Abstract
Purpose
The spinal penetration index (SPI) quantifies the portion of the rib cage occupied by vertebrae. When measured by computed tomography (CT) or magnetic resonance imaging, SPI can only be determined in the reclining position, which modifies spinal and thoracic morphology. CT results in high radiation exposure. The authors studied rib cage and spinal morphology using low-dose biplanar stereoradiography and their impact on respiratory function in adolescent idiopathic scoliosis (AIS).
Methods
In eighty thoracic AIS patients, a slot-scanning radiologic device allowing simultaneous acquisition of orthogonal images and 3D reconstructions with low exposure to radiation (EOS) was used to determine thoracic volume, mean spinal penetration index (SPIm), apical spinal penetration index (SPIa), main thoracic (MT) curve Cobb angle, T4–T12 kyphosis, and apical vertebral rotation (AVR).
Results
Thoracic volume was correlated with thoracic kyphosis (r = 0.31, p = 0.006), but not with SPI, MT Cobb angle, or AVR. SPIm and SPIa were negatively correlated with thoracic kyphosis. Forced vital capacity and forced expiratory volume in 1 s were significantly lower in the hypokyphotic patients (p = 0.04, p = 0.03, respectively) and correlated with thoracic volume and T4–T12 kyphosis. No correlation was found between spinal penetration indices and pulmonary function tests, but SPIm was significantly greater in patients with obstructive syndrome (p = 0.01).
Conclusions
With little radiation exposure, EOS biplanar stereoradiography permits routine imaging is a functional standing position. Hypokyphotic patients had significantly decreased FEV1 and FVC. SPIm was significantly higher in patients with obstructive syndrome.
Keywords: Adolescent idiopathic scoliosis, Rib cage, Spinal penetration index, Stereoradiography, 3D
Introduction
Scoliosis is a three-dimensional (3D) global deformity of the trunk consisting in structural modifications of the rib cage as well as abnormal frontal and sagittal spinal curvatures. Restrictive respiratory impairment has been reported in patients with thoracic adolescent idiopathic scoliosis (AIS), because of chest wall and diaphragmatic mechanical dysfunction [1]. Correlations between pulmonary function and frontal Cobb angle, sagittal diameter of the thoracic cage, and axial rotation of apical thoracic vertebrae have been found, suggesting the importance of evaluating thorax morphology in scoliotic patients [1–5]. In 2003, Dubousset et al. [6] first introduced the concept of spinal penetration index (SPI) in neuromuscular lordoscoliotic patients with airway compression, and described two humps associated with thoracic scoliosis: the visible or cosmetic exothoracic rib hump and a hidden or functional endothoracic vertebral hump. To assess 3D indices, computed tomography (CT) or magnetic resonance imaging is usually required. Both techniques have the disadvantage of being performed supine, which modifies the frontal and sagittal spinal curvatures and affects the thoracic shape [7]. Furthermore, CT scanning results in high radiation exposure, which proscribes it for routine clinical use.
The EOS device (EOS imaging, Paris, France) is a low-dose biplanar stereoradiographic system, which simultaneously acquires orthogonal imaging of patients in the standing position, and permits routine quantitative 3D reconstructions [8–11]. Moreover, 3D reconstruction of the thoracic cage by stereoradiography has also been developed and validated in small series of healthy volunteers, early onset scoliosis patients undergoing growing rod procedures, and patients with moderate AIS treated by bracing [12–14]. However, measurements of the SPI, and its relation to other clinical parameters, have never been reported in a large cohort of AIS patients. The purpose of this study was to measure the 3D thoracic volume and spinal parameters based on EOS 3D reconstructions in patients with AIS. Subsequently, we compared those results with respiratory parameters obtained during pulmonary function tests.
Materials and methods
Patients
Following institutional review board approval, 80 patients followed for thoracic AIS (Lenke 1–4) were prospectively included. Patients were divided into three groups, based on the main thoracic (MT) curve magnitude. Group 1 (26 patients) included patients with a MT Cobb angle between 20° and 40°, group 2 (32 patients) those with a MT curve between 40° and 65°, and group 3 (22 patients) those with a MT Cobb angle >65°. None of the patients had undergone spinal or thoracic surgery.
