Abstract
Multi-differentiation capability is an essential characteristic of bone marrow mesenchymal stem cells (BMSCs). Method on obtaining higher-quality stem cells with an improved differentiation potential has gained significant attention for the treatment of clinical diseases and developmental biology. In our study, we investigated the multipotential differentiation capacity of BMSCs under simulated microgravity (SMG) condition. F-actin staining found that cytoskeleton took on a time-dependent change under SMG condition, which caused spindle to round morphological change of the cultured cells. Quantitative PCR and Western Blotting showed the pluripotency marker OCT4 was up-regulated in the SMG condition especially after SMG of 72 h, which we observed would be the most appropriate SMG duration for enhancing pluripotency of BMSCs. After dividing BMSCs into normal gravity (NG) group and SMG group, we induced them respectively in endothelium oriented, adipogenic and neuronal induction media. Immunostaining and Western Blotting found that endothelium oriented differentiated BMSCs expressed higher VWF and CD31 in the SMG group than in the NG group. The neuron-like cells derived from BMSCs in the SMG group also expressed higher level of MAP2 and NF-H. Furthermore, the quantity of induced adipocytes increased in the SMG group compared to the NG group shown by Oil Red O staining, The expression of PPARγ2 increased significantly under SMG condition. Therefore, we demonstrated that SMG could promote BMSCs to differentiate into many kinds of cells and predicted that enhanced multi-potential differentiation capacity response in BMSCs following SMG might be relevant to the changes of cytoskeleton and the stem cell marker OCT4.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-013-9544-8) contains supplementary material, which is available to authorized users.
Keywords: Simulated microgravity, Bone marrow mesenchymal stem cells, Pluripotency, Differentiation, OCT4
Introduction
As an important member of the stem cell family, the mesenchymal stem cells (MSCs) have recently become a widespread focus of study for more and more researchers. It can be obtained from various tissues and the main source of isolated MSCs comes from bone marrow. Relatively easy to separate and culture is one of the main advantages of bone marrow mesenchymal stem cell (BMSC). In some specific conditions, BMSCs can differentiate into several distinct cell types, including chondrocytes, osteoblasts, smooth muscle cells, adipocytes, cardiac myocytes, endothelial cells and neurons (Pittenger et al. 1999; Oswald et al. 2004; Kodama et al. 2005, 2006; Tamama et al. 2006, 2008; Owen and Friedenstein 1988). Due to BMSC’s capabilities in regenerating tissue, self-renewal and multipotential differentiation, it is highly desirable to use them in therapeutic renewal methods following aging, disease or trauma. They can also play an important role in wound healing, organ donation and chronic immune-suppressive therapies (Pittenger et al. 1999; Barry and Murphy 2004; Phinney and Prockop 2007; Prockop 1997). However, the problems of the low induction efficiency and high risk of the stem cells in clinical application have not been addressed yet (Colter et al. 2000; Crisostomo et al. 2006). Therefore, the development of a method that can produce higher-quality stem cells with improved differentiation potential is highly significant.
A strong relationship between the morphology and function of stem cells has been demonstrated (Graziano et al. 2007; McBride et al. 2008). Geometric shape cues have shown to have great effect on the differentiation of human BMSCs (Kilian et al. 2010). McBeath and his coworkers recently confirmed that cell shape and size had a large effect on the fate of MSCs (McBeath et al. 2004). It has been reported that mechanical boundary conditions can change the gene expressing pattern and consequently the functional behavior of MSCs. For example, MSCs regulated angiogenesis according to their mechanical environment. Osteogenic proliferation and differentiation appeared when MSCs were stimulated by mechanical loading (Koike et al. 2005; Yoshikawa et al. 1997; Kasper et al. 2007; Mauney et al. 2004).
Microgravity, as one of the most essential elements of space flight, can induce extensive physiological changes in human (Basso et al. 2005). Our work concurred with previous observation that the shape of spindle stem cells derived from rat BMSCs turned round after simulated microgravity (SMG) (Chen et al. 2011). However, the significance of the shape change for the stem cell itself has not been explained sufficiently yet. Many studies have demonstrated that the change in geometric shape could bring cytoskeletal reorganization (Li et al. 2009), which could alter the multipotential differentiation fates of BMSCs by affecting the relative signaling pathways such as RhoA or the stem cell surface markers (Hosu et al. 2008; Yuge et al. 2011).
In this study, we report data obtained from SMG experiments performed with BMSCs. The objectives of this study were to investigate changes in morphology, cytoskeleton, the pluripotency marker and multipotential differentiation capacity of BMSCs under SMG condition and to examine the underlying relationships of these changes.
Materials and methods
Isolation and cell culture of BMSCs
Bone marrow mesenchymal stem cells were isolated by their adherence to plastic as described in our previously published paper (Chen et al. 2011), with slight modification. Both tibias and femurs were isolated from 2-week-old Sprague–Dawley (SD) rats according to standard outlined by the Chinese Ethics Committee. The bone marrow containing MSCs was washed out by ice-cold DMEM (low glucose, Gibco; Carlsbad, CA, USA) and supplemented with 15 % fetal bovine serum (FBS, Gibco). We then gathered and cultured the cells in 25 cm2 plastic flasks at 37 °C with 5 % CO2, removing the refreshing medium every 3–4 days. When the cells reached 70–80 % confluence, they were treated with 0.25 % trypsin and separated at a ratio of 1:3. Our study chose the 3rd to 6th passage cells for testing their surface makers CD44, CD45, CD90 and CD34 with Flow Cytometry. Every independent experiment began from cell culture of BMSCs. If n = 3, we needed to choose three SD rats to isolate and culture the BMSCs, respectively, and then perform the corresponding experiments as follows.
