Abstract
Background
Two-dimensional imaging is not adequate for evaluating ossification of the posterior longitudinal ligament (OPLL). This study was designed to evaluate the accuracy of a novel computed tomography (CT)-based three-dimensional (3D) analysis method that we had devised to measure volume changes in OPLL.
Subjects and methods
Twenty OPLL patients (12 male and 8 female; mean age 63.6 years) who were being followed conservatively were examined twice with an interval of at least 1 year between the two scans. The mean interval was 22 (range 12–45) months. A 3D model was created with DICOM data from CT images, using the MIMICS® software to calculate the volume. The mean ossification volume was determined from two measurements. Since ossification size varies widely, evaluation of change in volume is generally affected by the original size. Therefore, the change in ossification volume between the first and second CT examinations was calculated as the annual rate of progression.
Results
The type of OPLL was classified as continuous in 3 patients, segmented in 3, and mixed in 14. The mean ossification volume was 1,831.68 mm3 at the first examination and 1,928.31 mm3 at the second, showing a significant mean increase in ossification volume. The mean annual rate of lesion increase was 3.33 % (range 0.08–7.79 %).
Conclusion
The 3D method used allowed detailed OPLL classification and quantification of change in the ossified volume. Thus, this method appears to be very useful for quantitative evaluation of OPLL with only minimal measurement error.
Keywords: OPLL, 3D analysis, CT, Ossification
Introduction
Ossification of the posterior longitudinal ligament (OPLL) of the cervical spine is one of the main causes of myeloradiculopathy in Asian populations, especially in Japan [1–4]. Accurate determination of the size of OPLL and the nature of its growth in terms of length and thickness is important because these factors crucially relate to spinal canal stenosis and can cause myelopathy [5]. Evaluating the size and growth of OPLL is important to determine the timing of the operation and the risk factors for rapid progression of OPLL. Previous attempts have already been made to measure the size of OPLL [6–10]. However, these methods involved two-dimensional imaging. Recent technical improvements in computed tomography (CT)-based three-dimensional (3D) imaging analysis have made accurate 3D measurement of OPLL possible [5, 11]. Previous studies on lung cancer growth found that evaluation of the growth of lung cancer using a 3D measurement method was more reliable than using a 2D method [12–15]. The present study was designed to evaluate the accuracy of a novel CT-based method of 3D analysis we had previously devised, to measure changes in the volume of OPLL.
Materials and methods
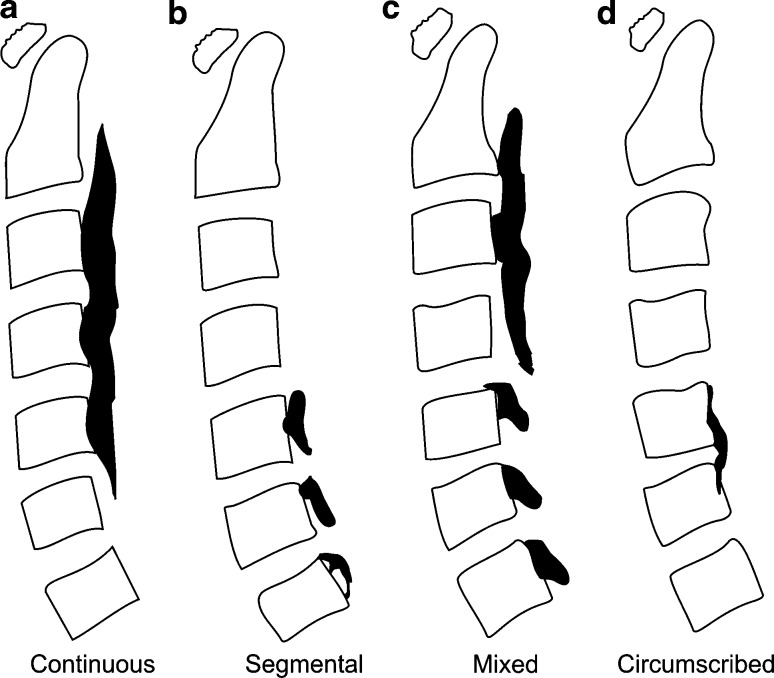
This study was approved by the ethics committee of the University Hospital. All patients gave their consent for participation. Twenty OPLL patients (12 male and 8 female; mean age 63.6 years; range 53–80 years) who had been followed conservatively at the University Hospital were included in the analysis. CT examinations were performed using a 16-row CT system (Light Speed QX/I; GE Healthcare Japan, Tokyo, Japan) until 2006, and thereafter using a 64-row multislice CT system (Aquilion; Toshiba Medical System Corporation, Tochigi, Japan). The imaging conditions with the former system were as follows: slice thickness, 1.25 mm; field of view, 14 mm; voltage, 120 kV; current, 178 mA. The conditions with the latter were as follows: slice thickness, 1.0 or 0.5 mm; field of