Imaging protocol and 3D modeling
Biplanar radiographs of patients in a standing position were obtained with the EOS system, which is used instead of conventional X-rays in our unit for routine clinical work-ups [11]. The system is a slot-scanning radiologic device consisting of two orthogonal X-ray sources, allowing simultaneous acquisition of two images and 3D reconstructions with very low exposure to radiation [8, 13]. Patients maintained their arms folded at 45° to avoid superposition with the spine. All images included the base of the skull and the upper third of the femurs. Scan time ranged from 6 to 10 s depending on the patient’s height.
Dedicated software developed jointly by the Laboratory for Imaging Research and Orthopedics (ETSCRCHUM, Montreal, Canada) and the Laboratoire de Biomécanique (Arts et Métiers ParisTech, Paris, France), was used by a trained physician, experimented with the method, to produce 3D reconstructions of the spine and rib cage [10, 12].
In brief, the spinal images were digitized as follows. Characteristic anatomic landmarks were identified on radiographs. For each rib, the most lateral point of the rib halfway between the top and bottom edge on the posteroanterior view and the most posterior point on the lateral radiograph were marked. These marks showed the lateral and posterior contours of the rib cage. Extremities of the first and fourth ribs were identified on the posteroanterior view, and the extremities of the tenth ribs were identified on both views yielding stereo-corresponding points. In addition, three sternal stereo-corresponding points were identified on the biplanar radiographs. For each non-stereo-corresponding point, the projection line between the X-ray source and points identified on the radiograph was determined, using the calibrated environment of the cabin.
Thoracic parameters
The following clinical parameters were computed:
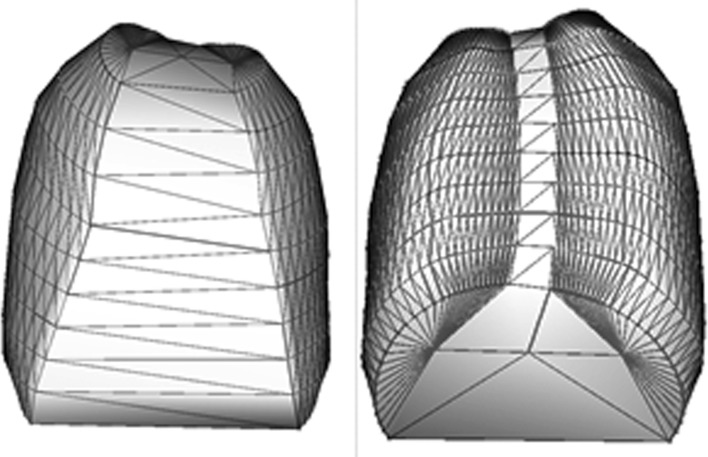
Thoracic volume (in mm3), obtained by joining the surfaces extracted from the rib reconstructions (Fig. 1).
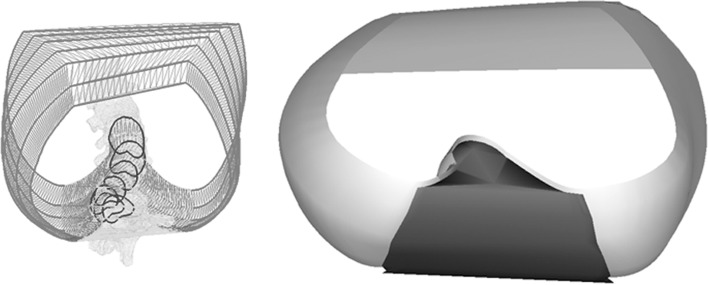
Mean spinal penetration index (SPIm), corresponding to the mean volume (in %) of the spine that penetrates the volume of the thoracic cage, calculated between the planes, best fit to the third and tenth rib pairs (Fig. 2).
Apical spinal penetration index (SPIa), corresponding to the surface of the spine (in %) penetrating the surface of the thoracic cage in the axial plane parallel to the ribs of the apical level, and passing through the geometric center of the apical vertebra of the MT curve (Fig. 3).
Fig. 1.
Thoracic volume obtained by joining the surfaces extracted from the ribs reconstructions
Fig. 2.
Mean spinal penetration index, corresponding to the mean volume (in %) of the spine that penetrates the volume of the thoracic cage
Fig. 3.
