Abstract
Cytotoxic and antimicrobial effects of Montivipera xanthina venom against LNCaP, MCF-7, HT-29, Saos-2, Hep3B, Vero cells and antimicrobial activity against selected bacterial and fungal species: Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, E. coli O157H7, Enterococcus faecalis 29212, Enterococcus faecium DSM 13590, Staphylococcus epidermidis ATCC 12228, S. typhimirium CCM 5445, Proteus vulgaris ATCC 6957 and Candida albicans ATCC 10239 were studied for evaluating the potential medical benefit of this snake venom. Cytotoxicity of venom was determined using MTT assay. Snake venom cytotoxicity was expressed as the venom dose that killed 50 % of the cells (IC50). The antimicrobial activity of venom was studied by minimal inhibitory concentration (MIC) and disc diffusion assay. MIC was determined using broth dilution method. The estimated IC50 values of venom varied from 3.8 to 12.7 or from 1.9 to 7.2 μg/ml after treatment with crude venom for 24 or 48 h for LNCaP, MCF-7, HT-29 and Saos-2 cells. There was no observable cytotoxic effect on Hep3B and Vero cells. Venom exhibited the most potent activity against C. albicans (MIC, 7.8 μg/ml and minimal fungicidal concentration, 62.5 μg/ml) and S. aureus (MIC, 31.25 μg/ml). This study is the first report showing the potential of M. xanthina venom as an alternative therapeutic approach due to its cytotoxic and antimicrobial effects.
Keywords: Snake venom, Cytotoxicity, Antimicrobial activity, Viper, Montivipera xanthina
Introduction
Snake venom is a natural biological resource that contains several active components with potential therapeutic value for the treatment of a variety of pathophysiological conditions, being used in folk medicine for centuries. (Davis et al. 1974; Francis and Markland 2001; Jamunaa et al. 2012; Koh et al. 2006; Macht 1940; Swenson et al. 2004; Zhou et al. 1999, 2000). Snake venom consists of several neurotoxin, cardiotoxin, cytotoxin, nerve growth factor, lectins, disintrigrins, haemorrhagins and many other different enzymes. These proteins not only cause death to animals and humans, but can also be used for the treatment of thrombosis, arthritis, cancer and many other diseases (Pal et al. 2002). Particularly non-toxic dose of snake venom has been shown to reduce the solid tumour size and to block the angiogenesis (Jamunaa et al. 2012; Koh et al. 2006; Macht 1940; Swenson et al. 2004; Zhou et al. 1999, 2000).
On the other hand, the majority of microorganisms have developed several ways to resist antibiotics. Pathogen microorganisms are becoming a serious clinical problem throughout the world (Pal et al. 2002; Ahmadi et al. 2010; Bastos et al. 2009; Mishra et al. 2007). Therefore, the discovery of new effective antibacterial agents or developing antibacterials with a new mechanism is continuously necessary. Natural products are an important source of medicinal compounds. A wide variety of organisms produce such bioactive compounds and some of these natural substances have been shown to be able to kill bacteria (Ahmadi et al. 2010; Bustillo et al. 2008; Samy et al. 2006). In recent years, venoms and venom components from different venomous animals have shown potential antibacterial activity. Therefore, these compounds may interact with specific molecules of some microorganisms while not affecting other strains. This includes snake and scorpion venoms (Bustillo et al. 2008; Samy et al. 2006).
The Ottoman Viper, Montivipera xanthina (Gray, 1849), an almost endemic species to Turkey, is known from the Central, Southern and Western Anatolia as well as some adjacent islands of Greece with vertical distribution to 2,000 m (Arikan et al. 2005; Samy et al. 2007; Göçmen et al. 2006).
In continuation to our previous studies with Turkey endemic species M. xanthina venom, the main purpose of this work was to investigate cytotoxic and antimicrobial effects of Ottoman viper venom on various cancer cells and on microbial cells to screen its potential use in medicine as a therapeutic agent.
