Abstract
Purpose
To explore how patients with sciatica rate the ‘bothersomeness’ of paresthesia (tingling and numbness) and weakness as compared with leg pain during 2 years of follow-up.
Methods
Observational cohort study including 380 patients with sciatica and lumbar disc herniation referred to secondary care. Using the Sciatica Bothersomeness Index paresthesia, weakness and leg pain were rated on a scale from 0 to 6. A symptom score of 4–6 was defined as bothersome.
Results
Along with leg pain, the bothersomeness of paresthesia and weakness both improved during follow-up. Those who received surgery (n = 121) reported larger improvements in both symptoms than did those who were treated without surgery. At 2 years, 18.2 % of the patients reported bothersome paresthesia, 16.6 % reported bothersome leg pain, and 11.5 % reported bothersome weakness. Among patients with no or little leg pain, 6.7 % reported bothersome paresthesia and 5.1 % bothersome weakness.
Conclusion
During 2 years of follow-up, patients considered paresthesia more bothersome than weakness. At 2 years, the percentage of patients who reported bothersome paresthesia was similar to the percentage who reported bothersome leg pain. Based on patients’ self-report, paresthesia and weakness are relevant aspects of disc-related sciatica.
Keywords: Sciatica, Lumbosacral radicular syndrome, Paresthesia, Weakness, Neuromuscular manifestations
Introduction
In the literature, ‘sciatica’ is an established term for pain radiating from the lower back or buttock into the leg [17]. It is assumed that about 90 % of cases of sciatica are caused by a herniated intervertebral disc in the lumbar column. Compression and inflammation of the herniated disc upon a lumbar nerve root, most commonly at the L5 and the S1 level, are accepted as the essential pathophysiological factors [23].
In Norway, a diagnosis of low back pain accounts for about 13 % of all patients on sick leave and 17 % of all compensation days. Of these claimants, 30 % have radiating pain [13]. In the general working population in Sweden [24], about 5 % sought health care for a new episode of low back pain during a 3-year period, and 25 % of these people reported radiating pain below the knee and had a positive straight leg raising test. Patients with radiating pain generally report more pain and have longer absences and lower rates of return to work compared with patients with non-specific low back pain [13, 21].
In addition to pain in the lower back and the leg, sensory and motor dysfunction are common in sciatica. In a large cohort of patients referred to secondary care in Maine, USA, about 40 % of patients had paresis and/or decreased cutaneous sensibility by clinical examination [1]. Similar or higher [20, 26] percentages have been reported among patients included in clinical trials. Immediate referral for surgery is recommended in cases of acute, severe, or progressive paresis [17]. Despite the common occurrence of both sensory and motor dysfunction and the potential severity of paresis, surprisingly little is known about how impactful these abnormalities are on patients. Traditionally, only leg pain and, to some extent, back pain have been utilized to rate symptom severity in sciatica. Difficulties in assessing the severity of sensory and motor symptoms arise in which descriptions and expressions are relevant and meaningful to patients and clinicians. One solution is to let patients report symptoms within the framework of ‘bothersomeness’. The use of bothersomeness in patient reported outcome measures was introduced in urology [2] and asthma [22] and has since been used in a broad spectrum of medical conditions [4]. In sciatica, the Sciatica Bothersomeness Index [19] provides an opportunity to investigate patients’ perceptions of sensory and motor function. The index includes self-reported ratings of the bothersomeness of (1) leg pain, (2) numbness or tingling in leg, foot, or groin, (3) weakness in leg/foot, and (4) back or leg pain while sitting. The Sciatica Bothersomeness Index has been used as a composite score in several studies [1, 20, 26], and three of these items were incorporated in the North American Spine Society outcome instrument [5]. We previously reported [12] baseline data from the cohort presented in this paper showing that the bothersomeness of weakness was associated with decreased physical functioning and the bothersomeness of paresthesia (numbness or tingling) was associated with impaired working status, physical functioning and emotional distress. Patients rated paresthesia 25 % less bothersome, and weakness 40 % less bothersome, than leg pain. With the exception of our previous paper [12], results generated from the Sciatica Bothersomeness Index have only been published as composite scores, resulting in a dearth of data for patients’ accounts of paresthesia and weakness.
Despite the fact that a patient’s assessment of his or her condition is important in the decision making for treatment and care of sciatica, the literature contains very little information about the clinical course of self-reported paresthesia and motor symptoms.
The first objective of this study was to explore the longitudinal changes in self-reported paresthesia and weakness for 2 years and to compare these with leg pain in patients with sciatica and lumbar disc herniation. The second objective was to identify baseline factors associated with bothersome paresthesia and bothersome weakness reported at 2 years.
Methods
Patients and setting
The details of the cohort presented in this paper have been published previously [12, 15]. During the 2-year study, we prospectively followed patients with sciatica and disc herniation who were referred to specialty back clinics at four public hospitals in south-eastern Norway from January 2005 to December 2006. The patients included were 18 years of age or older and had radiating pain or paresis below the knee and ipsilateral lumbar disc herniation at the corresponding level verified by a magnetic resonance imaging or computed tomography scan. The exclusion criteria were pregnancy, spinal fracture, tumor, infection, previous surgery to the affected disc and inability to communicate in written Norwegian. The patients were invited to participate in the study by the clinic staff. Study participation did not involve any specific type of intervention. The initial consultation included information about the condition and general advice to stay active and to use pain medication if necessary. In patients with severe symptoms, surgery was performed at the discretion of the individual surgeon.