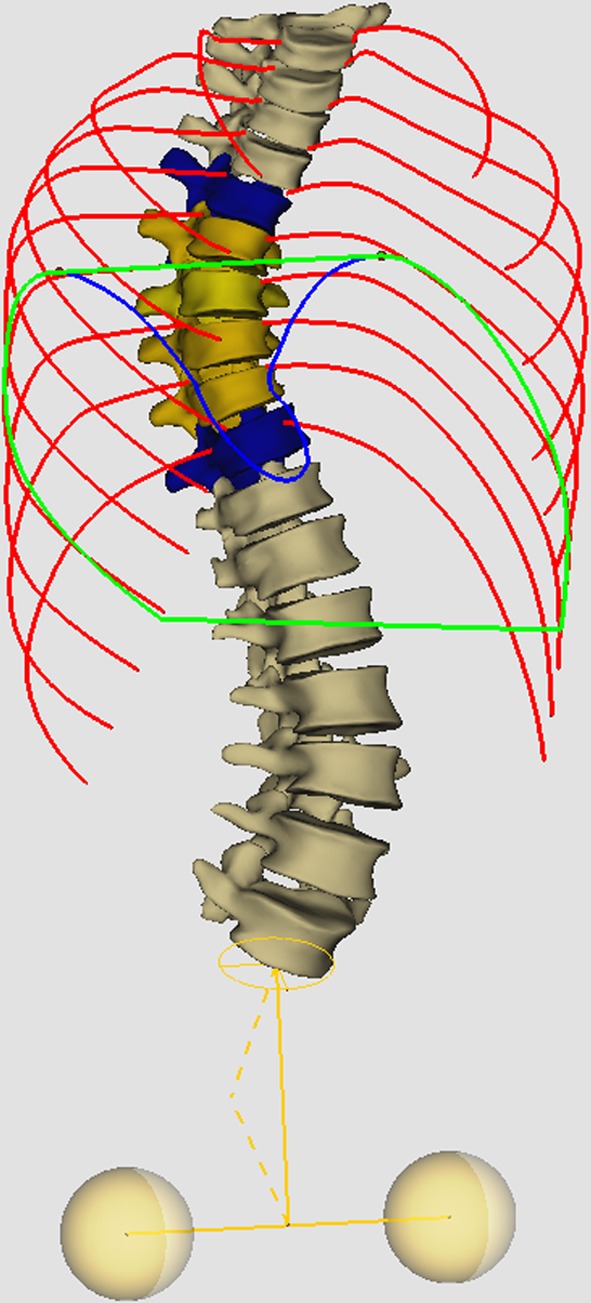
Apical spinal penetration index (SPIa) corresponding to the surface of the spine (in %) penetrating the surface of the thoracic cage in the axial plane parallel to the ribs of the apical level, and passing through the geometric center of the apical vertebra of the MT curve
Spinal parameters
Radiological measurements included the Cobb magnitude of the coronal plane MT curve, the sagittal plane Cobb angle between T4 and T12, and the degree of apical vertebral rotation (AVR) of the main thoracic curve.
Pulmonary function tests (arm span method)
Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1) and total lung capacity (TLC) were measured in patients with thoracic AIS >40° (groups 1 and 2), as part as of the work-up in these potential candidates for surgical treatment.
Statistical analysis
Groups were compared using two-sample t tests (assuming unequal variances). Pearson correlations were calculated between radiological parameters, pulmonary function tests and SPI values. A P value <0.05 was considered to be significant. All statistical analyses were conducted using the software Statview (SAS Institute Inc, Cary, NC, USA).
Results
Demographic data and reconstruction time
Demographic data and reconstruction times for the three groups are reported in Table 1. No significant difference was found between the groups, but a trend toward quicker reconstruction of the rib cage for severe curves (group 3) was observed. The mean calculated entrance surface doses obtained with stereoradiography were 0.18 mGy (±0.05 mGy) for the frontal view and 0.30 mGy (±0.07 mGy) for the lateral view, thus corresponding to an effective dose of 0.06 mSv.
Table 1.
Demographic data and reconstruction times of the 80 patients
| Group 1 (>65°, N = 22) | Group 2 (40–65°, N = 32) | Group 3 (20–40°, N = 26) | |
|---|---|---|---|
| Age | 15.1 years old ± 1.5 | 14.6 years old ± 1.2 | 14.7 years old ± 0.5 |
| Reconstruction time (spine) | 11 min 10 s (±2 min) | 10 min 50 s (±1.5 min) | 10 min 35 s (±1.5 min) |
| Reconstruction time (thoracic cage) | 5 min 10 s (±0.5 min) | 5 min 20 s (±1 min) | 5 min 50 s (±1 min) |
Values are presented with standard deviation
F female, M male
Spinal and thoracic parameters
Spinal and thoracic parameters of the cohort are summarized in Tables 2, 3, respectively. No significant difference was found between groups regarding T4–T12 thoracic kyphosis, thoracic volume and spinal penetration indices. AVR significantly increased with the MT Cobb angle (r = 0.804, p < 0.0001). Thoracic hypokyphosis (i.e. < 20°) was observed in 44 (55 %) of the patients.
