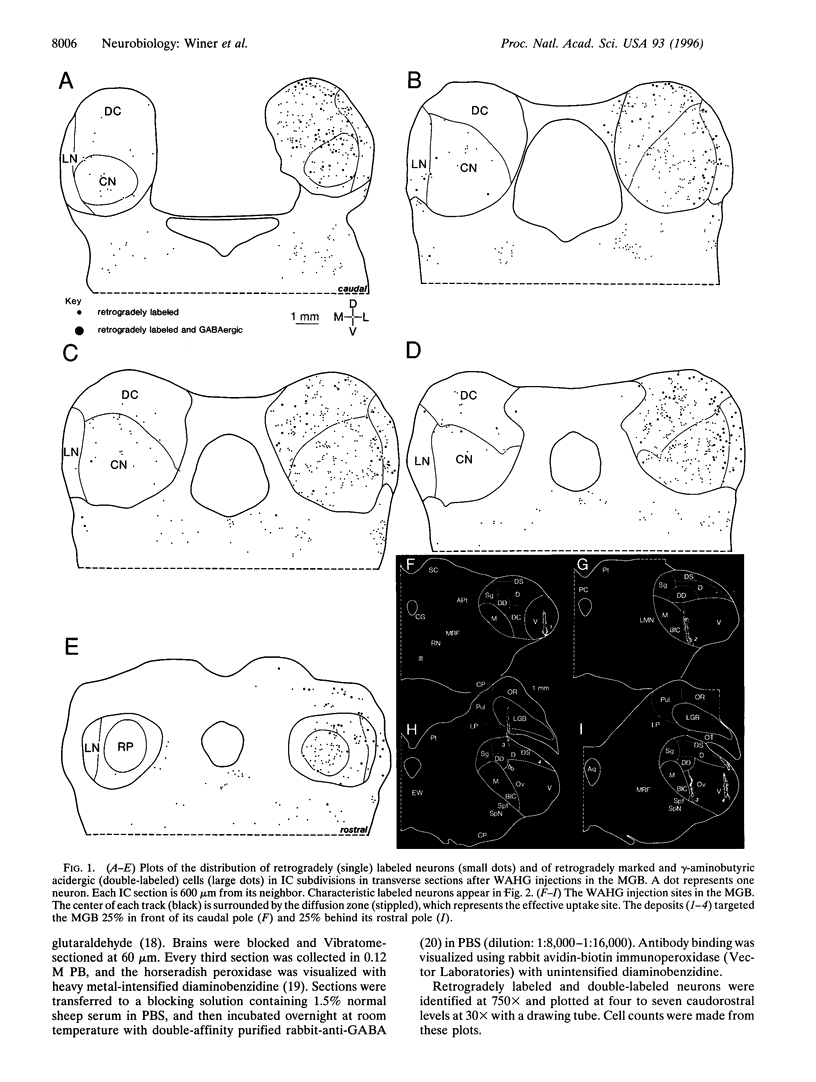
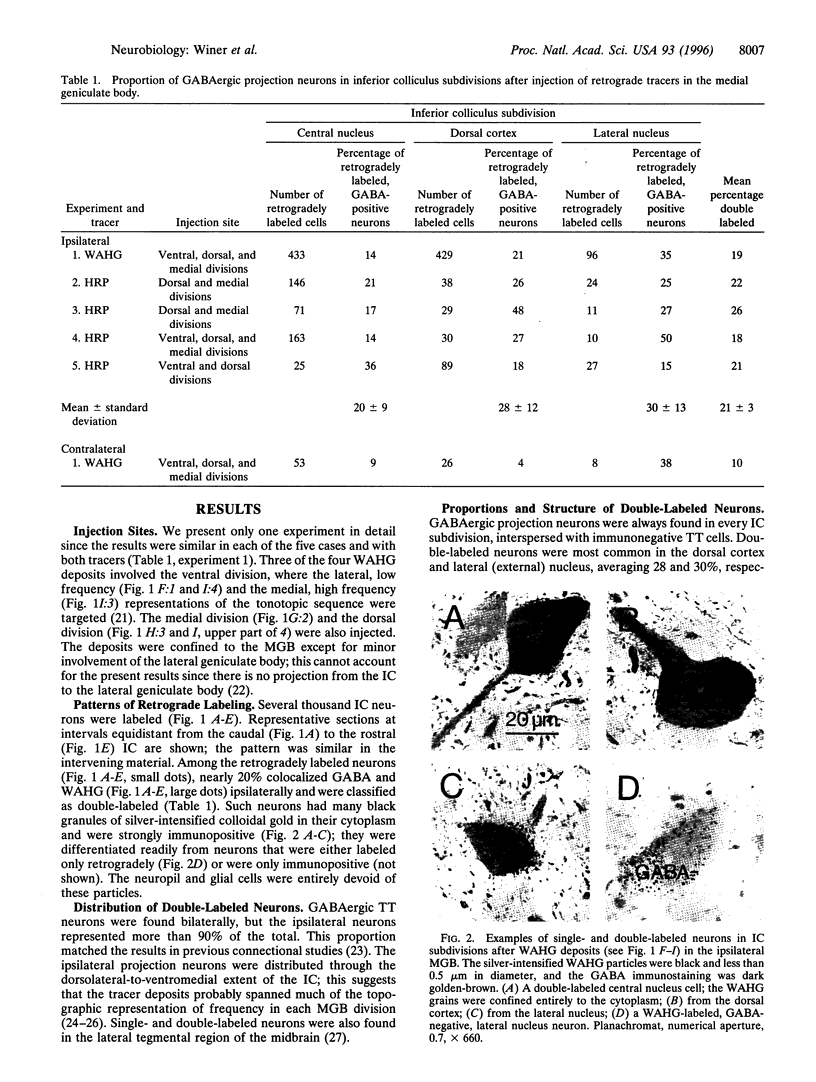
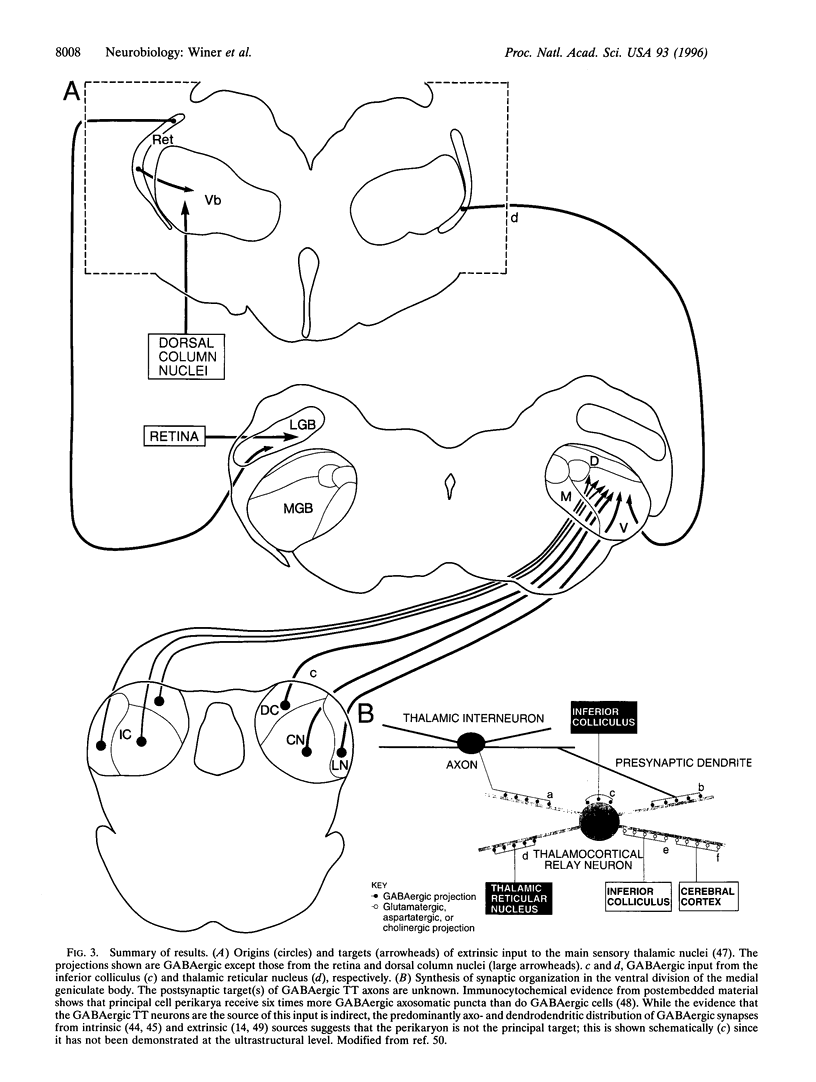
Abstract
A novel and robust projection from gamma-aminobutyric acid-containing (GABAergic) inferior colliculus neurons to the media] geniculate body (MGB) was discovered in the cat using axoplasmic transport methods combined with immunocytochemistry. This input travels with the classical inferior colliculus projection to the MGB, and it is a direct ascending GABAergic pathway to the sensory thalamus that may be inhibitory. This bilateral projection constitutes 10-30% of the neurons in the auditory tectothalamic system. Studies by others have shown that comparable input to the corresponding thalamic visual or somesthetic nuclei is absent. This suggests that monosynaptic inhibition or disinhibition is a prominent feature in the MGB and that differences in neural circuitry distinguish it from its thalamic visual and somesthetic counterparts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981 Jun;29(6):775–775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Aitkin L. M., Dunlop C. W. Inhibition in the medial geniculate body of the cat. Exp Brain Res. 1969;7(1):68–83. doi: 10.1007/BF00236108. [DOI] [PubMed] [Google Scholar]
- Aitkin L. M., Webster W. R. Medial geniculate body of the cat: organization and responses to tonal stimuli of neurons in ventral division. J Neurophysiol. 1972 May;35(3):365–380. doi: 10.1152/jn.1972.35.3.365. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., LEVICK W. R., VAKKUR G. J. The determination of the projection of the visual field on to the lateral geniculate nucleus in the cat. J Physiol. 1962 Oct;163:503–539. doi: 10.1113/jphysiol.1962.sp006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum A. I., Menetrey D. Wheat germ agglutinin-apoHRP gold: a new retrograde tracer for light- and electron-microscopic single- and double-label studies. J Comp Neurol. 1987 Jul 8;261(2):306–318. doi: 10.1002/cne.902610211. [DOI] [PubMed] [Google Scholar]