Materials and methods
Snake venom
All tests were performed with pooled venom from M. xanthina which is a venomous snake almost endemic to Turkey. Venom was obtained from Dr. B. Gocmen. Venom was extracted without applying any pressure on the venom glands, as described by Tare et al. (1986). Due to the fact that the venom extracts contained some dead cells, pooled venom was diluted in physiologic saline, centrifuged for 5 min at 600×g, filtered through a 0.22 μm cellulose acetate syringe filter and stored at −20 °C.
Protein content determination
Protein content was assayed triplicate for each diluted venom sample in saline, using bovine serum albumin as a standard by Bradford method (Bradford 1976) at 595 nm (Versamax, Molecular Devices, Sunnyvale, CA, USA).
Cell culture and in vitro cytotoxicity assay
Human colon adenocarcinoma (HT-29), human breast adenocarcinoma (MCF-7), human hepatoma (Hep3B), human osteoblastic osteosarcoma (Saos-2) and Vero (kidney epithelial cells from an African green monkey)—used as a non-cancerous cell line—cells were purchased from the HUKUK (Animal Cell Culture Collections) in Foot-and-Mouth Disease Institute (Ankara) of Ministry of Agriculture & Rural Affairs of Turkey. The cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10 % fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml of penicillin and 100 μg/ml of streptomycin (Biochrom AG, Berlin, Germany). Human prostate adenocarcinoma (LNCap) cells were a gift from Dr. Kemal Korkmaz (Ege University, Faculty of Engineering, Bioengineering Department, Bornova-Izmir, Turkey), maintained in RPMI 1640 medium supplemented with 10 % heat-inactivated FBS, 2 mM l-glutamine, 100 U/ml of penicillin and 100 μg/ml of streptomycin (Biochrom AG). The cultures were incubated at 37 °C in a humidified atmosphere of 5 % CO2. FBS content was decreased to 4 % while cells were challenged with different concentrations of venom.
Cytotoxicity of venom was determined with the general procedure used for the screening of cytotoxic agents based on cell viability using a modified MTT [3-(4, 5- Dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide)] assay (Mosmann 1983), which detects the activity of mitochondrial reductase of viable cells. The assay is based on cleavage of MTT that forms formazan crystals. This cleavage appears in living cells with succinate-dehydrogenase (a mitochondrial enzyme succinate). Adding dimethyl sulfoxide to wells helps formazan crystals to be resolved. The optical density is measured at 570 nm (reference filter, 690 nm) with U. V. visible spectrophotometer (VersaMax, Molecular Devices).
All cell lines were cultivated for 24 h in 96 well microplates with an initial concentration of 8 × 104 cells/ml. Then, the cultured cells were treated with different venom concentrations and incubated for 24 h and 48 h at 37 °C. Percentages of surviving cells in every culture were determined after venom treatment. The values of the blank wells were subtracted from each well of treated and control cells, inhibition of growth % 50 (IC50) found by comparing with untreated controls. The % viability was determined as formulated below by:
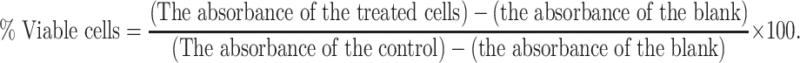 |
Determination of IC50
Cytotoxicity was expressed as mean percentage increase relative to the unexposed control ± SD. Control values were set at 0 % cytotoxicity. Cytotoxicity data (where appropriate) were fitted to a sigmoidal curve and a four parameters logistic model was used to calculate the IC50, which is the concentration of material causing 50 % inhibition in comparison to the untreated controls. The mean IC50 is the concentration of material that reduces cell growth by 50 % under the experimental conditions and is the average from at least three independent measurements that were reproducible and statistically significant. The IC50 values were reported at ±95 % confidence intervals (±95 % CI). This analysis was performed with Graph Pad Prism (San Diego, CA, USA).