Table 2.
Spinal parameters of the 80 patients
| Group 1 (>65°, N = 22) | Group 2 (40–65°, N = 32) | Group 3 (20–40°, N = 26) | |
|---|---|---|---|
| Main thoracic Cobb angle | 73.8° ± 9 | 49.2° ± 8 | 26.2° ± 5 |
| T4–12 kyphosis | 17.8° ± 11 | 16.3° ± 12 | 21° ± 13 |
| Apical vertebral rotation | 24.3° ± 8 | 15.5° ± 6 | 6.3° ± 3 |
Values are presented with standard deviation
Table 3.
Measurements of the thoracic parameters in the 80 patients
| Group 3 (>65°, N = 22) | Group 2 (40–65°, N = 32) | Group 1 (20–40°, N = 26) | |
|---|---|---|---|
| Thoracic volume | 5,059 ± 1,023 mm3 | 4,992 ± 771 mm3 | 4,936 ± 1,056 mm3 |
| SPIa | 13.6 ± 1.8 % | 13.3 ± 1.6 % | 12.8 ± 2.1 % |
| SPIm | 8.8 ± 1.1 % | 8.5 ± 1.3 % | 8.1 ± 1.1 % |
Values are presented with standard deviation
SPIa apical spinal penetration index, SPIm mean spinal penetration index
The variations in thoracic parameters, according to T4–T12 kyphosis, are summarized in Table 4. A significant correlation was found between thoracic volume and thoracic kyphosis (r = 0.31, p = 0.006). No correlation was observed between either spinal penetration index and MT Cobb angle or AVR (Table 5). In contrast, a significant negative correlation was found between both spinal penetration indices and thoracic kyphosis (Table 5).
Table 4.
Measurements of the thoracic parameters according to T4–T12 kyphosis
| Normokyphosis (N = 36) | Hypokyphosis (<20°, N = 44) | p | |
|---|---|---|---|
| Thoracic volume | 5,186 ± 996 mm3 | 4,818 ± 829 mm3 | 0.09 |
| SPIa | 12.4 ± 1.9 % | 13.8 ± 1.4 % | 0.0004 |
| SPIm | 7.9 ± 1.1 % | 8.9 ± 1.1 % | 0.0009 |
Values are presented with standard deviation
SPIa apical spinal penetration index, SPIm mean spinal penetration index
Table 5.
Correlations (with p values) between thoracic and spinal parameters in 80 patients
| MT Cobb angle | AVR | T4–T12 kyphosis | |
|---|---|---|---|
| SPIa | r = 0.14 | r = 0.13 | r = −0.51 |
| p = 0.22 | p = 0.26 | p < 0.0001 | |
| SPIm | r = 0.19 | r = 0.22 | r = −0.52 |
| p = 0.09 | p = 0.06 | p < 0.0001 |
MT main thoracic, AVR apical vertebral rotation, SPIa apical spinal penetration index, SPIm mean spinal penetration index
Pulmonary function tests
Pulmonary function tests were available in all patients included in groups 2 and 3 (54 patients). Mean FVC was 2.9 ± 0.6 L and FEV1 averaged 2.5 ± 0.5 L. A moderate obstructive syndrome (i.e. FEV1 <80 % predicted value) was observed in 26 patients (48 %), but clinically relevant dysfunction (i.e. FEV1 <65 % predicted value) was found in only 6 patients (11 %). MT Cobb angle in these six patients averaged 77° ± 12° and mean T4–T12 kyphosis was 18.8° ± 11°. FVC and FEV1 were correlated with thoracic volume measurement (r = 0.82 and r = 0.76, p < 0.0001) and T4–T12 kyphosis (r = 0.4 and r = 0.37, p < 0.0001). Both parameters were significantly lower in the hypokyphotic patients than in the other patients (p = 0.04 and p = 0.03, respectively). No correlation was found between spinal penetration indices and pulmonary function tests in the global cohort, but SPIm was significantly greater in the 26 patients with obstructive syndrome than in the other 28 patients (p = 0.01).