view, 14 mm; voltage, 120 kV; current, 75 mA. The examinations were performed twice in each patient, with an interval of at least 1 year between the two scans; the mean interval was 22 months (range 12–45 months). All ossifications of the vertebrae were identified semi-automatically by a single examiner based on DICOM data for CT images using the MIMICS® software (Materialise Japan Co. Ltd., Yokohama, Japan), and a 3D model for volume calculation was created automatically. The first step was to identify the affected vertebra and ossification using a threshold of 226–3,071 HU, as defined by MIMICS®, for the detection of bone. In the second step, the ossification was detached from the posterior aspect of the vertebral body between the bases of both pedicles, using both axial and sagittal slice images. In the third step, the region of ossification was isolated using the same threshold, and a 3D model was created. In the last step, the ossification volume was determined using the MIMICS® software, based on the 3D model. The types of OPLL based on the 3D form were classified as continuous, segmental, mixed, or circumscribed according to the criteria proposed by the Investigation Committee on Ossification of Spinal Ligaments of the Japanese Ministry of Public Health and Welfare (Fig. 1) [16]. To ensure the precision of volume calculation based on differences in slice thickness, images of a phantom (KYOTO KAGAKU Co., LTD.), 300 mm in length and 7.5 mm in diameter, were taken at slice thicknesses of 0.5 and 1.0 mm, and the errors in 10 measurements of individual data were estimated.
Fig. 1.
Classification of the types of OPLL. OPLL was classified into four types according to the classification established by the Investigation Committee on Ossification of Spinal Ligaments of the Japanese Ministry of Public Health and Welfare. a Continuous, b segmental, c mixed, d circumscribed
The ossification volume was calculated twice for each measurement to determine the mean volume, and to evaluate intraobserver error. Since ossification size varies widely, evaluation of change in ossification volume is affected by the original size. Therefore, the change in ossification volume between the first and second CT examinations was calculated as the rate of increase. The annual rate of increase was also calculated. The measurement error was defined as the difference from the average value, and the percentage error was also calculated and evaluated.
Classification of OPLL and range of ossification were evaluated with an X-ray and compared with those obtained during the CT. The length of the ossification lesion, maximum thickness and a spinal canal occupation rate of OPLL were measured in the lateral view of X-ray radiographs. The spinal canal occupation rate of OPLL was also measured using the axial view of a CT. The spinal canal occupation rate was expressed as the percentage ratio of the maximum thickness of ossification to the midsagittal diameter of cervical canal [2]. Correlation analysis was performed using the length, thickness of OPLL, spinal canal occupation rate (measured using X-ray and CT) and the rate of increase in the volume of OPLL.
Statistical analysis
The SPSS software package for Windows 2005 (Version 14.0; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. The slice thickness measurement error for the phantom and the two ossification volume measurements were analyzed using intra-class correlation coefficients (ICCs). Because ossification volume in the first and second measurements had similar variance, Student’s t test was applied to evaluate it, at a significance level of 0.05.