- Boos R., Müller F., Wässle H. Actions of excitatory amino acids on brisk ganglion cells in the cat retina. J Neurophysiol. 1990 Nov;64(5):1368–1379. doi: 10.1152/jn.1990.64.5.1368. [DOI] [PubMed] [Google Scholar]
- Calford M. B., Aitkin L. M. Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through thalamus. J Neurosci. 1983 Nov;3(11):2365–2380. doi: 10.1523/JNEUROSCI.03-11-02365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiaro J. B., Bickford M. E., Sherman S. M. A GABAergic projection from the pretectum to the dorsal lateral geniculate nucleus in the cat. Neuroscience. 1991;41(1):213–226. doi: 10.1016/0306-4522(91)90211-6. [DOI] [PubMed] [Google Scholar]
- De Biasi S., Rustioni A. Ultrastructural immunocytochemical localization of excitatory amino acids in the somatosensory system. J Histochem Cytochem. 1990 Dec;38(12):1745–1754. doi: 10.1177/38.12.1701456. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Lin C. S., Stanford L. R., Sherman S. M. Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol. 1981 Jul;46(1):80–129. doi: 10.1152/jn.1981.46.1.80. [DOI] [PubMed] [Google Scholar]
- Geisert E. E., Jr Cortical projections of the lateral geniculate nucleus in the cat. J Comp Neurol. 1980 Apr 15;190(4):793–812. doi: 10.1002/cne.901900410. [DOI] [PubMed] [Google Scholar]
- Henkel C. K. Evidence of sub-collicular auditory projections to the medial geniculate nucleus in the cat: an autoradiographic and horseradish peroxidase study. Brain Res. 1983 Jan 17;259(1):21–30. doi: 10.1016/0006-8993(83)91063-6. [DOI] [PubMed] [Google Scholar]
- Hu B., Senatorov V., Mooney D. Lemniscal and non-lemniscal synaptic transmission in rat auditory thalamus. J Physiol. 1994 Sep 1;479(Pt 2):217–231. doi: 10.1113/jphysiol.1994.sp020290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig T. J., Morel A. Tonotopic organization in ventral nucleus of medial geniculate body in the cat. J Neurophysiol. 1985 Jan;53(1):309–340. doi: 10.1152/jn.1985.53.1.309. [DOI] [PubMed] [Google Scholar]
- Ivarsson C., De Ribaupierre Y., De Ribaupierre F. Influence of auditory localization cues on neuronal activity in the auditory thalamus of the cat. J Neurophysiol. 1988 Feb;59(2):586–606. doi: 10.1152/jn.1988.59.2.586. [DOI] [PubMed] [Google Scholar]