TLC averaged 3.8 ± 0.8 L. Restrictive syndrome (i.e. TLC < 80 % predicted value) was observed in 23 patients (43 %), but significant restriction (i.e. TLC < 65 % predicted value) was found in only 2 patients (3.7 %). A significant correlation was found between thoracic volume measurement on 3D reconstructions and TLC (r = 0.8, p < 0.0001).
Discussion
Interest in 3D analysis
Interest in the potential clinical relevance and impact of 3D analysis of scoliotic deformities is growing [15, 16]. Hong et al. [15] have reported significant correlations between 3D spinal parameters and clinical outcomes. Structural modifications of the thoracic cage in scoliosis have also been investigated, but most reports have focused on rib deformity analysis and the anterior or posterior chest wall shape, as cosmetic issues for the patients [2, 15, 17, 18]. Takahashi et al. [5] emphasized the need to consider not only the spinal curvature, but also rotational deformity of the rib cage, and evaluated in two dimensions the thoracic area and sagittal diameter of the thorax at all vertebral levels. A significant correlation was found between pulmonary vital capacity and these measurements at the levels T8 and T9. Formerly, CT scanning was the most frequently used imaging tool for thorax analysis but at the expense of high radiation exposure, and with the limitation of being performed in the supine position. These drawbacks can be avoided by the use of low-dose biplanar radiographs of standing AIS patients to obtain 3D reconstructions, the validity of which in routine use has been established at present [11].
Spinal penetration index
Based on computed tomography, Dubousset et al. [6] introduced the concept of SPI to evaluate the influence of scoliosis on the 3D shape of the rib cage and quantify in 2D (SPIa) and 3D (SPIm), the protrusion of vertebral bodies into the thorax. In the present cohort of AIS patients, the endothoracic hump was assessed using stereoradiography. Interestingly, reconstruction times for severe scolioses were not longer than those for moderate scoliosis for a trained operator. On posteroanterior views, the time necessary to mark anatomical landmarks was equivalent in the three groups of patients, and on lateral views, because of the rib hump, the landmarks were easier to distinguish (except for the three proximal ribs) in patients with severe deformity. On lateral views, the horizontal portion of the ribs on the concave side was perpendicular to the X-ray source, giving the ribs a rounded shape. Work is in progress on semi-automatic processing to further reduce the 3D reconstruction time.
The present study is the first to measure pulmonary functional consequences of 2D or 3D SPI determined in patients in a standing position. Both SPIa and SPIm were correlated to the spinal sagittal alignment, i.e. significantly increased in hypokyphotic patients (p = 0.0004 and p = 0.0009, respectively). SPIm ranged from 6.3 to 12.8 %, and was significantly higher in patients with obstructive syndrome (p = 0.01), but not in patients with restrictive syndrome.
Pulmonary function and spinal parameters
Respiratory impairment in AIS patients is more frequent than previously believed, with pulmonary function tests under 65 % of predicted values reported in 19 to 41 % [1, 19–21]. Johnston et al. [1] reported correlations between respiratory impairment and the severity of both hypokyphosis and the MT Cobb angle. In the current study, pulmonary function tests were significantly correlated with T4–T12 kyphosis, increased respiratory impairment being associated with sagittal flattening. Hypokyphotic patients had significantly decreased FEV1 and FVC (p = 0.04 and p = 0.03, respectively). The present results are consistent with previous reports [1, 19, 20], and emphasize again the importance not only of correcting scoliosis in the frontal plane, but also of restoring a physiological sagittal alignment. No correlation was found between pulmonary function tests and AVR or MT Cobb angle, although mean MT Cobb angle in patients with clinically relevant restrictive syndrome (<65 % predicted values) averaged 77°, consistent with the tidemark reported by Johnston et al. [1] (>70°) separating minimal from severe pulmonary dysfunction. No significant correlation was found between any of the spinal penetration indices and the pulmonary function tests.