Results
All patients were eligible for disease classification and measurement of ossification volume. The 3D model showed a change in the vertebral form associated with growth of the distal part of the lesion and increase in the volume of the lesion on the left (Fig. 2), and allowed detailed classification. The type of OPLL was classified as continuous in 3 patients, segmented in 3, and mixed in 14 (Table 1). There were no changes in disease classification in any of the 20 patients during the study period. OPLL was identified in the cervical vertebrae C2–7 in 15 cases and were evaluated using X-rays. In nine cases, the classification done using X-rays corresponded with classification done using a CT, and the range of ossification lesions corresponded with CT findings in three cases. Thus, a CT was able to confirm the range of the ossification lesion more finely.
Fig. 2.
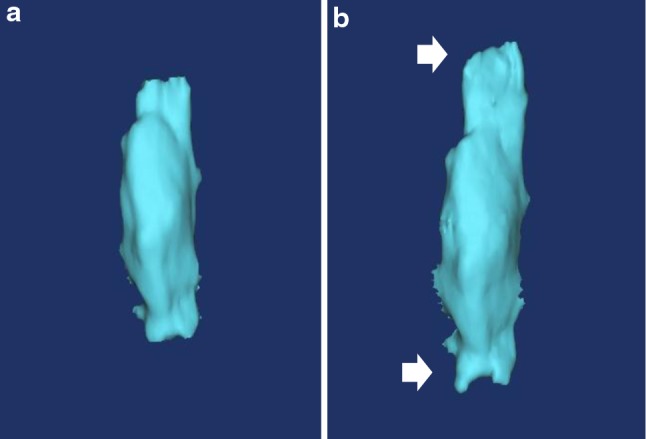
Image of the 3D model. a Length 54.72 mm, Volume 3,654.09 mm3. b Length 66.75 mm, Volume 4,283.70 mm3, Increase 629.61 mm3. The rate of increase was 17.23 %, and the annual rate of increase was 3.09 %
Table 1.
Patient demographic data and measurement value
| Age | Sex | Type | Area | Interval (M) | Volume at first examination (mm3) | Volume at second examination (mm3) | Rate of increase (%) | Annual rate of increase (%) | Increase part | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 62 | M | Co | C2–4 | 36 | 3,654.09 | 4,092.72 | 12.00 | 4.00 | Local |
| Case 2 | 68 | F | Mi | C3–7 | 25 | 2,248.55 | 2,469.90 | 9.84 | 4.73 | Diffuse |
| Case 3 | 65 | F | Ci | C5 | 12 | 478.09 | 483.22 | 1.07 | 1.07 | Diffuse |
| Case 4 | 62 | M | Co | C2–4 | 24 | 809.81 | 828.29 | 2.28 | 1.14 | Diffuse |
| Case 5 | 69 | M | Mi | C3–6 | 24 | 1,130.69 | 1,203.90 | 6.47 | 3.24 | Diffuse |
| Case 6 | 57 | F | Mi | C3–6 | 45 | 1,908.68 | 2,171.56 | 13.77 | 3.67 | Diffuse |
| Case 7 | 73 | M | Mi | C2–7 | 24 | 4,967.98 | 5,087.29 | 2.40 | 1.20 | Intervertebral |
| Case 8 | 74 | M | Mi | C3–7 | 12 | 2,580.11 | 2,667.31 | 3.38 | 3.38 | Intervertebral |
| Case 9 | 53 | F | Co | C2–3 | 39 | 1,749.72 | 1,935.91 | 10.64 | 4.73 | Diffuse |
| Case 10 | 68 | M | S | C5–6 | 36 | 151.85 | 174.76 | 15.09 | 5.03 | Local |
| Case 11 | 62 | M | Mi | C2–6 | 12 | 1,387.09 | 1,461.49 | 5.36 | 5.36 | Intervertebral |
| Case 12 | 57 | F | Mi | C2–4 | 24 | 410.35 | 438.16 | 6.78 | 3.39 | Local |
| Case 13 | 63 | M | Mi | C2–7 | 12 | 2,510.77 | 2,512.88 | 0.08 | 0.08 | Diffuse |
| Case 14 | 67 | M | Mi | C2–7 | 12 | 3,673.29 | 3,836.25 | 4.44 | 4.44 | Local |
| Case 15 | 58 | M | Mi | C2–6 | 12 | 2,653.84 | 2,690.66 | 1.39 | 1.39 | Diffuse |
| Case 16 | 58 | M | Mi | C3–7 | 17 | 1,751.41 | 1,777.10 | 1.47 | 1.04 | Local |
| Case 17 | 58 | F | Mi | C3–7 | 18 | 431.60 | 482.05 | 11.69 | 7.79 | Local |
| Case 18 | 60 | F | Ci | C6 | 12 | 25.81 | 27.14 | 5.15 | 5.15 | Diffuse |
| Case 19 | 58 | F | Mi | C3–7 | 12 | 2,208.59 | 2,265.15 | 2.56 | 2.56 | Local |
| Case 20 | 80 | M | Mi | C4–7 | 12 | 1,901.31 | 1,960.41 | 3.11 | 3.11 | Diffuse |
| Mean | 63.6 | 22.0 | 1,831.68 | 1,928.31 | 5.95 | 3.33 |