From January 2005 to December 2006, a total of 466 patients were enrolled of whom 380 (81.5 %) patients responded at the 2-year follow-up. During the follow-up period, 121/380 (31.8 %) patients received surgical therapy.
Procedures
On the day of inclusion, the participants were given a comprehensive baseline questionnaire at the clinic, and a clinical examination was conducted by a physician or physiotherapist. Follow-ups were conducted at 3, 6, 12 and 24 months with mailed questionnaires, which were completed at home and returned in prepaid envelopes. Patients who had not responded 2 weeks after the scheduled date were contacted by telephone or a text message. A reminder was sent to non-responders if no reply was obtained after 3 weeks. The follow-up assessments included the outcome measures used at baseline and questions about any treatment received since the previous follow-up.
Clinical tests
The clinical examination performed at baseline included the passive straight leg raising test (deemed abnormal if leg pain emerged at <60°), sensibility (impaired if reduced reaction to dermatomal light hand touch), reflexes (reduced if depressed patellar or Achilles reflex) and muscular paresis (deemed present if one of the following were abnormal: single limb stance, tiptoe or heel walking, supine knee or ankle flexion/extension, or big toe extension).
Measures
Questionnaires included measures of educational level, smoking, work status, back pain and sciatica history. The Hopkins Symptom Check List-25 [16] was used to assess emotional distress. Self-reported comorbidity was measured by the Subjective Health Complaints Inventory [6] by grading the intensity of 29 complaints experienced in the previous month on a four-point scale: not at all (0), a little (1), some (2) and severe (3). In this study, the responses were dichotomized into absent (0) or present (1, 2 or 3), and a score was calculated by summing all the complaints reported as present. Two items—low back pain and leg pain during exercise—were excluded, reducing the maximum obtainable score from 29 to 27. Fear-avoidance beliefs about movement/reinjury were assessed by the Tampa Scale of Kinesiophobia [14] and general health and physical functioning by subscales of the SF-36 [25].
Outcome measures
Patients’ ratings on the Sciatica Bothersomeness Index [11, 19] were used to establish values for paresthesia (numbness or tingling in the leg, foot or groin), weakness (in the leg or foot) and leg pain. The severity of each symptom is rated on a scale from 0 to 6, with anchors at 0 (not bothersome), 3 (somewhat bothersome) and 6 (extremely bothersome). In this study, a symptom score of 4–6 was defined as bothersome.
Ethics
The protocol was approved by the Regional Committee for Medical Research Ethics and The Ombudsman for Privacy in Research at the Norwegian Social Science Data Services.
Statistical analysis
Group differences were tested by Mann–Whitney U tests, t tests or Chi-square tests according to the type of the data. Wilcoxon’s signed-rank sum test or McNemar’s test was used to compare symptoms at baseline and 2 years. Correlations were assessed by Spearman’s ρ. Multivariate associations were analyzed by logistic regression models using the report of bothersome paresthesia and bothersome weakness at 2 years as dependent variables. Models were built by including independent variables with p values <0.2 in the univariate analyses. Variables with the highest p value were removed one by one until all remaining variables had p values <0.05. Surgical therapy received during the follow-up period was entered as yes/no, and final models were presented with adjustments for surgery. All data analyses were performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL).
Results
Compared with those who completed the 2-year follow-up (n = 380), patients who did not complete the 2-year follow-up (n = 86) were significantly younger and more likely to be single or divorced, to be smoking, to have a positive straight leg raising test and to report worse general health. There were no significant differences between responders and non-responders in terms of baseline sensibility, muscular paresis, self-reported paresthesia or weakness.
Baseline characteristics for the patients who completed the 2-year follow-up are given in Table 1. The clinical examination revealed impaired sensibility in 59.0 % of the patients and paresis in 44.5 %. Those with impaired sensibility reported a mean (SD) paresthesia score of 3.8 (1.6) compared with 2.5 (1.9) among patients with normal sensibility (p < 0.01). Patients with and without paresis at the clinical examination reported a mean weakness score (SD) of 3.5 (1.8) and 1.6 (1.7), respectively (p < 0.01).
Table 1.