Thoracic volume
Low-dose stereoradiography also allowed 3D thoracic volume assessment, which was significantly correlated with pulmonary function tests. In particular, thoracic volume was highly correlated with TLC (r = 0.8, p < 0.0001), which corresponds to the maximum volume of the lungs with the greatest possible effort of inhalation. However, the measurement given by low-dose biplanar radiographs remained morphological, and must be distinguished from the vital capacity, which depends not only on the volume, but also on the related function of thoracic muscles, including the diaphragm, and the stiffness of other chest wall structures (ribs, joints). Similar to the series of Takahashi et al. [5], the present hypokyphotic patients tended to have a smaller thoracic volume than the others, but the difference did not reach statistical significance.
Limitations of the study
There are several weaknesses in this study. First, the patients were requested not to move during the stereoradiographic examination, but whether they should hold their breath after inspiration or expiration was not specified. This needs to be standardized in the future studies. Second, the sample size was relatively small and there was no control group of nonscoliotic patients. Third, pulmonary function tests were not performed in the group of patients with moderate scoliosis (20°–40°). In addition, the thoracic volume reported in the present study represented only the volume generated by the reconstructions, and therefore might differ from the real physiological volume influenced by the position of the diaphragm. Finally, the present study was a retrospective descriptive analysis of data prospectively collected from adolescents, but the measurement errors of spinal penetration indices need to be further assessed to validate the method. Further investigation on younger patients with progressive curves might provide useful information on thoracic growth and confirm the correlation between spinal penetration indices and spinal sagittal alignment.
In conclusion, with low levels of radiation exposure, low-dose biplanar stereoradiography permits thorough investigation of trunk deformities in young patients. The spinal penetration index, assessed for the first time in a functional standing position, significantly increased with sagittal flattening of the thoracic spine and the hypokyphotic patients had significantly decreased FEV1 and FVC (p = 0.04 and p = 0.03, respectively). No significant correlation was found between SPI and any pulmonary function parameter in the global cohort, but SPIm was significantly increased in patients with obstructive syndrome (FEV1 < 80 % predicted values).
Conflict of interest
None.
References
- 1.Johnston CE, Richards BS, Sucato DJ, Bridwell KH, Lenke LG, Erickson M. Correlation of preoperative deformity magnitude and pulmonary function tests in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2011;36:1096–1102. doi: 10.1097/BRS.0b013e3181f8c931. [DOI] [PubMed] [Google Scholar]
- 2.Erkula G, Sponseller PD, Kiter AE. Rib deformity in scoliosis. Eur Spine J. 2003;12:281–287. doi: 10.1007/s00586-002-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lonner BS, Auerbach JD, Estreicher MB, Betz RR, Crawford AH, Lenke LG, Newton PO. Pulmonary function changes after various anterior approaches in the treatment of adolescent idiopathic scoliosis. J Spinal Disord Tech. 2009;22:551–558. doi: 10.1097/BSD.0b013e318192d8ad. [DOI] [PubMed] [Google Scholar]
- 4.Qiu Y, Sun GQ, Zhu F, Wang WJ, Zhu ZZ. Rib length discrepancy in patients with adolescent idiopathic scoliosis. Stud Health Technol Inform. 2010;158:63–66. [PubMed] [Google Scholar]
- 5.Takahashi S, Suzuki N, Asazuma T, Kono K, Ono T, Toyama Y. Factors of thoracic cage deformity that affect pulmonary function in adolescent idiopathic thoracic scoliosis. Spine (Phila Pa 1976) 2007;32:106–112. doi: 10.1097/01.brs.0000251005.31255.25. [DOI] [PubMed] [Google Scholar]
- 6.Dubousset J, Wicart P, Pomero V, Barois A, Estournet B. Spinal penetration index: new three-dimensional quantified reference for lordoscoliosis and other spinal deformities. J Orthop Sci. 2003;8:41–49. doi: 10.1007/s007760300007. [DOI] [PubMed] [Google Scholar]
