Abstract
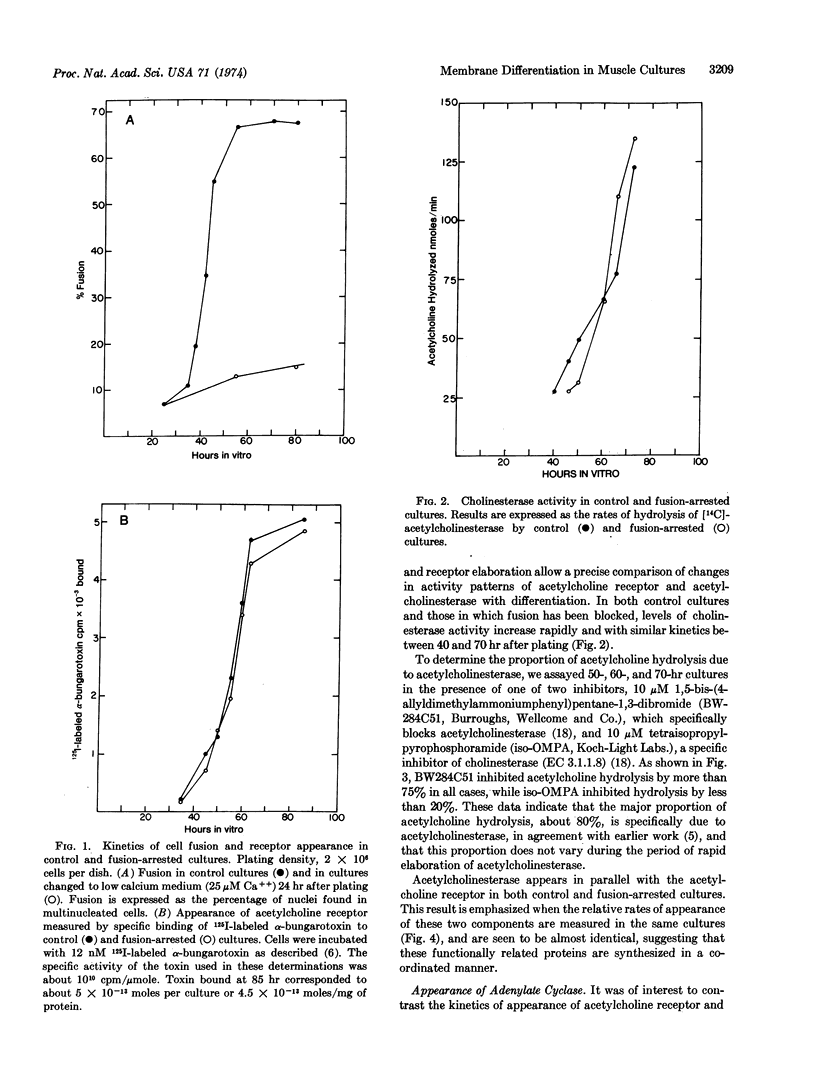
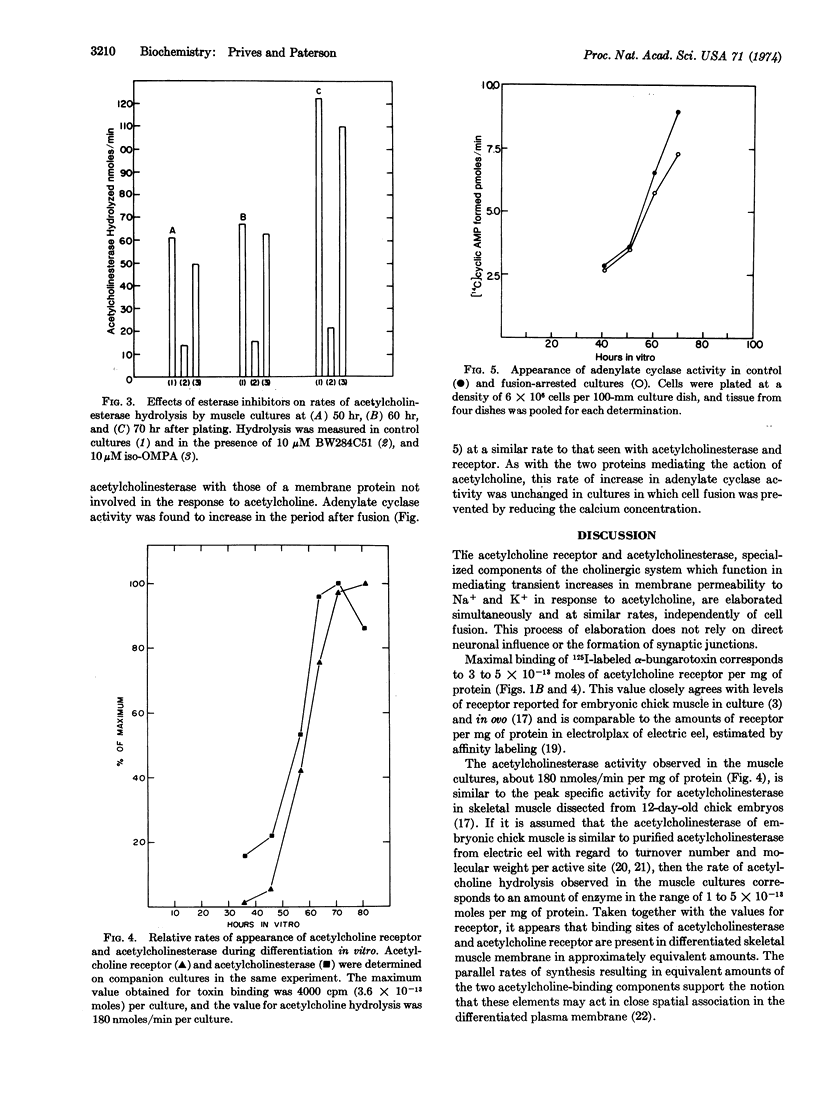
Three components of differentiated muscle membrane, the acetylcholine receptor, acetylcholinesterase (EC 3.1.1.7; acetylcholine hydrolase), and adenylate cyclase [EC 4.6.1.1; ATP pyrophosphate-lyase (cyclizing)], appear simultaneously during myogenesis in cultures of embryonic chick muscle, after the main period of rapid cell fusion. However, unlike the cytoplasmic proteins of differentiated muscle, the elaboration of these membrane components is unaltered when fusion is blocked by lowering the calcium concentration in the medium. These results suggest that membrane differentiation and cytoplasmic differentiation are regulated independently during muscle development.