Demographic and clinical characteristics, n = 380
| Baseline | 2 years | |
|---|---|---|
| Males, n (%) | 212 (55.8) | |
| Age, years | 44.5 (11.3) | |
| Education, years | 13.1 (3.1) | |
| Married/cohabitant, n (%) | 286 (75.5) | |
| Working full time, n (%) | 77 (20.3) | 249 (65.9)* |
| Current smoker, n (%) | 154 (40.8) | |
| Duration current episode <3 months, n (%) | 161 (42.5) | |
| First sciatica episode, n (%) | 172 (45.4) | |
| Duration back problems >1 year, n (%) | 266 (70.0) | |
| Clinical findings, n (%) | ||
| Positive straight leg raising test | 205 (54.8) | |
| Sensory impairment | 219 (58.1) | |
| Paresis | 170 (45.7) | |
| Reflexes decreased | 176 (46.9) | |
| Paresthesia (numbness and tingling) (0–6) | 3.3 (1.8) | 1.6 (1.8)* |
| Weakness (0–6) | 2.5 (2.0 | 1.1 (1.6)* |
| Leg pain (0–6) | 4.5 (1.5) | 1.7 (1.8)* |
| Back pain (0–6) | 2.9 (1.8) | 2.1 (1.7)* |
| Bothersome paresthesia, n (%)a | 192 (51.2) | 68 (18.2)* |
| Bothersome weakness, n (%)a | 126 (33.5) | 43 (11.5)* |
| Bothersome leg pain, n (%)a | 290 (76.5) | 62 (16.6)* |
| SF-36 general health (0–100) | 70.4 (20.1) | 70.7 (22.0) |
| SF-36 physical functioning (0–100) | 50.2 (25.1) | 80.3 (20.2)* |
| Emotional distress (1–4) | 1.6 (0.4) | 1.4 (0.5)* |
| Subjective health complaints (0–27) | 7.4 (4.3) | 7.6 (5.3) |
| Fear of movement/reinjury (13–52) | 27.1 (7.0) | 23.9 (7.6)* |
Values are means (SD) if not otherwise stated
* p < 0.01 between baseline and 2 years
aScore 4–6 on the Sciatica Bothersomeness Index (0–6)
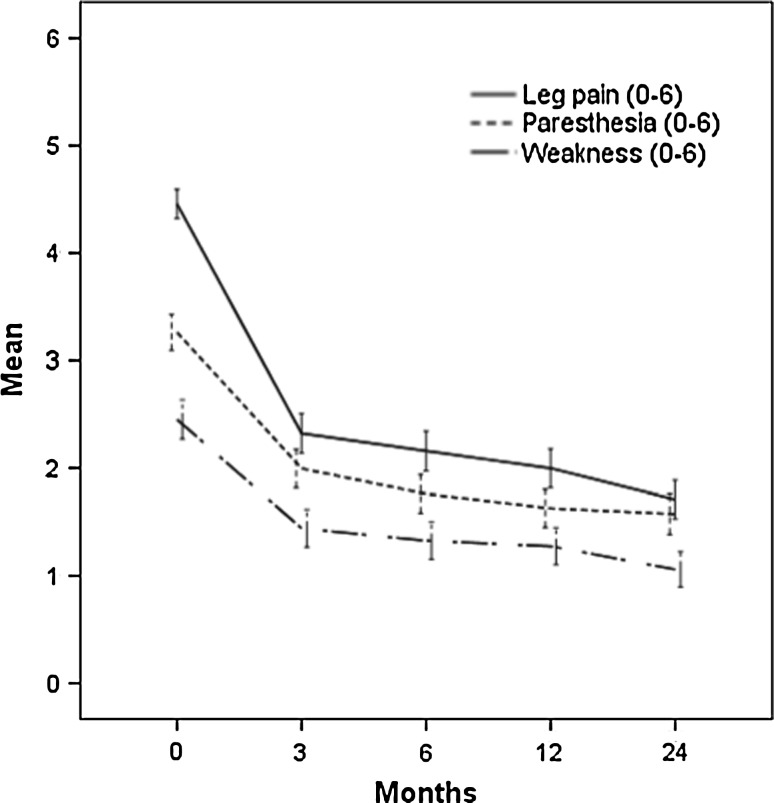
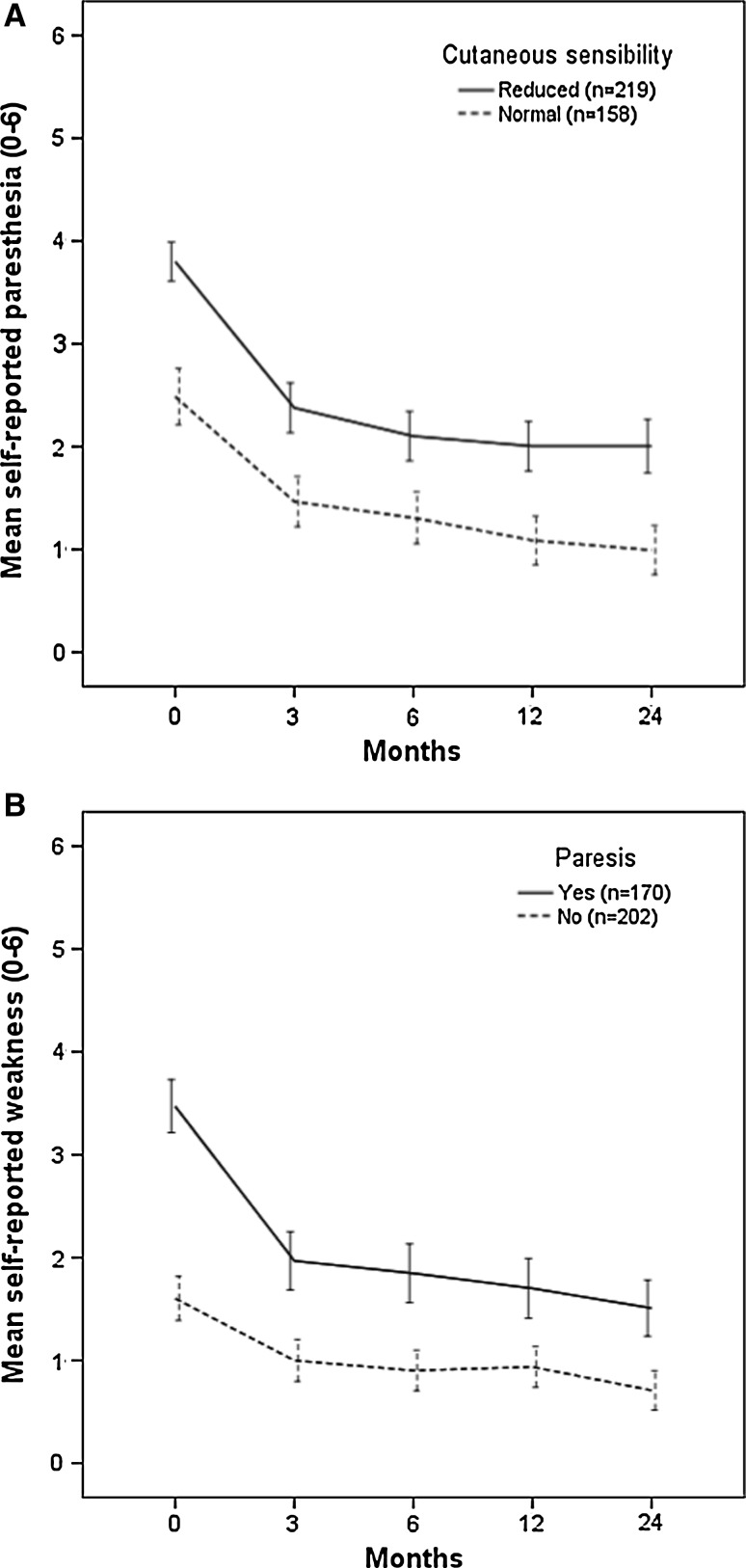
Paresthesia, weakness and leg pain improved significantly (p < 0.01) during 2 years (Table 1); the largest changes occurred between the baseline and 3 months (Fig. 1). Paresthesia and weakness improved both for patients with normal and abnormal clinical examination findings at baseline (Fig. 2a, b). At 2 years, the mean (SD) paresthesia scores for patients with impaired and normal baseline sensibility were 2.0 (1.9) and 1.0 (1.5), respectively (p < 0.01). For patients with and without paresis, the mean weakness (SD) scores were 1.5 (1.8) and 0.7 (1.4), respectively (p < 0.01).
Fig. 1.
The 2-year course of self-reported leg pain, paresthesia and weakness. Symptom scores range from 0 (not bothersome) to 6 (extremely bothersome)
Fig. 2.
a Self-reported paresthesia during the 2 years according to clinical finding of cutaneous sensibility at baseline. b Self-reported weakness during the 2 years according to clinical finding of paresis at baseline
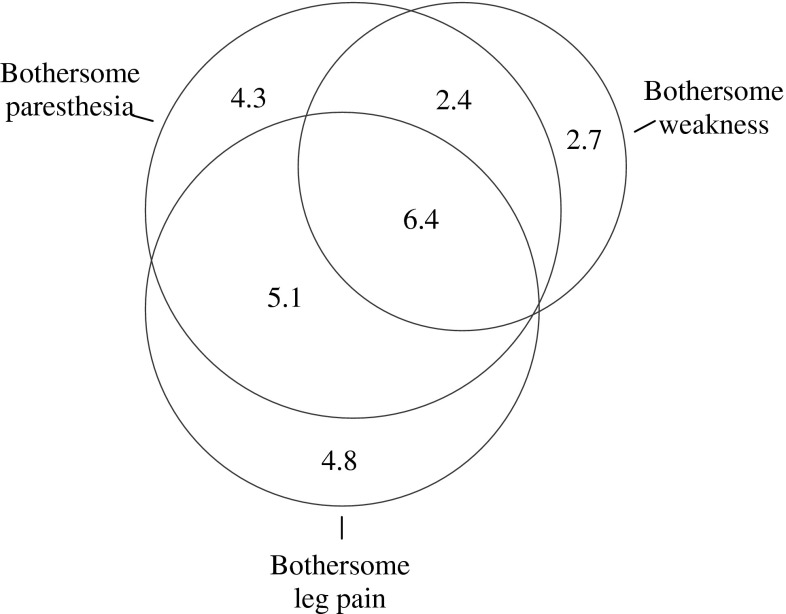
At the 2-year follow-up, 18.2 % of the patients reported bothersome paresthesia, 11.5 % reported bothersome weakness, and 8.8 % reported both bothersome paresthesia and bothersome weakness. The symptom overlap including 16.6 % of the patients who reported bothersome leg pain is illustrated in Fig. 3. Among those with little or no leg pain, 6.7 % reported bothersome paresthesia and 5.1 % reported bothersome weakness. The correlation coefficients (ρ) were: paresthesia and weakness, 0.69; paresthesia and leg pain, 0.73; and weakness and leg pain, 0.63.
Fig. 3.
Diagram depicting the overlap between bothersome paresthesia, bothersome weakness and bothersome leg pain at 2 years. Numbers are percent
Univariate associations between baseline variables and bothersome paresthesia and bothersome weakness at 2 years are shown in Table 2. In the multivariate models, both bothersome paresthesia and bothersome weakness were independently and significantly associated with baseline impaired sensibility, bothersome back pain and bothersome weakness (see Table 3). A longer duration of back problems and older age were significantly associated with bothersome paresthesia.
