Abstract
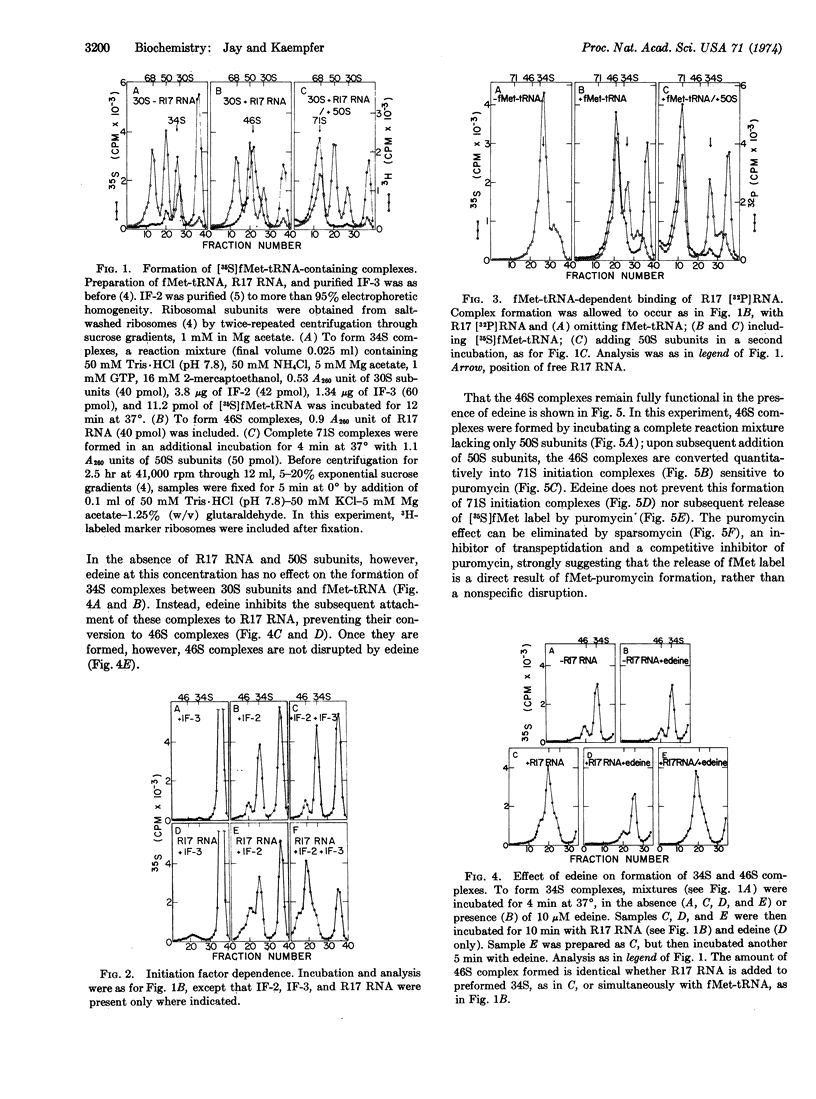
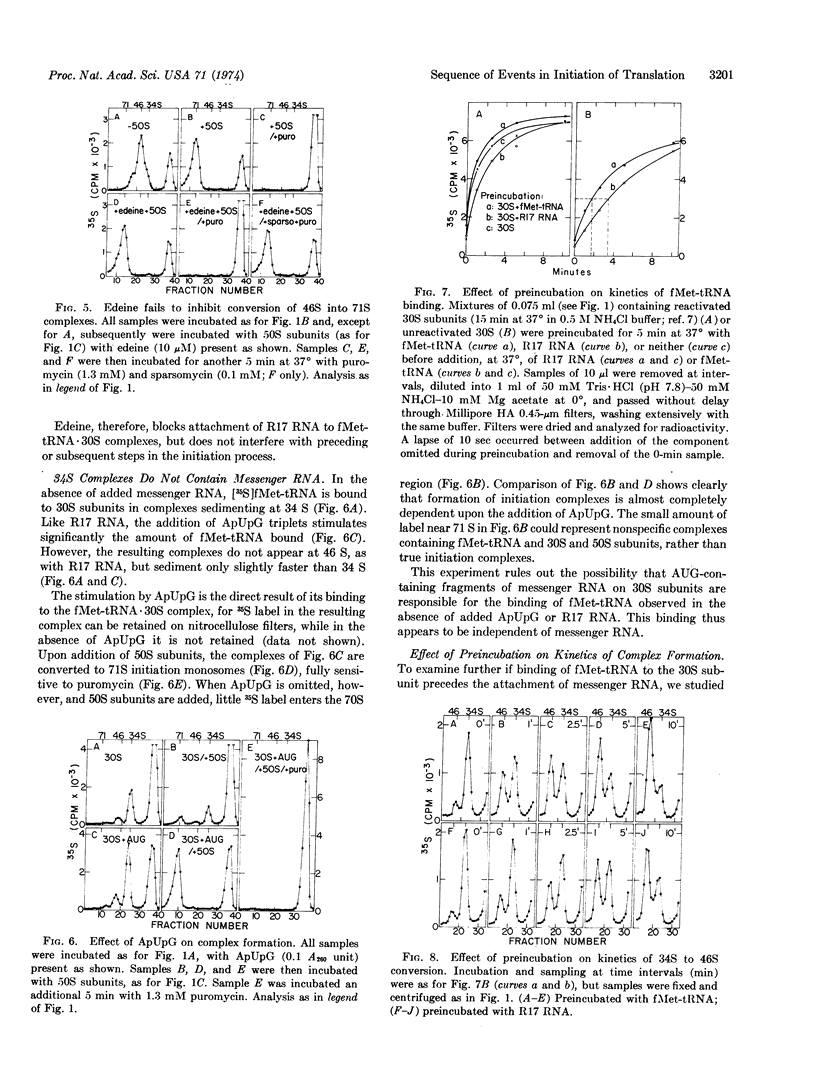
It is shown that initiation of translation involves several steps. (i) Binding of fMet-tRNAfMet to the bacterial 30S ribosomal subunit in the absence of messenger RNA, yielding a 34S complex. This binding is rapid and dependent on initiation factor 2 but not on initiation factor 3. (ii) Binding of messenger RNA to the 34S complex. This binding is slower and depends on initiation factor 3. If R17 RNA is used as messenger, the resulting complex sediments at 46 S. (iii) Joining of a 50S subunit to yield a complete initiation complex. Binding of fMet-tRNAfMet not only precedes, but is necessary for, correct binding of messenger RNA to ribosomes. Thus, initiator tRNA may play an active role in the selection of initiation sites in messenger RNA.
Keywords: fMet-tRNAfMet binding, messenger RNA binding, initiation factors, edeine
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C. Heterogeneity of the small ribosomal subunit and mechanism of chain initiation in eukaryotes. Biochim Biophys Acta. 1972 Nov 16;287(1):189–193. doi: 10.1016/0005-2787(72)90341-3. [DOI] [PubMed] [Google Scholar]
- Benne R., Ebes F., Voorma H. O. Sequence of events in initiation of protein synthesis. Eur J Biochem. 1973 Oct 5;38(2):265–273. doi: 10.1111/j.1432-1033.1973.tb03058.x. [DOI] [PubMed] [Google Scholar]
- Benne R., Naaktgeboren N., Gubbens J., Voorma H. O. Recycling of initiation factors IF-1, IF-2 and IF-3. Eur J Biochem. 1973 Jan 15;32(2):372–380. doi: 10.1111/j.1432-1033.1973.tb02619.x. [DOI] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- Greenshpan H., Revel M. Initiator protein dependent binding of messenger RNA to ribosomes. Nature. 1969 Oct 25;224(5217):331–335. doi: 10.1038/224331a0. [DOI] [PubMed] [Google Scholar]
- Haselkorn R., Rothman-Denes L. B. Protein synthesis. Annu Rev Biochem. 1973;42:397–438. doi: 10.1146/annurev.bi.42.070173.002145. [DOI] [PubMed] [Google Scholar]