Co continuous, S segmented, Mi mixed, Ci circumscribed
The 10 measurements of the phantom at slice thicknesses of 0.5 and 1.0 mm showed a coefficient of variation of 0.16 and 0.13 %, respectively, an inter-slice error of 0.12 %, and an ICC of 0.856 (p < 0.01). The mean error in the two measurements for each patient was 1.16 %, and the ICC was 0.999 (p < 0.01). The mean ossification volume was 1,831.68 ± 1,302.12 mm3 at the first examination and 1,928.31 ± 1,363.15 mm3 at the second, showing a significant mean increase in ossification volume (96.63 mm3; p = 0.0002) over 22 months. The mean rate of increase in volume between the first and second CT examinations was 5.95 % (range 0.08–15.09 %). The mean annual rate of lesion increase was 3.33 % (range 0.08–7.79 %). The location and direction of the increased ossification were confirmed three-dimensionally in some patients with a larger increase in volume. In these patients, longitudinal progression of the ossification in the C2-3 intervertebral space was observed. However, the progression could not be evaluated in detail in patients with a smaller increase in volume.
The measurement of the length of OPLL using X-rays was possible in 10 cases. The maximum thickness was recorded in 19 cases. OPLL could not be identified clearly in X-rays because of overlap with shoulder in the lateral view. Therefore, the measurement of the length and the thickness were possible in all cases. The mean progression of length was 1.22 mm (0.07–3.22), thickness was 0.30 mm (0.01–0.65), and spinal canal occupation rate was 1.78 % (0.08–7.79) (Table 2). Measurement of spinal canal occupation rate using CT was possible in all cases. The mean progression of the spinal canal occupation rate was 1.84 % (0.02–5.40) (Table 2). There was no correlation between the rate of increase in volume of OPLL progression of length, thickness, spinal canal occupation rate using either X-ray or CT.
Table 2.
Measurement value of X-rays and CT
| Length at first examination (mm) | Length at second examination (mm) | Length at increase (mm) | Thickness at first examination (mm) | Thickness at second examination (mm) | Thickness of increase (mm) | Canal stenosis ratio at first examination (%) (X-ray) | Canal stenosis ratio at second examination (%) (X-ray) | Canal stenosis ratio of increase (%) (X-ray) | Canal stenosis ratio at first examination (%) (CT) | Canal stenosis ratio at second examination (%) (CT) | Canal stenosis ratio of increase (%) (CT) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 63.50 | 66.37 | 2.87 | 10.11 | 10.22 | 0.11 | 77.06 | 77.42 | 0.37 | 71.81 | 74.41 | 2.60 |
| Case 2 | 82.75 | 86.63 | 3.88 | 6.36 | 6.40 | 0.04 | 53.63 | 53.83 | 0.20 | 56.69 | 62.09 | 5.40 |
| Case 3 | – | – | – | 5.58 | 5.59 | 0.01 | 44.29 | 45.12 | 0.83 | 51.41 | 51.50 | 0.09 |
| Case 4 | 42.70 | 43.95 | 1.25 | 4.93 | 5.50 | 0.57 | 39.69 | 43.17 | 3.48 | 38.01 | 41.18 | 3.17 |
| Case 5 | – | – | – | 6.57 | 6.81 | 0.24 | 41.29 | 41.42 | 0.13 | 38.85 | 41.77 | 2.92 |
| Case 6 | – | – | – | 6.70 | 7.32 | 0.62 | 61.87 | 62.09 | 0.22 | 54.81 | 58.01 | 3.20 |