Microorganisms
Gram-positive and gram-negative bacteria and yeast were used for antimicrobial activity studies. The Gram-negative bacteria used were Escherichia coli ATCC 25922, Escherichiacoli 0157H7, Proteus vulgaris ATCC 6957 and Salmonella thyphimurium CCM 5445. The Gram positive bacteria used were Bacillus cereus ATCC 7064, Enterococcus faecalis ATCC 29212, Enterococcus faecium DSM 13590, Staphylococcus aureus ATCC 25923, and Staphylococcus epidermidis ATCC 12228 and Candida albicans ATCC 10239 was used as yeast. The lyophilized bacteria and yeast were obtained from Ege University, Faculty of Science, Department of Basic and Industrial Microbiology.
Antimicrobial assay
Antimicrobial susceptibility testing was performed as described by Bauer et al. (1966), and NCCLS (2009), with some modifications. For the agar diffusion assay, test microorganisms were grown on Mueller–Hinton agar plates (Merck Darmstadt, Germany), and suspended in 5 ml of sterile MH broth (Merck Darmstadt, Germany). Turbidity was measured at 600 nm and adjusted to an absorbance of 0.5, corresponding to 1.5 × 106 colony forming units (cfu)/ml. Microorganisms were spread by using a sterile cotton swab onto 20 ml of sterile MH agar plates (90-mm diameter). The surface of the medium was allowed to dry for about 3 min. Sterile paper discs (6-mm diameter) were then placed onto the MH agar surface and 20 μl venom sample (1,000 μg/ml) were added per disc. A blank with sterile saline solution instead of venom served as negative control. Ampicillin (Sigma Aldrich St. Louis, MO, USA) and nystatin (NS20) were used as a positive control or reference, respectively. Diameters of the bacterial growth inhibition zones were measured. Each assay was performed in triplicate.
Minimum inhibitory concentration (MIC) by microdilution susceptibility test
Test microorganisms were grown in MH broth for 5 h (exponential phase) and adjusted to 0.5 McFarland turbidity standard (A600 = 1.0), corresponding to 1.5 × 106 cfu/ml. MICs were determined according to the National Committee for Clinical Laboratory Standards (2000). Serial dilutions of venom (0.9–500 μg/ml) were prepared in 96-well microtiter trays, at a final volume of 80 μl. Then, 20 μl of the adjusted bacterial inocula (1.5 × 105 cfu/ml) were added to each well and incubated at 37 °C for 24 h. Inhibition of microorganisms growth was determined by visual observation. The MIC was defined as the lowest concentration of venom required to inhibit microbial growth. Each dilution series included control wells, which consisted of 80 μl of it and 80 μl of Mueller–Hinton broth. Ampicillin and flucytosine were used as standard drugs for comparison. All assays were run using 3 replicates. In addition, minimal bactericidal concentration (MBC) and minimal fungicidal concentration (MFC) were defined as the lowest concentration of venom required to kill the bacteria and yeast, as determined by subculturing 30 μl from the broth MIC tests to agar media.
Data analysis
Values were presented as mean ± standard error of the mean (SEM). IC50 calculation and variance analysis (standard deviation calculation) were performed with Graph Pad Prism.
Results
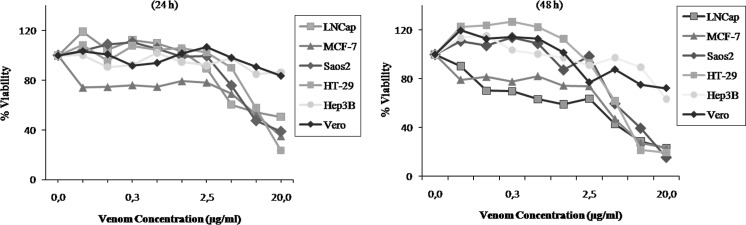
The diluted (1:2,000) crude venom protein concentration was estimated as 8.28 mg/ml for M. xanthina. The cytotoxic effect and IC50 value of venom on cell lines were investigated by using different concentrations of the venom. The MTT assay results showed that venom inhibits cell proliferation in a dose-dependent manner and enhances cell proliferation at lower concentrations (Fig. 1).
