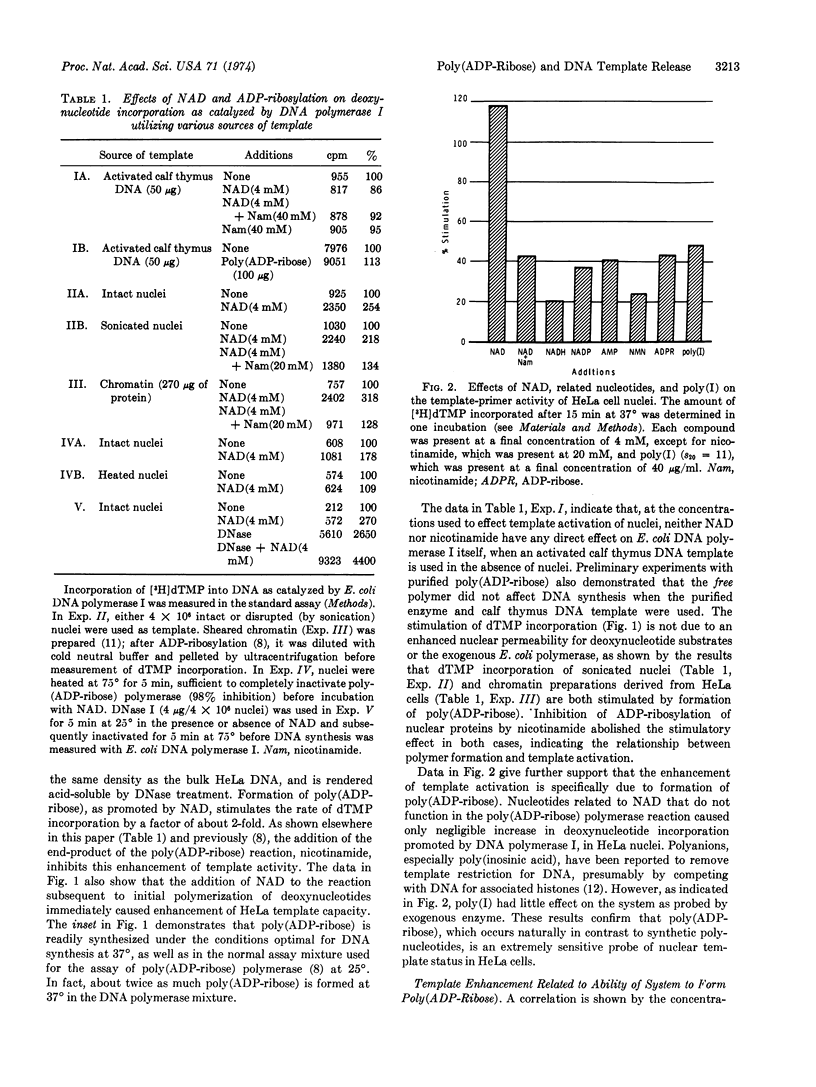
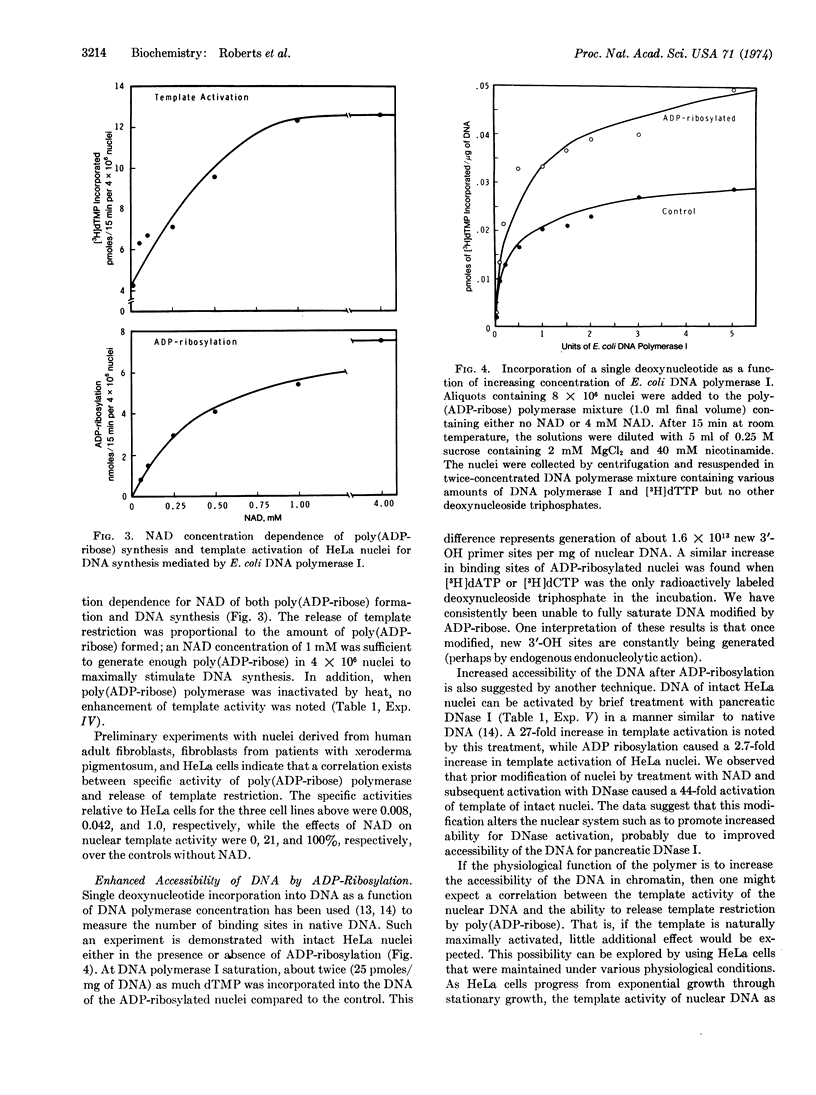
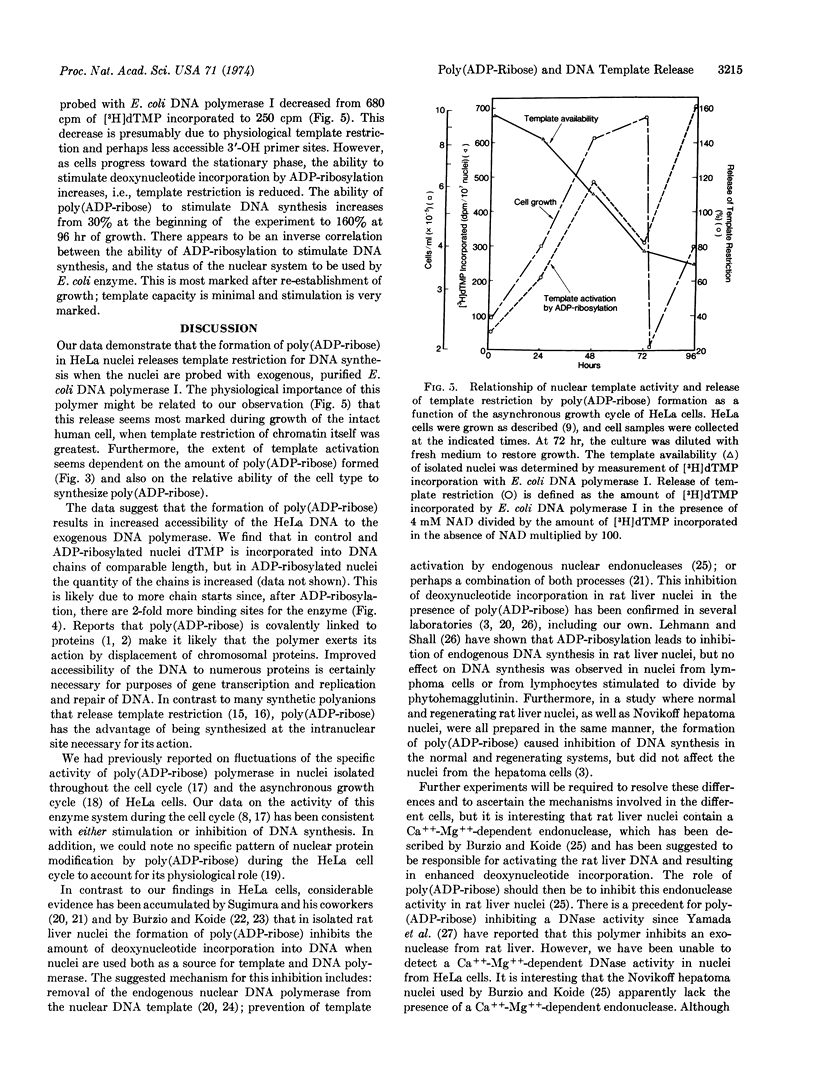
Abstract
Evidence is presented to show that ADP-ribosylation of nuclear proteins by poly(ADP-ribose) polymerase enhances template-primer activity of HeLa cell nuclear DNA. We used Escherichia coli DNA polymerase I (EC 2.7.7.7; DNA nucleotidyltransferase) as an exogenous probe of nuclear DNA status. Neither NAD nor free poly(ADP-ribose) acts directly on the bacterial enzyme. The stimulation is specific for formation of ADP-ribosylated proteins and is not a generalized polyanion or nucleotide effect on chromatin. The release of template restriction is proportional to the capacity of a given cell line to synthesize poly(ADP-ribose); both the stimulation and poly(ADP-ribose) formation are coordinately proportional to NAD concentration. Binding studies with DNA polymerase indicate exposure or generation of additional 3′-OH primer sites due to ADP-ribosylation in intact nuclei. With intact cells, there appears a correlation among growth, physiological template restriction, and the above effects of ADP-ribosylation.