| Case 7 | 108.87 | 109.32 | 0.45 | 5.81 | 6.04 | 0.23 | 42.60 | 43.77 | 1.17 | 31.74 | 34.77 | 3.02 |
| Case 8 | 61.58 | 61.95 | 0.37 | 5.93 | 6.57 | 0.64 | 55.21 | 61.52 | 6.30 | 66.70 | 69.26 | 2.55 |
| Case 9 | 50.99 | 52.05 | 1.06 | 5.74 | 6.08 | 0.34 | 40.94 | 43.37 | 2.43 | 40.89 | 40.91 | 0.02 |
| Case 10 | 5.56 | 5.74 | 0.18 | 3.56 | 3.87 | 0.31 | 25.56 | 27.98 | 2.43 | 19.45 | 20.78 | 1.33 |
| Case 11 | – | – | – | 5.18 | 5.34 | 0.16 | 38.54 | 39.38 | 0.84 | 40.57 | 41.68 | 1.11 |
| Case 12 | – | – | – | 2.55 | 3.20 | 0.65 | 20.95 | 26.40 | 5.45 | 26.32 | 26.37 | 0.05 |
| Case 13 | – | – | – | 8.65 | 8.85 | 0.20 | 51.03 | 52.06 | 1.03 | 47.24 | 50.10 | 2.87 |
| Case 14 | 51.79 | 53.51 | 1.72 | 7.43 | 7.50 | 0.07 | 57.64 | 57.69 | 0.05 | 57.15 | 57.87 | 0.72 |
| Case 15 | 77.42 | 77.80 | 0.38 | 6.57 | 6.87 | 0.30 | 49.55 | 50.85 | 1.30 | 46.52 | 47.44 | 0.92 |
| Case 16 | – | – | – | – | – | – | – | – | – | 51.29 | 54.05 | 2.75 |
| Case 17 | – | – | – | 4.79 | 5.10 | 0.31 | 38.72 | 41.06 | 2.34 | 36.39 | 39.38 | 2.99 |
| Case 18 | 5.42 | 5.49 | 0.07 | 3.18 | 3.20 | 0.02 | 24.96 | 25.00 | 0.04 | 25.13 | 25.66 | 0.53 |
| Case 19 | – | – | – | 5.32 | 5.86 | 0.54 | 34.52 | 37.78 | 3.26 | 30.29 | 30.37 | 0.07 |
| Case 20 | – | – | – | 8.97 | 9.27 | 0.30 | 71.70 | 73.57 | 1.87 | 62.81 | 63.36 | 0.54 |
| Mean | 55.06 | 56.28 | 1.22 | 5.83 | 6.13 | 0.30 | 44.34 | 46.11 | 1.77 | 44.70 | 46.55 | 1.84 |
Discussion
Our method of using CT imaging is novel and can precisely evaluate the volume of OPLL at one point and provide information about the progression of OPLL during a given period with minimal analytical error. Chiba et al. [6, 7] reported the incidence of ossification progression based on computer analysis of X-ray images in patients who had undergone laminoplasty. Measurements of ossification length and thickness using 2D X-ray images are possible to a certain extent [17, 18]. However, its accuracy is reduced due to errors associated with the imaging procedure. For example, ossification width and volume are not measurable. Other disadvantages of X-ray analysis include inability to identify small-area ossifications and to accurately locate the ossification. When evaluating a pulmonary nodule, 3D measurement is more reliable in determining changes in growth [12–15].
Ossification not identifiable by X-ray examination can be evaluated on CT images, and therefore, the classification discrimination may differ from that obtained by X-ray imaging. Multidimensional evaluation of ossification can be achieved with CT images, but it is difficult to evaluate continuity on 2D X-ray images. On 3D images, however, evaluation of continuity and classification of ossification are comparatively simple [19]. With CT images, changes in ossification form and thickness on the caudal side can be evaluated in detail in patients with ossifications growing toward the cranial side. Slight graininess of the images associated with slice thickness is seen at ossification boundaries. In this study, a thinner slice produced clearer boundaries on 3D images.