Fig. 1.
Cytotoxic effect of M. xanthina crude venom on cancer and normal cells after 24 and 48-h exposure to different venom concentrations. Cell viability was determined by MTT assay, control was exposed to vehicle only which was taken as 100 % viability. Data are expressed as mean ± SD
The venom IC50 values for cell lines that are affected by different concentrations of venom are given in Table 1 for 24 and 48 h. Untreated cancer cells were equivalently distributed in the culture plates showing a polygonal shape with distinct boundaries. The morphological changes were observed throughout the post-treatment with venom both for 24 and 48 h exposure. Increasing venom concentrations resulted in increased number of rounded cells, growth inhibition and the incidence of various morphological abnormalities with larger areas devoid of cells when compared with the untreated control cells, (Figs. 2 and 3).
Table 1.
Montivipera xanthina Venom IC50 values for cell lines following crude venom exposure
| Cell lines | IC50 (24 h) μg/ml |
IC50 (48 h) μg/ml |
|---|---|---|
| HT-29 | 12.7 | 6.3 |
| Saos-2 | 10 | 7.2 |
| MCF-7 | 4.2 | 4.1 |
| LNCap | 3.8 | 1.9 |
| Hep3B | – | – |
| Vero | – | – |
– Not detected
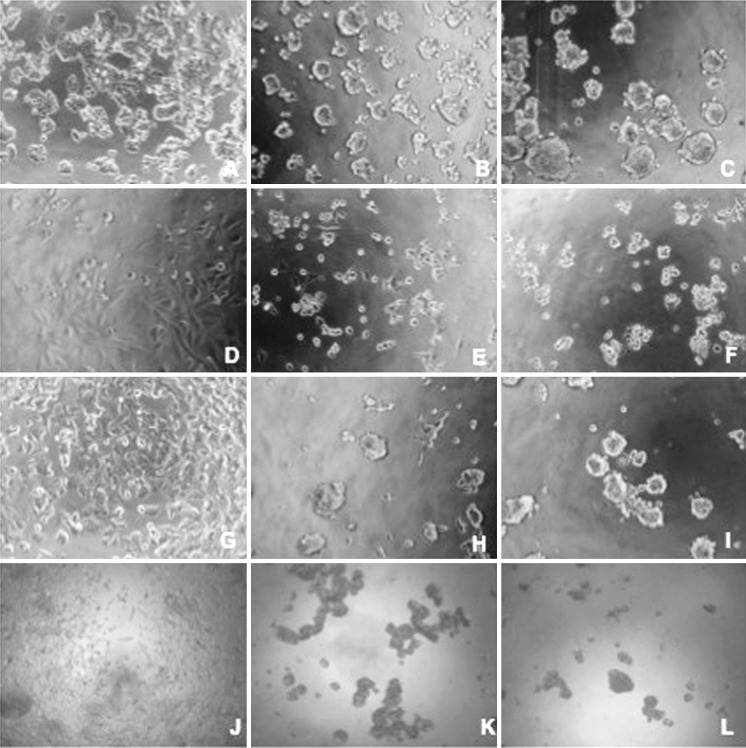
Fig. 2.