Table 2.
Percentage of patients who reported bothersome paresthesia or weakness at 2 years according to baseline characteristics
| Bothersome paresthesiaa | p | Bothersome weaknessa | p | |
|---|---|---|---|---|
| Sex | ||||
| Males | 18.4 | 0.90 | 12.7 | 0.40 |
| Females | 17.9 | 9.9 | ||
| Age | ||||
| <50 years | 14.2 | <0.01 | 9.6 | 0.08 |
| ≥50 years | 27.4 | 15.9 | ||
| Education | ||||
| <12 years | 21.3 | 0.10 | 12.0 | 0.79 |
| ≥12 years | 14.7 | 11.1 | ||
| Smoking | ||||
| No | 15.1 | 0.07 | 11.1 | 0.69 |
| Yes | 22.4 | 12.4 | ||
| Working full time | ||||
| No | 19.4 | 0.22 | 12.5 | 0.27 |
| Yes | 13.3 | 7.9 | ||
| Duration back problems | ||||
| <1 year | 9.9 | 0.01 | 8.0 | 0.17 |
| ≥1 year | 21.7 | 13.0 | ||
| Previous sciatica episodes | ||||
| No | 16.1 | 0.33 | 8.9 | 0.15 |
| Yes | 20.0 | 13.7 | ||
| Duration current sciatica episode | ||||
| <3 months | 16.4 | 0.42 | 13.4 | 0.35 |
| ≥3 months | 19.6 | 10.2 | ||
| Straight leg raising test | ||||
| Normal | 18.8 | 0.89 | 12.8 | 0.56 |
| Abnormal | 18.2 | 10.8 | ||
| Sensibility | ||||
| Normal | 7.7 | <0.01 | 7.1 | 0.02 |
| Abnormal | 25.9 | 15.0 | ||
| Muscular paresis | ||||
| No | 12.1 | <0.01 | 7.1 | <0.01 |
| Yes | 26.2 | 17.2 | ||
| Reflexes | ||||
| Normal | 17.4 | 0.70 | 10.8 | 0.68 |
| Decreased | 19.0 | 12.1 | ||
| Back pain (0–6) | ||||
| Score 0–3 | 12.9 | <0.01 | 7.4 | <0.01 |
| Score 4–6a | 26.4 | 17.9 | ||
| Leg pain (0–6) | ||||
| Score 0–3 | 19.1 | 0.81 | 12.4 | 0.79 |
| Score 4–6a | 18.0 | 11.3 | ||
| Paresthesia (0–6) | ||||
| Score 0–3 | 11.6 | <0.01 | 8.3 | 0.10 |
| Score 4–6a | 24.3 | 13.8 | ||
| Weakness (0–6) | ||||
| Score 0–3 | 13.0 | <0.01 | 6.5 | <0.01 |
| Score 4–6a | 28.2 | 21.0 | ||
| Emotional distressb (0–4) | ||||
| <1.75 | 17.3 | 0.41 | 10.3 | 0.21 |
| ≥1.75 | 21.0 | 15.0 | ||
| Fear of movement/reinjury (13–52) | ||||
| <Median (27) | 15.5 | 0.16 | 11.6 | 0.98 |
| >Median (27) | 21.2 | 11.7 | ||
| Subjective health complaints (0–27) | ||||
| <Median (7) | 13.6 | 0.03 | 9.7 | 0.30 |
| >Median (7) | 22.2 | 13.1 | ||
| General health (0–100)c | ||||
| <Median (72) | 21.2 | 0.18 | 12.1 | 0.75 |
| >Median (72) | 15.8 | 11.1 | ||
| Physical functioning (0–100)c | ||||
| <Median (50) | 23.4 | 0.02 | 15.8 | 0.02 |
| >Median (50) | 14.1 | 8.2 | ||
aScore 4–6 on the Sciatica Bothersomeness Index (0–6)
bHopkins Symptom Check List-25
cHigher score indicates better health
Table 3.