By calculating exact volumes, it was possible to derive an accurate numerical estimate of both overall absolute value of the volume of ossification and its rate of progression over time, and to compare these volumes with those derived from subsequent scans. Considering that the error of volume calculation associated with slice thickness was minimal (0.12 %), the precision of the measurements was obviously high and only a slight degree of intra-examiner error was evident. The ossification volume increased annually to some extent in all the studied patients. Since ossification size varies markedly among individuals, the absolute value of the increase in ossification volume can differ considerably depending on the original size. Therefore, we used the rate of increase in volume, since comparable evaluation of increased ossification among all the patients based on the absolute volume would have been difficult. The evaluation based on the rate of increase in volume was not affected by the original size of the ossification, and, therefore, was useful for comparative study. The mean annual rate of increase in volume was 3.33 %, implying that the spinal canal narrows gradually with time. In this study, there was no correlation between the canal stenosis ratio of maximum thickness and annual progression rate. Ossification volume did not increase, but the maximum thickness did which increased lesions. A high rate of progression is a potential risk factor for myelopathy due to spinal canal stenosis. Further analyses of the location and the direction of increased ossification are necessary to elucidate the detailed history of progression and to establish the preventive methods and the effective treatment. This method may be useful to examine the risk factors for progression of OPLL, to determine the timing for initiating the drug therapy for prevention of OPLL that may be available in the near future, and to identify the progression of OPLL in different surgical procedures. The authors believe that their novel method allows detailed evaluation of ossification progression, which cannot be achieved using X-ray evaluation.
There were several potential limitations to this study. First, a threshold of 226–3,071 HU was set as an appropriate value for detection. As this threshold setting was defined by MIMICS for the detection of bone, and was not specific for ossification, the volume of the ossification might have been over- or underestimated in some cases. However, identification of ossification and volume calculation were done at the same CT examination with the same threshold setting, and thus evaluation based on comparison of volumes would have been valid. Second, the method used for identification of ossification was not completely automatic, and thus accidental errors might have occurred. However, the ICC calculated in this study was obviously high, suggesting that the evaluation of ossification volume was accurate and valid.
The next step is to analyze the risk factors for OPLL progression using our novel method, to verify those that have been described in previous studies [6, 17].
Conclusion
We measured ossification volume based on a novel method involving creation of a 3D model from DICOM data obtained from CT images. The novel method described here appears to be very useful for quantitative evaluation of OPLL with only minimal measurement error. It is also expected to be useful for identifying the risk factors associated with progression of OPLL, to determine the timing for preventive therapy, and to identify progression during surgical procedures.
Acknowledgments
This work was supported by Health and Labour Sciences Research Grants.
Conflict of interest
None.