- 7.Yazici M, Acaroglu ER, Alanay A, Deviren V, Cila A, Surat A. Measurement of vertebral rotation in standing versus supine position in adolescent idiopathic scoliosis. J Pediatr Orthop. 2001;21:252–256. [PubMed] [Google Scholar]
- 8.Deschenes S, Charron G, Beaudoin G, Labelle H, Dubois J, Miron MC, Parent S. Diagnostic imaging of spinal deformities: reducing patients radiation dose with a new slot-scanning X-ray imager. Spine (Phila Pa 1976) 2010;35:989–994. doi: 10.1097/BRS.0b013e3181bdcaa4. [DOI] [PubMed] [Google Scholar]
- 9.Dubousset J, Charpak G, Skalli W, Kalifa G, Lazennec JY. EOS stereo-radiography system: whole-body simultaneous anteroposterior and lateral radiographs with very low radiation dose. Rev Chir Orthop Reparatrice Appar Mot. 2007;93:141–143. doi: 10.1016/S0035-1040(07)92729-4. [DOI] [PubMed] [Google Scholar]
- 10.Humbert L, De Guise JA, Aubert B, Godbout B, Skalli W. 3D reconstruction of the spine from biplanar X-rays using parametric models based on transversal and longitudinal inferences. Med Eng Phys. 2009;31:681–687. doi: 10.1016/j.medengphy.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Ilharreborde B, Steffen JS, Nectoux E, Vital JM, Mazda K, Skalli W, Obeid I. Angle measurement reproducibility using EOS three-dimensional reconstructions in adolescent idiopathic scoliosis treated by posterior instrumentation. Spine (Phila Pa 1976) 2011;36:E1306–E1313. doi: 10.1097/BRS.0b013e3182293548. [DOI] [PubMed] [Google Scholar]
- 12.Jolivet E, Sandoz B, Laporte S, Mitton D, Skalli W. Fast 3D reconstruction of the rib cage from biplanar radiographs. Med Biol Eng Comput. 2010;48:821–828. doi: 10.1007/s11517-010-0610-5. [DOI] [PubMed] [Google Scholar]
- 13.Mitton D, Zhao K, Bertrand S, Zhao C, Laporte S, Yang C, An KN, Skalli W. 3D reconstruction of the ribs from lateral and frontal X-rays in comparison to 3D CT-scan reconstruction. J Biomech. 2008;41:706–710. doi: 10.1016/j.jbiomech.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Sabourin M, Jolivet E, Miladi L, Wicart P, Rampal V, Skalli W. Three-dimensional stereoradiographic modeling of rib cage before and after spinal growing rod procedures in early-onset scoliosis. Clin Biomech (Bristol, Avon) 2010;25:284–291. doi: 10.1016/j.clinbiomech.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Hong JY, Suh SW, Easwar TR, Modi HN, Yang JH, Park JH. Evaluation of the three-dimensional deformities in scoliosis surgery with computed tomography: efficacy and relationship with clinical outcomes. Spine (Phila Pa 1976) 2011;36:E1259–E1265. doi: 10.1097/BRS.0b013e318205e413. [DOI] [PubMed] [Google Scholar]
- 16.Labelle H, Aubin CE, Jackson R, Lenke L, Newton P, Parent S. Seeing the spine in 3D: how will it change what we do? J Pediatr Orthop. 2011;31:S37–S45. doi: 10.1097/BPO.0b013e3181fd8801. [DOI] [PubMed] [Google Scholar]
- 17.Mao SH, Qiu Y, Zhu ZZ, Zhu F, Liu Z, Wang B. Clinical evaluation of the anterior chest wall deformity in thoracic adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2012;37:E540–E548. doi: 10.1097/BRS.0b013e31823a05e6. [DOI] [PubMed] [Google Scholar]
- 18.Trawicki M, Liu XC, Tassone C, Thometz J, Lyon R. Improvements in three-dimensional back contour after spinal fusion for idiopathic scoliosis. Stud Health Technol Inform. 2010;158:19–23. [PubMed] [Google Scholar]
- 19.Kim YJ, Lenke LG, Bridwell KH, Cheh G, Whorton J, Sides B. Prospective pulmonary function comparison following posterior segmental spinal instrumentation and fusion of adolescent idiopathic scoliosis: is there a relationship between major thoracic curve correction and pulmonary function test improvement? Spine (Phila Pa 1976) 2007;32:2685–2693. doi: 10.1097/BRS.0b013e31815a7b17. [DOI] [PubMed] [Google Scholar]
- 20.Newton PO, Faro FD, Gollogly S, Betz RR, Lenke LG, Lowe TG. Results of preoperative pulmonary function testing of adolescents with idiopathic scoliosis. A study of six hundred and thirty-one patients. J Bone Joint Surg Am. 2005;87:1937–1946. doi: 10.2106/JBJS.D.02209. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein SL. Natural history. Spine (Phila Pa 1976) 1999;24:2592–2600. doi: 10.1097/00007632-199912150-00006. [DOI] [PubMed] [Google Scholar]