Odds ratios for reporting bothersome paresthesia and weakness at 2 years
| Baseline characteristics | Bothersome paresthesiaa | Bothersome weaknessa | ||
|---|---|---|---|---|
| OR | 95 % CI | OR | 95 % CI | |
| Age >50 years | 2.3 | 1.3, 4.2 | – | |
| Abnormal sensibility | 4.6 | 2.2, 9.5 | 2.3 | 1.1, 5.1 |
| Duration back problems ≥1 year | 2.1 | 1.0, 4.4 | – | |
| Bothersome back pain | 2.5 | 1.4, 4.6 | 2.7 | 1.3, 5.3 |
| Bothersome weakness | 2.1 | 1.1, 3.7 | 3.1 | 1.6, 6.3 |
| Surgical treatment | 0.6 | 0.3, 1.1 | 0.4 | 0.2, 1.0 |
Results of multivariate logistic regression models
aScore 4–6 on the Sciatica Bothersomeness Index (0–6)
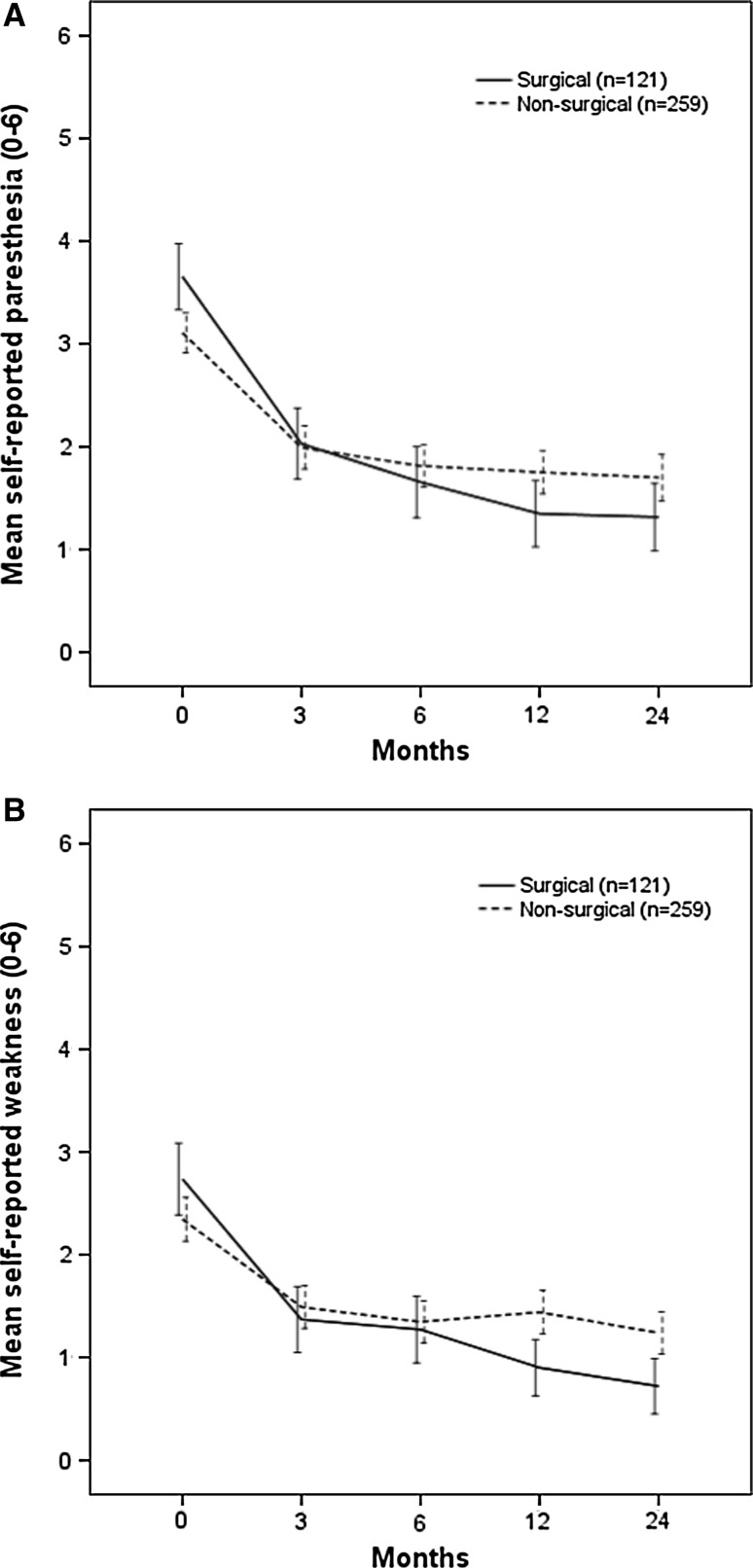
At baseline, the patients who were treated surgically (n = 121) during follow-up reported significantly more paresthesia (3.7 (SD 1.9) vs. 3.1 (SD 1.8), p < 0.01) but not significantly more weakness [2.7 (SD 2.0) vs. 2.4 (SD 2.0), p = 0.14] than the non-surgical patients. Those who received surgery reported larger changes in scores for both symptoms from baseline to 2 years than did those who were treated without surgery. The mean differences in the change from baseline to 2 years between surgical and non-surgical patients were: paresthesia, 1.0 [95 % confidence interval (CI) 0.5, 1.5] and weakness, 0.9 (95 % CI 0.5, 1.3) (Fig. 4a, b). In univariate analyses, surgical treatment was not significantly associated with any of the two outcomes: bothersome paresthesia, odds ratio (OR) 0.72 (95 % CI 0.40, 1.30) and bothersome weakness, OR 0.60 (95 % CI 0.28, 1.26). When surgical treatment was added to the multivariate models, this variable became borderline significantly negatively associated with bothersome weakness (OR 0.42, 95 % CI 0.19, 0.96) but not with bothersome paresthesia (Table 3).
Fig. 4.
a Self-reported paresthesia during the 2 years according to surgical treatment status. b Self-reported weakness during the 2 years according to surgical treatment status
Discussion
The main finding of the present study was that self-reported paresthesia and weakness improved from baseline to year 2 generally in accordance with improvement in leg pain. At the 2-year follow-up, the percentage of patients who reported bothersome paresthesia (18.2 %) was similar to the percentage who reported bothersome leg pain (16.6 %). Among patients with no or little leg pain, 6.7 % reported bothersome paresthesia and 5.1 % reported bothersome weakness.
