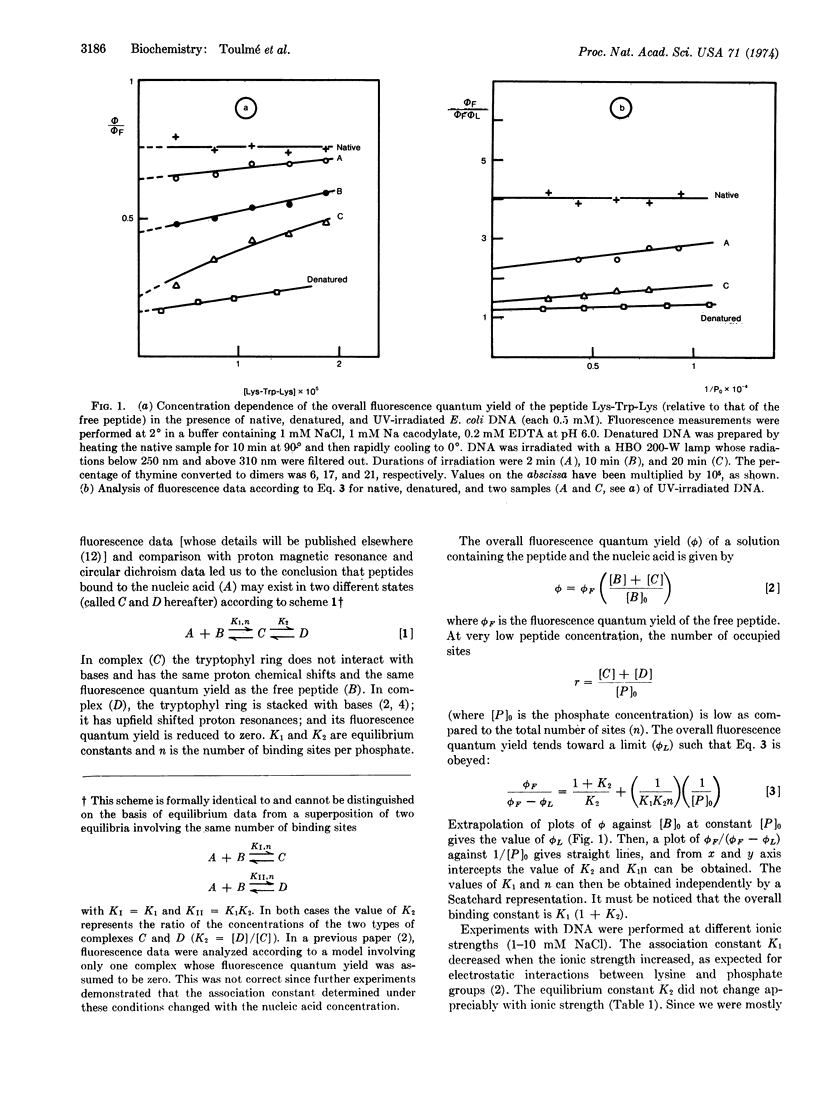
Fluorescence studies of the binding of peptides containing lysyl and tryptophyl residues to nucleic acids show that two types of complexes are formed. One of them involves a direct interaction of the tryptophyl ring with nucleic acid bases, which leads to fluorescence quenching. Comparison with proton magnetic resonance and circular dichroism data indicates that this fluorescence quenching is due to a stacking of the indole ring with bases. Quantitative analysis of fluorescence data leads to the conclusion that stacking is favored in single-stranded regions of DNAs, which are produced either by heating or by UV-irradiation of the native DNA sample. The binding of the peptide Lys-Trp-Lys is about ten times tighter in these single-stranded regions as compared with double-stranded ones. A short tripeptide such as Lys-Trp-Lys is, therefore, able to discriminate between single-stranded and double-stranded regions. Moreover, bound peptide molecules photosensitize the splitting of thymine dimers in UV-irradiated DNA, thus providing a model for DNA photoreactivation.
Keywords: protein-nucleic acid interaction, photoreactivation, fluorescence
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crick F. General model for the chromosomes of higher organisms. Nature. 1971 Nov 5;234(5323):25–27. doi: 10.1038/234025a0. [DOI] [PubMed] [Google Scholar]
- Dimicoli J. L., Hélène C. Interactions of aromatic residues of proteins with nucleic acid. II. Proton magnetic resonance studies of the binding of tyramine and tyrosine-containing peptides to poly(adenylic acid) and deoxyribonucleic acid. Biochemistry. 1974 Feb 12;13(4):724–730. doi: 10.1021/bi00701a014. [DOI] [PubMed] [Google Scholar]
- Gabbay E. J., Sanford K., Baxter C. S., Kapicak L. Specific interaction of peptides with nucleic acids. Evidence for a "selective bookmark" recognition hypothesis. Biochemistry. 1973 Oct 9;12(21):4021–4029. doi: 10.1021/bi00745a001. [DOI] [PubMed] [Google Scholar]
- Gabbay E. J., Sanford K., Baxter C. S. Specific interaction of peptides with nucleic acids. Biochemistry. 1972 Aug 29;11(18):3429–3435. doi: 10.1021/bi00768a016. [DOI] [PubMed] [Google Scholar]
- Helene C., Charlier M. Photosensitized splitting of pyrimidine dimers by indole derivatives. Biochem Biophys Res Commun. 1971 Apr 16;43(2):252–257. doi: 10.1016/0006-291x(71)90745-5. [DOI] [PubMed] [Google Scholar]
- Hélène C., Dimicoli J. L., Brun F. Binding of tryptamine and 5-hydroxytryptamine (serotonin) to nucleic acids. Fluorescence and proton magnetic resonance studies. Biochemistry. 1971 Sep 28;10(20):3802–3809. doi: 10.1021/bi00796a025. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Kushner S. R., Grossman L. Enzymatic repair of DNA. 3. Properties of the UV-endonuclease and UV-exonuclease. Biochemistry. 1971 Aug 31;10(18):3315–3324. doi: 10.1021/bi00794a001. [DOI] [PubMed] [Google Scholar]
- Sellini H., Maurizot J. C., Dimicoli J. L., Helene C. Hydrogen bonding of amino acid side chains to nucleic acid bases. FEBS Lett. 1973 Mar 1;30(2):219–224. doi: 10.1016/0014-5793(73)80655-6. [DOI] [PubMed] [Google Scholar]
- Vigny P. Fluorescence de l'acide polyacénylique à température ambiante. C R Acad Sci Hebd Seances Acad Sci D. 1973 Nov 5;277(18):1941–1944. [PubMed] [Google Scholar]
- Von Hippel P. H., Wong K. Y. Dynamic aspects of native DNA structure: kinetics of the formaldehyde reaction with calf thymus DNA. J Mol Biol. 1971 Nov 14;61(3):587–613. doi: 10.1016/0022-2836(71)90066-0. [DOI] [PubMed] [Google Scholar]