Dose-dependent venom induced morphologic changes of LNCaP, MCF-7, HT-29 and Saos-2 cells viewed by inverted microscope. Cells were treated with different concentrations of crude venom (0–20 μg/ml) for 48 h at 37 °C. a HT-29, untreated, b HT-29, 10 μg/ml, c HT-29, 20 μg/ml, d Saos2, untreated, e Saos-2, 10 μg/ml, f Saos-2, 20 μg/ml, g MCF-7, untreated, h MCF-7,10 μg/ml, i MCF-7, 20 μg/ml, j LNCap, untreated, k LNCap, 10 μg/ml, l LNCap, 20 μg/ml
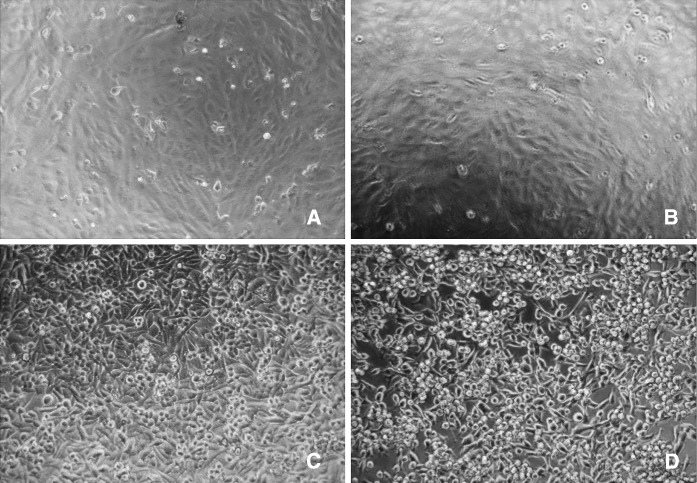
Fig. 3.
Effect of crude venom on Hep3B and Vero cells observed by inverted microscope. Cells were treated with different concentrations of crude venom (0–20 μg/ml) for 48 h at 37 °C. a Vero, untreated, b Vero, 20 μg/ml, c Hep3B, untreated, d Hep3B, 20 μg/ml
Antimicrobial activity was evaluated by disc diffusion assay in MH agar for Gram-positive and Gram-negative bacteria and yeast. M. xanthina venom was able to inhibit the growth of C.Albicans (ATCC 10239) and S. aureus (ATCC 25923) with 10 and 8 mm inhibition zone, respectively, but not that of other microorganisms. MIC was determined using the broth dilution method (0.9–500 μg/mL). Venom exhibited the most potent activity against C. albicans (MIC 7.8 μg/ml and MFC 62.5 μg/ml) and S. aureus (MIC 31.25 μg/ml). The positive control (flucytosine and ampicillin) had a MIC of 7.8 and 3.9 μg/ml, respectively (Table 2).
Table 2.
M. xanthina venom MIC values for microorganisms following crude venom exposure
| Microorganisms | MIC (μg/ml) values for venom and antimicrobial agents | ||
|---|---|---|---|
| Montivipera xanthina crude venom | Ampicillin | Flucytosine | |
| E. coli ATCC 25922 | – | 1.9 | – |
| E. coli O157:H7 | – | 3.9 | – |
| P. vulgaris ATCC 6957 | – | 3.9 | – |
| S. thyphimurium CCM 5445 | – | 3.9 | – |
| S. aureus ATCC 25923 | 31.25 | 3.9 | – |
| B. cereus ATCC 7064 | – | 7.8 | – |
| E. faecalis ATCC 29212 | – | 7.8 | – |
| E. faecium DSM 13590 | – | 3.9 | – |
| S. epidermidis ATCC 12228 | – | 1.9 | – |
| C. albicans ATCC 10239 | 7.8 | – | 7.8 |
– Not detected
Discussion
Snake venoms contain many biologically active proteinaceous components such as neurotoxins (pre- and postsynaptic), cardiotoxins, myotoxins, cytotoxins, proteases, nucleases, L-amino acid oxidase and phospholipase (Davis et al. 1974; Nair et al. 2007; Chellapandi and Jebakumar 2008; Kaufmann and Vaux 2003). They can be useful and valuable as pharmacological tools in drug research, potential drug design templates and therapeutic agents (Davis et al. 1974; Ahmadi et al. 2010). In this study, cytotoxic and antimicrobial properties of M. xanthina venom on various cancer and microbial cells were investigated in order to evaluate its potential for the development of therapeutic agents.