Currently, most outcome measures for patients with sciatica only inquire about pain or pain-related disability [15]. Our findings suggest that self-reported paresthesia and weakness are still important to patients 2 years after an initial episode of sciatica and should therefore be included in the outcome measures for sciatica. The Sciatica Bothersomeness Index represents an alternative to the pain-only outcome measures.
The literature on self-reported weakness and paresthesia in sciatica is sparse. In a qualitative study of patients’ own account of sciatica, participants highlighted the unpleasantness of other symptoms such as numbness, deadness, and pins and needles in addition to pain severity [18], but the severity of each particular symptom was not investigated.
It is not clear why the perception of paresthesia is so strongly associated with the perception of weakness. One explanation may be central sensorimotor integration [8]. In both the brain and spinal cord, sensory information and motor control are tightly bound together [9]. Possibly, this functional intertwinement influences how numbness or tingling and weakness are perceived and consequently the ability to discriminate between the two. Such difficulties may also have a linguistic counterpart; for example, English-speaking patients agree poorly on the meaning of the terms “numbness” and “weakness” [27].
One may speculate that patients who report bothersome sciatica symptoms have a lower threshold for reporting symptoms in general. To assess patients’ tendency to report non-sciatica symptoms, we measured their number of subjective health complaints. The baseline subjective health complaint score was associated with bothersome paresthesia at 2 years in the univariate but not in the multivariate analyses, and no association was found with bothersome weakness. Because sensory loss has been reported in painful conditions without evidence of neuropathy [10], we cannot know if all the paresthesia reported in this study is of neuropathic origin. Both numbness and weakness have been associated with pain in general [7].
The nomenclature of unpleasant sensory experiences may be somewhat confusing, especially the distinction between paresthesia and dysaesthesia. The International Association for the Study of Pain [3] recommends that dysaesthesia should be used to describe an unpleasant abnormal sensation and paresthesia to describe an abnormal sensation that is not unpleasant. However, because paresthesia was used in a previous paper on this topic [12], we have chosen to be consistent and use the same terminology here.
Patients who received surgery reported more paresthesia and weakness at baseline and more improvement in these symptoms than did non-surgical patients, but the differences were generally small. In the multivariate analyses, surgical treatment was borderline significantly associated with bothersome weakness at 2 years but not with paresthesia. The observational design of this study precludes the ability to draw causal inferences about treatment effects.
The strengths of this study are the relatively large number of patients with high rates of follow-up, a multicenter design and a systematic registration of self-reported paresthesia and weakness using a validated questionnaire. The procedure for the clinical examination at baseline represents a potential limitation. Because findings were rated only as normal or abnormal, we cannot say whether patients with more bothersome symptoms had more abnormal clinical findings. The study took place at specialty back clinics and the clinical examinations were performed by experienced doctors and physiotherapists. However, the reliability of clinical test results or inter-observer agreement was not formally assessed.
In conclusion, self-reported paresthesia and weakness both improved during 2 years of follow-up. Patients considered that paresthesia was somewhat more bothersome than weakness. At the end of the 2-year follow-up period, the percentage of patients who reported bothersome paresthesia was similar to the percentage who reported bothersome leg pain. Information about paresthesia and weakness is important in the assessment of how sciatica affects patients.
Acknowledgments
Funding for this study was received from the South-Eastern Norway Regional Health Authority; no support was received from commercial sources. We thank Anne Keller, Eli Molde Hagen, Dag Soldal, Knut Morten Huneide, Anett Bjørnødegard Hångmann, and Bjarte Justnæs for their help with collecting data and Professor Leiv Sandvik for statistical advice.
Conflict of interest
None.
References
- 1.Atlas SJ, Deyo RA, Keller RB, Chapin AM, Patrick DL, Long JM, Singer DE. The Maine Lumbar Spine Study, Part II. 1-year outcomes of surgical and nonsurgical management of sciatica. Spine. 1996;21:1777–1786. doi: 10.1097/00007632-199608010-00011. [DOI] [PubMed] [Google Scholar]
- 2.Barry MJ, Fowler FJ, Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 3.Boyd D, Butler M, Carr D, Cohen M, Devor M, Dworkin R, Greenspan J et al (2011) IASP taxonomy. http://www.iasppain.org/Content/NavigationMenu/GeneralResourceLinks/PainDefinitions/default.htm. Accessed 20 Nov 2012
- 4.Cramer JA, Silberstein SD, Winner P. Development and validation of the Headache Needs Assessment (HANA) survey. Headache. 2001;41:402–409. doi: 10.1046/j.1526-4610.2001.111006402.x. [DOI] [PubMed] [Google Scholar]