Keywords: acetylcholine receptor, acetylcholinesterase, adenylate cyclase, cell fusion
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTIN L., BERRY W. K. Two selective inhibitors of cholinesterase. Biochem J. 1953 Jul;54(4):695–700. [PMC free article] [PubMed] [Google Scholar]
- Blume A., Gilbert F., Wilson S., Farber J., Rosenberg R., Nirenberg M. Regulation of acetylcholinesterase in neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Oct;67(2):786–792. doi: 10.1073/pnas.67.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y., Silman I., Kalderon N., Blumberg S. Purification by affinity chromatography of acetylcholinesterase from electric organ tissue of the electric eel subsequent to tryptic treatment. Biochim Biophys Acta. 1972 Apr 7;268(1):138–157. doi: 10.1016/0005-2744(72)90208-2. [DOI] [PubMed] [Google Scholar]
- Fambrough D., Rash J. E. Development of acetylcholine sensitivity during myogenesis. Dev Biol. 1971 Sep;26(1):55–68. doi: 10.1016/0012-1606(71)90107-2. [DOI] [PubMed] [Google Scholar]
- Fluck R. A., Strohman R. C. Acetylcholinesterase activity in developing skeletal muscle cells. Dev Biol. 1973 Aug;33(2):417–428. doi: 10.1016/0012-1606(73)90147-4. [DOI] [PubMed] [Google Scholar]
- Giacobini G., Filogamo G., Weber M., Boquet P., Changeux J. P. Effects of a snake alpha-neurotoxin on the development of innervated skeletal muscles in chick embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1708–1712. doi: 10.1073/pnas.70.6.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Prives J., Deal W., Winnik M. Affinity labeling of the acetylcholine receptor in the electroplax. J Mol Biol. 1971 Oct 14;61(1):175–188. doi: 10.1016/0022-2836(71)90214-2. [DOI] [PubMed] [Google Scholar]
- Krug F., Parikh I., Illiano G., Cuatrecasas P. , -methylene-adenosine 5'-triphosphate. A competitive inhibitor of adenylate cyclase in fat and liver cell membranes. J Biol Chem. 1973 Feb 25;248(4):1203–1206. [PubMed] [Google Scholar]
- Levitzki A., Atlas D., Steer M. L. The binding characteristics and number of beta-adrenergic receptors on the turkey erythrocyte. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2773–2776. doi: 10.1073/pnas.71.7.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E., Nachmansohn D., Katchalsky A. An attempt at an integral interpretation of nerve excitability. Proc Natl Acad Sci U S A. 1973 Mar;70(3):727–731. doi: 10.1073/pnas.70.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh T. H., Johnson D. D. Effects of acetyl- -methylcholine on development of acetylcholinesterase and butyrylcholinesterase activities in cultured chick embryonic skeletal muscle. Exp Neurol. 1972 Nov;37(2):360–370. doi: 10.1016/0014-4886(72)90080-5. [DOI] [PubMed] [Google Scholar]
- Paterson B., Prives J. Appearance of acetylcholine receptor in differentiating cultures of embryonic chick breast muscle. J Cell Biol. 1973 Oct;59(1):241–245. doi: 10.1083/jcb.59.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin synthesis in cultures of differentiating chicken embryo skeletal muscle. Dev Biol. 1972 Oct;29(2):113–138. doi: 10.1016/0012-1606(72)90050-4. [DOI] [PubMed] [Google Scholar]
- Patrick J., Heinemann S. F., Lindstrom J., Schubert D., Steinbach J. H. Appearance of acetylcholine receptors during differentiation of a myogenic cell line. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2762–2766. doi: 10.1073/pnas.69.10.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberry T. L., Chang H. W., Chen Y. T. Purification of acetylcholinesterase by affinity chromatography and determination of active site stoichiometry. J Biol Chem. 1972 Mar 10;247(5):1555–1565. [PubMed] [Google Scholar]
- Severson D. L., Drummond G. I., Sulakhe P. V. Adenylate cyclase in skeletal muscle. Kinetic properties and hormonal stimulation. J Biol Chem. 1972 May 10;247(9):2949–2958. [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. A., Zenser T. V. Separation of cyclic 3',5'-nucleoside monophosphates from other nucleotides on aluminum oxide columns. Application to the assay of adenyl cyclase and guanyl cyclase. Anal Biochem. 1971 Jun;41(2):372–396. doi: 10.1016/0003-2697(71)90156-4. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Schrier B. K., Farber J. L., Thompson E. J., Rosenberg R. N., Blume A. J., Nirenberg M. W. Markers for gene expression in cultured cells from the nervous system. J Biol Chem. 1972 May 25;247(10):3159–3169. [PubMed] [Google Scholar]
- Wilson S. H., Schrier B. K., Farber J. L., Thompson E. J., Rosenberg R. N., Blume A. J., Nirenberg M. W. Markers for gene expression in cultured cells from the nervous system. J Biol Chem. 1972 May 25;247(10):3159–3169. [PubMed] [Google Scholar]