Cancer is one of the most important causes of death worldwide in spite of considerable progress in its treatment. Today’s conventional cancer therapies such as radio and chemotherapies, achieve their therapeutic effect indirectly by promoting apoptosis through the intrinsic pathway as they induce cellular DNA damage, however they cannot differentiate between malignant and normal cell types (Wilson et al. 2009; Braganca and Hospattankar 1978). Attributable to difference in biochemical levels such as several important enzymatic and signal transduction cascade participate in metabolic pathway, altered gene expression, programmed cell death and plasma membtane changes between normal and cancerous cells, treatment strategy have attracted much attention on specific therapy selection of effective drugs and its concentration. This helps to destroy tumor cells for longer period and to avoid deterioration of normal cells. A substantial progression has been made to treat cancer patients with cytotoxic drugs irrespective of the disease progression stages. Snake venom is a natural source of molecules displaying cytotoxic, anti-tumor and apoptosis-inducing agents acting on different cancer cell lines, therefore it is the subject of molecular studies in the discovery of new therapeutic drugs (Zhou et al. 2000; Samel et al. 2012; Shebl et al. 2012; Nalbantsoy et al. 2012).
In the present study, cancer cell proliferation after M. xanthina snake venom treatment was analyzed by the MTT assay. The venom displayed cytotoxic activity on LNCaP, MCF-7, HT-29 and Saos-2 cells after 24 and 48 h treatment, in a time- and dose-dependent manner. No significant effects were observed on the proliferation of Hep3B cancer and normal Vero cells after venom treatment. IC50 values of venom showed variation depending on the cancer cell line and duration of treatment. Accordingly, notable cytotoxicity to MCF-7 and LNCaP cells were observed, where the venom IC50 values were higher than in HT-29 and Saos-2 cells; regarding time, venom IC50 values were lower for 48 h of treatment compared to 24 h for all affected cells. Consistent with the previous studies, cytotoxic effects of M. xanthina venom on cancer cells were concentration and time dependent (Jamunaa et al. 2012; Koh et al. 2006; Nalbantsoy et al. 2012; Shebl et al. 2012; Swenson et al. 2004; Zare et al. 2008; Zhou et al. 2000). On the other hand, venom did not show any cytotoxicity against Vero cells even at the highest concentration. This difference is significant for the selective effect of venom between cancer cells and normal cells.
Montivipera xanthina venom has shown species-specific antimicrobial activity. The venom exhibited most potent activity against Gram positive strains of S. aureus and a yeast strain of C. albicans, whereas it did not show any activity against other tested Gram-positive and Gram-negative bacteria. This selective antibacterial activity may be due to several factors, such as differences between species, including charge density and structure of lipopolysaccharides in the case of Gram-negative bacteria, or lipid composition of the cytoplasmic membrane and the electrostatic potential across membrane in Gram-positive bacteria. Moreover, peptide transport and efflux mechanisms may also lead to species-selectivity of such antibacterial proteins (San et al. 2010).
In conclusion, further studies should focus on the purification of the active components of this venom and to investigate the possible mode of action of venom-induced cytotoxicity and antimicrobial activity to obtain a better understanding of their potential use as cytotoxic and antimicrobial agents. Additionally proteomic studies are required for active peptides/proteins uses as a prototype for potential development of new anticancer drugs.
Contributor Information
Husniye Tansel Yalcın, Phone: +90-232-3112436, FAX: +90-232-3881036, Email: tansel.yalcin@ege.edu.tr.
Ayse Nalbantsoy, Phone: +90-232-3884955, FAX: +90-232-3884955, Email: analbantsoy@gmail.com.