- Jay G., Kaempfer R. Host interference with viral gene expression: mode of action of bacterial factor i. J Mol Biol. 1974 Jan 15;82(2):193–212. doi: 10.1016/0022-2836(74)90341-6. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Kyner D., Acs G. Protein initiation in eukaryotes: formation and function of a ternary complex composed of a partially purified ribosomal factor, methionyl transfer RNA, and guanosine triphosphate. Proc Natl Acad Sci U S A. 1973 Jan;70(1):41–45. doi: 10.1073/pnas.70.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M., Noll H. Mechanism and control of initiation in the translation of R17 RNA. Nat New Biol. 1972 Aug 23;238(86):225–228. doi: 10.1038/newbio238225a0. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Thach R. E. A new method for the purification of initiation factor F2 in high yield, and an estimation of stoichiometry in the binding reaction. J Biol Chem. 1970 Nov 10;245(21):5737–5742. [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of eukaryotic protein synthesis: (Met-tRNA f -40S ribosome) initiation complex catalysed by purified initiation factors in the absence of mRNA. Nat New Biol. 1973 Mar 14;242(115):35–38. doi: 10.1038/newbio242035a0. [DOI] [PubMed] [Google Scholar]
- Szer W., Kurylo-Borowska Z. Effect of edeine on aminoacyl-tRNA binding to ribosomes and its relationship to ribosomal binding sites. Biochim Biophys Acta. 1970 Dec 14;224(2):477–486. doi: 10.1016/0005-2787(70)90580-0. [DOI] [PubMed] [Google Scholar]
- Vermeer C., de Kievit R. J., van Alphen W. J., Bosch L. Recycling of the initiation factor IF-3 on 30 S ribosomal subunits of E. coli. FEBS Lett. 1973 May 1;31(3):273–276. doi: 10.1016/0014-5793(73)80120-6. [DOI] [PubMed] [Google Scholar]
- Zamir A., Miskin R., Elson D. Interconversions between inactive and active forms of ribosomal subunits. FEBS Lett. 1969 Apr;3(1):85–88. doi: 10.1016/0014-5793(69)80103-1. [DOI] [PubMed] [Google Scholar]