- Lugo-García N., Blanco R. E. Localization of GAD- and GABA-like immunoreactivity in ground squirrel retina: retrograde labeling demonstrates GAD-positive ganglion cells. Brain Res. 1991 Nov 8;564(1):19–26. doi: 10.1016/0006-8993(91)91346-3. [DOI] [PubMed] [Google Scholar]
- MOREST D. K. THE NEURONAL ARCHITECTURE OF THE MEDIAL GENICULATE BODY OF THE CAT. J Anat. 1964 Oct;98:611–630. [PMC free article] [PubMed] [Google Scholar]
- MOUNTCASTLE V., HENNEMAN E. Pattern of tactile representation in thalamus of cat. J Neurophysiol. 1949 Mar;12(2):85–100. doi: 10.1152/jn.1949.12.2.85. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Reid M. D. Representation of the cochlea within the inferior colliculus of the cat. Brain Res. 1974 Sep 13;77(3):397–415. doi: 10.1016/0006-8993(74)90630-1. [DOI] [PubMed] [Google Scholar]
- Montero V. M. Quantitative immunogold analysis reveals high glutamate levels in synaptic terminals of retino-geniculate, cortico-geniculate, and geniculo-cortical axons in the cat. Vis Neurosci. 1990 May;4(5):437–443. doi: 10.1017/s0952523800005198. [DOI] [PubMed] [Google Scholar]
- Morest D. K. Dendrodendritic synapses of cells that have axons: the fine structure of the Golgi type II cell in the medial geniculate body of the cat. Z Anat Entwicklungsgesch. 1971;133(2):216–246. doi: 10.1007/BF00528025. [DOI] [PubMed] [Google Scholar]
- Morest D. K. Synaptic relationships of Golgi type II cells in the medial geniculate body of the cat. J Comp Neurol. 1975 Jul 15;162(2):157–193. doi: 10.1002/cne.901620202. [DOI] [PubMed] [Google Scholar]
- Niimi K., Matsuoka H. Thalamocortical organization of the auditory system in the cat studied by retrograde axonal transport of horseradish peroxidase. Adv Anat Embryol Cell Biol. 1979;57:1–56. doi: 10.1007/978-3-642-67353-5. [DOI] [PubMed] [Google Scholar]
- Oliver D. L., Winer J. A., Beckius G. E., Saint Marie R. L. Morphology of GABAergic neurons in the inferior colliculus of the cat. J Comp Neurol. 1994 Feb 1;340(1):27–42. doi: 10.1002/cne.903400104. [DOI] [PubMed] [Google Scholar]