Keywords: NAD, nuclear proteins, DNA polymerase, DNA primer sites
Full text
PDF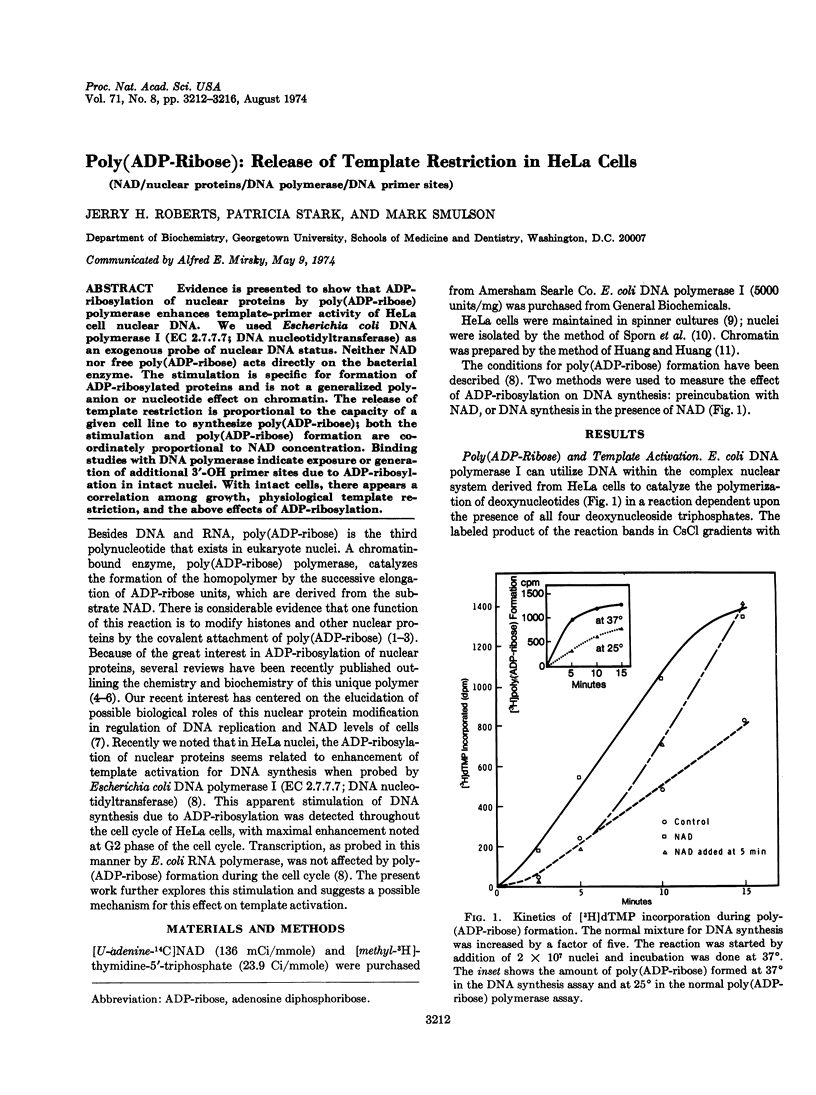

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Lehman I. R., Bessman M. J., Simms E. S., Kornberg A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. IV. LINKAGE OF SINGLE DEOXYNUCLEOTIDES TO THE DEOXYNUCLEOSIDE ENDS OF DEOXYRIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):641–647. doi: 10.1073/pnas.44.7.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. G., Coffey D. S. Effects of polyinosinic acid and polycytidylic acid on the deoxyribonucleic acid template activity of isolated nuclei and soluble chromatin from rat liver. J Biol Chem. 1972 Dec 10;247(23):7674–7683. [PubMed] [Google Scholar]
- Burzio L., Koide S. S. A functional role of polyADPR in DNA synthesis. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1013–1020. doi: 10.1016/0006-291x(70)90894-6. [DOI] [PubMed] [Google Scholar]
- Burzio L., Koide S. S. Activation of the template activity of isolated rat liver nuclei for DNA synthesis and its inhibition by NAD. Biochem Biophys Res Commun. 1973 Jul 17;53(2):572–579. doi: 10.1016/0006-291x(73)90700-6. [DOI] [PubMed] [Google Scholar]
- Burzio L., Koide S. S. In vitro effect of NAD on DNa synthesis in isolated nuclei from regenerating rat liver and novikoff hepatoma. FEBS Lett. 1972 Jan 15;20(1):29–32. doi: 10.1016/0014-5793(72)80009-7. [DOI] [PubMed] [Google Scholar]
- Burzio L., Koide S. S. Mode of inhibition of DNA synthesis induced by adenosine diphosphoribosylation of nuclear protein. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1185–1190. doi: 10.1016/0006-291x(71)90031-3. [DOI] [PubMed] [Google Scholar]
- Honjo T., Hayaishi O. Enzymatic ADP-ribosylation of proteins and regulation of cellular activity. Curr Top Cell Regul. 1973;7:87–127. doi: 10.1016/b978-0-12-152807-2.50011-5. [DOI] [PubMed] [Google Scholar]
- Huang R. C., Huang P. C. Effect of protein-bound RNA associated with chick embryo chromatin on template specificity of the chromatin. J Mol Biol. 1969 Jan;39(2):365–378. doi: 10.1016/0022-2836(69)90323-4. [DOI] [PubMed] [Google Scholar]
- Kraemer R. J., Coffey D. S. The interaction of natural and synthetic polyanions with mammalian nucleo. I. DNA synthesis. Biochim Biophys Acta. 1970 Dec 14;224(2):553–567. [PubMed] [Google Scholar]
- Lehmann A. R., Shall S. No inhibition of endogenous DNA polymerase by synthesis of poly (ADP-ribose) in nuclei from lymphoid cells. FEBS Lett. 1972 Oct 1;26(1):181–184. doi: 10.1016/0014-5793(72)80568-4. [DOI] [PubMed] [Google Scholar]
- NEMETH A. M., DICKERMAN H. Pyridine nucleotides and diphosphopyridine nucleotidase in developing mammalian tissues. J Biol Chem. 1960 Jun;235:1761–1764. [PubMed] [Google Scholar]