References
- Ahmadi AJ, Fathi B, Jamshidi A, Zolfagharian H, Mirakabbadi AZ. Investigation of the antibacterial effect of venom of the Iranian Snake Echis carinatus. Iran J Vet Sci Technol. 2010;2:93–100. [Google Scholar]
- Arikan H, Göçmen B, Mermer A, Bahar H. An electrophoretic comparison of the venoms of a colubrid and various viperid snakes from Turkey and Cyprus, with some taxonomic and phylogenetic implications. Zootaxa. 2005;1038:1–10. [Google Scholar]
- Bastos MLA, Lima MRF, Conserva LM, Andrade VS, Rocha EMM, Lemos RPL. Studies on the antimicrobial activity and brine shrimp toxicity of Zeyheria tuberculosa (Vell.) Bur. (Bignoniaceae) extracts and their main constituents. Ann Clin Microbiol Antimicrob. 2009;8:1–6. doi: 10.1186/1476-0711-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braganca BM, Hospattankar AV. Potentiating Action of Cobra venom cytotoxin on the antitumour effects of alkylating agents (Melphalan) Eur J Cancer. 1978;14:707–712. doi: 10.1016/0014-2964(78)90307-9. [DOI] [PubMed] [Google Scholar]
- Bustillo L, Leiva LC, Merino L, Acosta O, Joffé EBdK, Gorodner OJ (2008) Antimicrobial activity of Bothrops alternatus venom from the Northeast of Argentine. Rev Latinoam Microbiol 50:79–82
- Chellapandi P, Jebakumar SRD. Purification and antibacterial activity of Indian Cobra and Viper Venoms. eJBio. 2008;4:11–16. [Google Scholar]
- Davis HL, Ramirez G, Ellerby RA, Ansfield FJ. Five-drug therapy in advanced breast cancer. Factors Influencing Toxicity and Response. Cancer. 1974;34:239–245. doi: 10.1002/1097-0142(197408)34:2<239::AID-CNCR2820340202>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Francis S, Markland A. Novel Snake venom disintegrin that inhibits human ovarian cancer dissemination and angiogenesis in an orthotopic nude mouse model. Haemostasis. 2001;31:183–191. doi: 10.1159/000048062. [DOI] [PubMed] [Google Scholar]
- Göçmen B, Arıkan H, Mermer A, Langerwerf B, Bahar H. Morphological, hemipenial and venom electrophoresis comparisons of Levantine Viper, Macrovipera lebetina (Linnaeus, 1758) from Cyprus and Southern Anatolia. Turk J Zool. 2006;30:225–234. [Google Scholar]
- Jamunaa A, Vejayan J, Halijah I, Sharifah SH, Ambu S. Cytotoxicity of Southeast Asian snake venoms. J Venom Anim Toxins incl Trop Dis. 2012;18:150–156. doi: 10.1590/S1678-91992012000200004. [DOI] [Google Scholar]
- Kaufmann SH, Vaux DL. Alterations in the apoptotic machinery and their potential role in anticancer drug resistance. Oncogene. 2003;22:7414–7430. doi: 10.1038/sj.onc.1206945. [DOI] [PubMed] [Google Scholar]
- Koh DC, Armugam A, Jeyaseelan K. Snake venom components and their applications in biomedicine. Cell Mol Life Sci. 2006;63:3030–3041. doi: 10.1007/s00018-006-6315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macht DI (1940) New developments in pharmacology and therapeutics of cobra venom. Trans Am Ther Soc 40:62
- Mishra NN, Prasad T, Sharma N, Paysi A, Prasad R, Gupta DK, Singh R. Pathogenicity and drug resistance in Candida albicans and other yeast species. A review. Acta Microbiol Immunol Hung. 2007;54:201–235. doi: 10.1556/AMicr.54.2007.3.1. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nair DG, Fry BG, Alewood P, Prakash P, Kumar PP, Kini RM. Antimicrobial activity of omwaprin, a new member of the waprin family of snake venom proteins. Biochem. J. 2007;402:93–104. doi: 10.1042/BJ20060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbantsoy A, Karabay-Yavasoglu NU, Sayım F, Deliloglu-Gurhan I, Gocmen B, Arıkan H, Yildiz MZ. Determination of in vivo toxicity and in vitro cytotoxicity of venom from the Cypriot blunt-nosed viper Macrovipera lebetina lebetina and antivenom production. J Venom Anim Toxins incl Trop Dis. 2012;18:208–216. doi: 10.1590/S1678-91992012000200011. [DOI] [Google Scholar]