- Ottersen O. P., Storm-Mathisen J. Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol. 1984 Nov 1;229(3):374–392. doi: 10.1002/cne.902290308. [DOI] [PubMed] [Google Scholar]
- Saint Marie R. L., Benson C. G., Ostapoff E. M., Morest D. K. Glycine immunoreactive projections from the dorsal to the anteroventral cochlear nucleus. Hear Res. 1991 Jan;51(1):11–28. doi: 10.1016/0378-5955(91)90003-r. [DOI] [PubMed] [Google Scholar]
- Saint Marie R. L., Ostapoff E. M., Morest D. K., Wenthold R. J. Glycine-immunoreactive projection of the cat lateral superior olive: possible role in midbrain ear dominance. J Comp Neurol. 1989 Jan 15;279(3):382–396. doi: 10.1002/cne.902790305. [DOI] [PubMed] [Google Scholar]
- Spreafico R., Hayes N. L., Rustioni A. Thalamic projections to the primary and secondary somatosensory cortices in cat: single and double retrograde tracer studies. J Comp Neurol. 1981 Nov 20;203(1):67–90. doi: 10.1002/cne.902030107. [DOI] [PubMed] [Google Scholar]
- Wenthold R. J., Huie D., Altschuler R. A., Reeks K. A. Glycine immunoreactivity localized in the cochlear nucleus and superior olivary complex. Neuroscience. 1987 Sep;22(3):897–912. doi: 10.1016/0306-4522(87)92968-x. [DOI] [PubMed] [Google Scholar]
- Winer J. A., Larue D. T. Evolution of GABAergic circuitry in the mammalian medial geniculate body. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):3083–3087. doi: 10.1073/pnas.93.7.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer J. A., Larue D. T., Pollak G. D. GABA and glycine in the central auditory system of the mustache bat: structural substrates for inhibitory neuronal organization. J Comp Neurol. 1995 May 8;355(3):317–353. doi: 10.1002/cne.903550302. [DOI] [PubMed] [Google Scholar]
- Winer J. A., Morest D. K. The medial division of the medial geniculate body of the cat: implications for thalamic organization. J Neurosci. 1983 Dec;3(12):2629–2651. doi: 10.1523/JNEUROSCI.03-12-02629.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]