- 5.Daltroy LH, Cats-Baril WL, Katz JN, Fossel AH, Liang MH. The North American Spine Society lumbar spine outcome assessment Instrument: reliability and validity tests. Spine. 1996;21:741–749. doi: 10.1097/00007632-199603150-00017. [DOI] [PubMed] [Google Scholar]
- 6.Eriksen HR, Ihlebaek C, Ursin H. A scoring system for subjective health complaints (SHC) Scand J Public Health. 1999;27:63–72. doi: 10.1177/14034948990270010401. [DOI] [PubMed] [Google Scholar]
- 7.Fishbain DA, Cole B, Cutler RB, Lewis J, Rosomoff HL, Rosomoff RS. A structured evidence-based review on the meaning of nonorganic physical signs: Waddell signs. Pain Med. 2003;4:141–181. doi: 10.1046/j.1526-4637.2003.03015.x. [DOI] [PubMed] [Google Scholar]
- 8.Forss N, Jousmaki V. Sensorimotor integration in human primary and secondary somatosensory cortices. Brain Res. 1998;781:259–267. doi: 10.1016/S0006-8993(97)01240-7. [DOI] [PubMed] [Google Scholar]
- 9.Freund HJ. The parietal lobe as a sensorimotor interface: a perspective from clinical and neuroimaging data. Neuroimage. 2001;14:S142–S146. doi: 10.1006/nimg.2001.0863. [DOI] [PubMed] [Google Scholar]
- 10.Geber C, Magerl W, Fondel R, Fechir M, Rolke R, Vogt T, Treede RD, Birklein F. Numbness in clinical and experimental pain–a cross-sectional study exploring the mechanisms of reduced tactile function. Pain. 2008;139:73–81. doi: 10.1016/j.pain.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Grovle L, Haugen AJ, Keller A, Natvig B, Brox JI, Grotle M. Reliability, validity, and responsiveness of the Norwegian versions of the Maine-Seattle Back questionnaire and the sciatica bothersomeness and frequency indices. Spine. 2008;33:2347–2353. doi: 10.1097/BRS.0b013e31818047d6. [DOI] [PubMed] [Google Scholar]
- 12.Grovle L, Haugen AJ, Keller A, Natvig B, Brox JI, Grotle M. The bothersomeness of sciatica: patients’ self-report of paresthesia, weakness and leg pain. Eur Spine J. 2010;19:263–269. doi: 10.1007/s00586-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagen KB, Thune O. Work incapacity from low back pain in the general population. Spine. 1998;23:2091–2095. doi: 10.1097/00007632-199810010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Haugen AJ, Grovle L, Keller A, Grotle M. Cross-cultural adaptation and validation of the Norwegian version of the Tampa scale for kinesiophobia. Spine. 2008;33:E595–E601. doi: 10.1097/BRS.0b013e31817c6c4b. [DOI] [PubMed] [Google Scholar]
- 15.Haugen AJ, Grovle L, Brox JI, Natvig B, Keller A, Soldal D, Grotle M. Estimates of success in patients with sciatica due to lumbar disc herniation depend upon outcome measure. Eur Spine J. 2011;20:1669–1675. doi: 10.1007/s00586-011-1809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hesbacher PT, Rickels K, Morris RJ, Newman H, Rosenfeld H. Psychiatric illness in family practice. J Clin Psychiatry. 1980;41:6–10. [PubMed] [Google Scholar]
- 17.Koes BW, van Tulder MW, Peul WC. Diagnosis and treatment of sciatica. BMJ. 2007;334:1313–1317. doi: 10.1136/bmj.39223.428495.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong BN, Konstantinou K, Corbett M, Hay E. Patients’ own accounts of sciatica: a qualitative study. Spine. 2011;36:1251–1256. doi: 10.1097/BRS.0b013e318204f7a2. [DOI] [PubMed] [Google Scholar]
- 19.Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB. Assessing health-related quality of life in patients with sciatica. Spine. 1995;20:1899–1908. doi: 10.1097/00007632-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Peul WC, van Houwelingen HC, van den Hout WB, Brand R, Eekhof JA, Tans JT, Thomeer RT, Koes BW, Leiden-The Hague Spine Intervention Prognostic Study Group Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356:2245–2256. doi: 10.1056/NEJMoa064039. [DOI] [PubMed] [Google Scholar]
- 21.Selim AJ, Ren XS, Fincke G, Deyo RA, Rogers W, Miller D, Linzer M, Kazis L. The importance of radiating leg pain in assessing health outcomes among patients with low back pain. Results from the Veterans Health Study. Spine. 1998;23:470–474. doi: 10.1097/00007632-199802150-00013. [DOI] [PubMed] [Google Scholar]
- 22.Steen N, Hutchinson A, McColl E, Eccles MP, Hewison J, Meadows KA, Blades SM, Fowler P. Development of a symptom based outcome measure for asthma. BMJ. 1994;309:1065. doi: 10.1136/bmj.309.6961.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi N, Yabuki S, Aoki Y, Kikuchi S. Pathomechanisms of nerve root injury caused by disc herniation: an experimental study of mechanical compression and chemical irritation. Spine. 2003;28:435–441. doi: 10.1097/01.BRS.0000048645.33118.02. [DOI] [PubMed] [Google Scholar]
- 24.Vingard E, Mortimer M, Wiktorin C, Pernold RPTG, Fredriksson K, Nemeth G, Alfredsson L, Musculoskeletal Intervention Center-Norrtalje Study Group Seeking care for low back pain in the general population: a two-year follow-up study: results from the MUSIC-Norrtalje Study. Spine. 2002;27:2159–2165. doi: 10.1097/00007632-200210010-00016. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE. SF-36 health survey update. Spine. 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Hanscom B, Skinner JS, Abdu WA, Hilibrand AS, Boden SD, Deyo RA. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296:2441–2450. doi: 10.1001/jama.296.20.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright V, Hopkins R. Communicating with the rheumatic patient. Rheumatol Rehabil. 1977;16:107–118. doi: 10.1093/rheumatology/16.2.107. [DOI] [PubMed] [Google Scholar]