- NCCLS (2009) Performance standards for antimicrobial susceptibility testing, nineteenth informational supplement. Approved Standard M100-S19 National Committee for Clinical Standards. Wayne PA January
- Pal SK, Gomes A, Dasgupta SC, Gomes A. Snake venom as therapeutic agents: from toxin to drug development. Indian J Exp Biol. 2002;40:1353–1358. [PubMed] [Google Scholar]
- Samel M, Trummal K, Siigur E, Siigur J. Effect of HUVEC apoptosis inducing proteinase from Vipera lebetina venom (VLAIP) on viability of cancer cells and on platelet aggregation. Toxicon. 2012;60:648–655. doi: 10.1016/j.toxicon.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Samy RP, Pachiappan A, Gopalakrishnakone P, Thwin MM, Hian YE, Chow VTK, Bow H, Weng JT. In vitro antimicrobial activity of natural toxins and animal venoms tested against Burkholderia pseudomallei. BMC Infect Dis. 2006;6:1–16. doi: 10.1186/1471-2334-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samy RP, Gopalakrishnakone P, Thwin MM, Chow TKV, Bow H, Yap EH, Thong TWJ. Antibacterial activity of snake, scorpion and bee venoms: a comparison with purified venom phospholipase A2 enzymes. J Appl Microbiol. 2007;102:650–659. doi: 10.1111/j.1365-2672.2006.03161.x. [DOI] [PubMed] [Google Scholar]
- San TM, Vejayan J, Shanmugan K, Ibrahim H. Screening antimicrobial activity of venoms from snakes commonly found in Malasia. J Appl Sci. 2010;10:2328–2332. doi: 10.3923/jas.2010.2328.2332. [DOI] [Google Scholar]
- Shebl RI, Mohamed AF, Ali AE, Amin MA. Cerastes cerastes and Vipera lebetina Snake Venoms apoptotic—stimulating activity to human breast cancer cells and related gene modulation. J Cancer Sci Ther. 2012;4:317–323. [Google Scholar]
- Swenson S, Costa F, Minea R, Sherwin RP, Ernst W, Fujii G, Yang D, Markland FS Jr (2004) Intravenous liposomal delivery of the snake venom disintegrin contortrostatin limits breast cancer progression. Mol Cancer Ther 3:499–511 [PubMed]
- Tare TG, Sutar NK, Renapurkar DM. A study of snake venom yield by different methods of venom extraction. Amphib Reptile. 1986;7:187–191. doi: 10.1163/156853886X00406. [DOI] [Google Scholar]
- Wilson TR, Johnston PG, Longley DB. Anti-apoptotic mechanisms of drug resistance in cancer. Curr Cancer Drug Targets. 2009;9:307–319. doi: 10.2174/156800909788166547. [DOI] [PubMed] [Google Scholar]
- Zare MA, Mahdavi S, Koohi MK, Taghavian M. Cytotoxic effect of ICD-85 (venom- derived peptides) on MDA-MB-231 cell line. J Venom Anim Toxins incl Trop Dis. 2008;14:180–186. [Google Scholar]
- Zhou Q, Nakada MT, Arnold C, Markland FS. Contortrostatin, a dimeric disintegrin from Agkistrodon contortrix contortrix, inbibits angiogenesis. Angiogenesis. 1999;3:259–269. doi: 10.1023/A:1009059210733. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wulfkuhle J, Ouatas T, Fukushima P, Stetler-Stevenson M, Miller FR, Steeg PS (2000) Contortrostatin, a dimeric disintegrin from agkistrodo contortrix, inbibit s breast cancer progression. Breast Cancer Res Treat 61:249–260