- Nagao M., Yamada M., Miwa M., Sugimura T. Depression of DNA polymerase activity in chromatin by incubation with NAD. Biochem Biophys Res Commun. 1972 Jul 11;48(1):219–225. doi: 10.1016/0006-291x(72)90366-x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Honjo T., Hayaishi O. Enzymic adenosine diphosphate ribosylation of histone and poly adenosine diphosphate ribose synthesis in rat liver nuclei. J Biol Chem. 1968 Jul 10;243(13):3765–3767. [PubMed] [Google Scholar]
- Otake H., Miwa M., Fujimura S., Sugimura T. Binding of ADP-ribose polymer with histone. J Biochem. 1969 Jan;65(1):145–146. [PubMed] [Google Scholar]
- Roberts J. H., Stark P., Smulson M. Stimulation of DNA synthesis by adenosine diphosphoribosylation of HeLa nuclear proteins during the cell cycle. Biochem Biophys Res Commun. 1973 May 1;52(1):43–50. doi: 10.1016/0006-291x(73)90951-0. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Wang T. S., Korn D. Purification and properties of nuclear and cytoplasmic deoxyribonucleic acid polymerases from human KB cells. J Biol Chem. 1972 Aug 25;247(16):5026–5033. [PubMed] [Google Scholar]
- Smulson M. E., Thomas J. Ribonucleic acid biosynthesis of human cells during amino acid deprivation. J Biol Chem. 1969 Oct 10;244(19):5309–5312. [PubMed] [Google Scholar]
- Smulson M., Henriksen O., Rideau C. Activity of polyadenosine diphosphoribose polymerase during the human cell cycle. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1266–1273. doi: 10.1016/s0006-291x(71)80009-8. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Berkowitz D. M., Glinski R. P., Ash A., Stevens C. L. Irreversible inhibition of nuclear exoribonuclease by thymidine-3'-fluorophosphate and p-haloacetamidophenyl nucleotides. Science. 1969 Jun 20;164(3886):1408–1410. doi: 10.1126/science.164.3886.1408. [DOI] [PubMed] [Google Scholar]
- Yamada M., Nagao M., Hidaka T., Sugimura T. Effect of poly(ADP-ribose) formation on DNA synthesis and DNA fragmentation in nuclei of rat liver and rat ascites hepatoma AH-130 cells. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1567–1572. doi: 10.1016/0006-291x(73)91165-0. [DOI] [PubMed] [Google Scholar]
- Yamada M., Nagao M., Miwa M., Sugimura T. Inhibition of deoxyribonuclease in an extract of rat liver nuclei by poly(ADP-ribose). Biochem Biophys Res Commun. 1974 Feb 27;56(4):1093–1099. doi: 10.1016/s0006-291x(74)80300-1. [DOI] [PubMed] [Google Scholar]
- Yoshihara K., Koide S. S. Release of DNA polymerase from rat liver chromatin on incubation with NAD. FEBS Lett. 1973 Sep 15;35(2):262–264. doi: 10.1016/0014-5793(73)80300-x. [DOI] [PubMed] [Google Scholar]