References
- 1.Bakay L, Cares HL, Smith RJ. Ossification in the region of the posterior longitudinal ligament as a cause of cervical myelopathy. J Neurol Neurosurg Psychiatry. 1970;33:263–268. doi: 10.1136/jnnp.33.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayakumar PN, Kolluri VR, Vasudev MK, Srikanth SG. Ossification of the posterior longitudinal ligament of the cervical spine in Asian Indians: a multiracial comparison. Clin Neurol Neurosurg. 1996;98:142–148. doi: 10.1016/0303-8467(96)00004-2. [DOI] [PubMed] [Google Scholar]
- 3.Lee T, Chacha PB, Khoo J. Ossification of posterior longitudinal ligament of the cervical spine in non-Japanese Asians. Surg Neurol. 1991;35:40–44. doi: 10.1016/0090-3019(91)90200-S. [DOI] [PubMed] [Google Scholar]
- 4.Ogata N, Kawaguchi H. Ossification of the posterior longitudinal ligament of spine (OPLL) Clin Calcium. 2004;14:42–48. [PubMed] [Google Scholar]
- 5.Matsunaga S, Nakamura K, Seichi A, et al. Radiographic predictors for the development of myelopathy in patients with ossification of the posterior longitudinal ligament: a multicenter cohort study. Spine (Phila Pa 1976) 2008;33:2648–2650. doi: 10.1097/BRS.0b013e31817f988c. [DOI] [PubMed] [Google Scholar]
- 6.Chiba K, Yamamoto I, Hirabayashi H, Iwasaki M, Goto H, Yonenobu K, Toyama Y. Multicenter study investigating the postoperative progression of ossification of the posterior longitudinal ligament in the cervical spine: a new computer-assisted measurement. J Neurosurg Spine. 2005;3:17–23. doi: 10.3171/spi.2005.3.1.0017. [DOI] [PubMed] [Google Scholar]
- 7.Chiba K, Kato Y, Tsuzuki N, Nagata K, Toyama Y, Iwasaki M, Yonenobu K. Computer-assisted measurement of the size of ossification in patients with ossification of the posterior longitudinal ligament in the cervical spine. J Orthop Sci. 2005;10:451–456. doi: 10.1007/s00776-005-0925-5. [DOI] [PubMed] [Google Scholar]
- 8.Hori T, Kawaguchi Y, Kimura T. How does the ossification area of the posterior longitudinal ligament progress after cervical laminoplasty? Spine (Phila Pa 1976) 2006;31:2807–2812. doi: 10.1097/01.brs.0000245870.97231.65. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi Y, Kanamori M, Ishida H, et al. Progression of posterior longitudinal ligament following cervical laminoplasty. J Bone Joint Surg Am. 2001;83-A:1798–1802. doi: 10.2106/00004623-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Yonenobu K, Tsuzuki N, Nagata K, Toyama Y, Kato Y, Iwasaki M. Computer-assisted measurement of ossified lesion in ossification of the posterior longitudinal ligament of the cervical spine. Bone. 2002;16:283–286. [Google Scholar]
- 11.Seichi A. Updates on ossification of posterior longitudinal ligament. Image diagnosis of ossification of posterior longitudinal ligament and associated diseases. Clin Calcium. 2009;19:1426–1434. [PubMed] [Google Scholar]
- 12.Honda O, Kawai M, Gyobu T, et al. Reproducibility of temporal volume change in CT of lung cancer: comparison of computer software and manual assessment. Br J Radiol. 2009;82:742–747. doi: 10.1259/bjr/67746844. [DOI] [PubMed] [Google Scholar]
- 13.Revel MP, Bissery A, Bienvenu M, et al. Are two-dimensional CT measurements of small non-calcified pulmonary nodules reliable? Radiology. 2004;231:453–458. doi: 10.1148/radiol.2312030167. [DOI] [PubMed] [Google Scholar]
- 14.Revl MP, Lefort C, Bissery A, et al. Pulmonary nodules: preliminary experience with three-dimensional evaluation. Radiology. 2004;231:459–466. doi: 10.1148/radiol.2312030241. [DOI] [PubMed] [Google Scholar]
- 15.Yankelevits DF, Reeves AP, Kotis WL, et al. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology. 2000;217:251–256. doi: 10.1148/radiology.217.1.r00oc33251. [DOI] [PubMed] [Google Scholar]
- 16.Investigation Committee on OPLL of the Japanese Ministry of Public Health and Welfare The ossification of the posterior longitudinal ligament of the spine (OPLL) J Jpn Orthop Assoc. 1981;55:425–440. [PubMed] [Google Scholar]
- 17.Kawaguchi Y, Kanamori M, Ishida H, et al. Progression of posterior longitudinal ligament following en bloc cervical laminoplasty. Orthop Surg. 2004;45:192–196. doi: 10.2106/00004623-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Takatsu T, Ishida Y, Suzuki K, Inoue H. Radiological study of cervical ossification of the posterior longitudinal ligament. J Spinal Disord. 1999;12:271–273. [PubMed] [Google Scholar]
- 19.Chang H, Kong CG, Won HY, Kim JH, Park JB. Inter- and intra-observer variability of a cervical OPLL classification using reconstructed CT images. Clin Orthop Surg. 2010;2:8–12. doi: 10.4055/cios.2010.2.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]