Culture under simulated microgravity condition
Simulated microgravity (SMG) was operated according to a previously published paper (Li et al. 2009). A rotating vessel was used to create the weightless environment which is often referred to as “SMG”. This equipment with high aspect ratio vessels was designed to provide a vector-averaged gravity environment through the medium that contained cells rotating at the same speed with the vessel wall (Fig. 1D). Clinorotation technology (30 rpm) that models microgravity actually prevented the cell from feeling the effects of gravity, the gravity vector thus escaped its detection machinery. In our previous study, we found cell configuration, the cytoskeleton, apoptotic rate and the expression of OCT4 of the BMSCs have no change in different NG durations (0, 48, 72, 120 h) (Supplementary material 1). So in our study, we have chosen to culture the BMSCs in the NG condition for 72 h to represent the NG group. We divided the vessels randomly. Normal gravity group vessels were kept in the same room as the machine only without clinorotation. SMG group vessels were rotated with different durations (48, 72, 120 h). Flow Cytometry texted the surface markers CD44, CD45, CD90 and CD34 of SMG 72 h group.
Fig. 1.
A(a) Flow Cytometry analysis of CD44, CD90, CD45 and CD34 in normal BMSCs; (b) flow Cytometry analysis of CD44, CD90, CD45 and CD34 in BMSCs which were cultured under SMG for 72 h. B Phase-contrast microscopic analysis of the effect of SMG on the morphology of BMSCs. The scale bar is 40 μm. C The changes of the ratio of the width/length of BMSCs in the different groups. * Denotes P < 0.05, versus the NG group. # Denotes P < 0.05, versus the SMG 48 h group, n = 10. D The clinostat system modeled microgravity
Flow Cytometry analysis of the apoptotic rate
The effect of SMG on BMSCs apoptosis was investigated in this step. After the cells were cultured under NG and SMG conditions of different rotation durations, they were treated with 0.25 % trypsin. Their density was adjusted to 106/mL, 100 μL was transferred to 5 mL centrifuge tubes and then two groups cells were stained with the Annexin V-FITC and PI (BD Pharmingen, San Diego, CA, USA) for 15 min without light. The results were analyzed by fluorescence activated cell sorter (FACScan) cytometer (Elite ESP, Miami, FL, USA) and WinList software (Verity Software House, Topsham, ME, USA).
F-actin staining
The changes of cytoskeleton were observed after microgravity stimulations. Briefly, the cells were fixed in 4 % paraformaldehyde for 30 min and then washed in PBS 3 times. After being immersed in 0.01 % Triton X-100 for 30 min, they were covered with 1 % BSA at room temperature for 1 h to block the non-specific bindings. They were subsequently incubated with FITC-Phalloidin (5 μg/mL, Sigma, USA) for 2 h at 37 °C. BMSCs were then stained with DAPI (1:500, Sigma) for 7 min for nucleolus staining. Cells were imaged using IPLab software (Scanalytics, Fairfax, VA, USA).
Quantitative PCR
The expression of the pluripotency marker OCT4 at gene level was detected after SMG simulations. Total RNAs were isolated from SMG groups of different durations (0, 48, 72 and 120 h) using Trizol reagent (Invitrogen), and then reverse-transcribed into cDNA. Real-time PCR technique quantified the cDNAs using a SYBR green PCR mix, directed by the ABI Prism 7900HT. The sequences of the specific primers were: forward, 5′-GAACAGTTTGCCAAGCTGCTG-3′, and reverse, 5′-CCGGTTACAGAACCATACTCG-3′, Real-time PCR were performed using a thermal cycler consisting of an initial holding at 42 °C for 2 min, then 37 °C for 15 min, 85 °C 5 s. This was then followed by a two-step PCR program: 95 °C for 5 s and 60 °C for 34 s for 45 cycles. Quantitative analyses of the data were carried out using an ABI PRISM 7900 sequence detection system (Applied Biosystems). The values were expressed as fold change relative to the expression of GAPDH.
Endothelium oriented differentiation of BMSCs
As described previously (Oswald et al. 2004), the BMSCs of NG group and SMG 72 h group were cultivated in DMEM, supplemented with 10 % FBS and 100 ng/mL vascular endothelial growth factor (VEGF, Invitrogen), 50 ng/mL epidermal growth factor (Invitrogen) and 1 μg/mL hydrocortisone (Sigma-Aldrich) for 14 days, aiming to induce endothelial differentiation. An inverted phase-contrast microscope (Eclipse TE 300; Nikon Co., Tokyo, Japan) was subsequently used to examine the morphological changes. The expression level of VWF and CD31 were analyzed by Immunofluorescent and Western Blotting.
Adipogenic differentiation of BMSCs
Both of the NG group cells and the SMG 72 h group cells were treated with adipogenic medium composed of 1 μmol/L dexamethasone (Sigma), 0.5 mmol/L isobutylmethylxanthine (IBMX, Sigma), l0 μmol/L bovine insulin (Sigma), 200 μmoL/L indomethacin (Sigma) and DMEM + 10 % FBS. After 7 days, a phase-contrast microscope showed a change in shape. Here, Oil Red O staining was applied to examine the efficiency of adipogenic induction as described previously (Xiang et al. 2007). Western Blotting was used to analyzed the expression level of PPARγ2.
Neuron oriented differentiation of BMSCs
After the stimulation of SMG 72 h, the two groups BMSCs were induced to differentiate into neuron-like cells as previously described (Chen et al. 2011). Briefly, the cells were cultured in neuronal induction medium consisting of DMEM + 10 % FBS, 10 μg/L basic fibroblast growth factor (b-FGF, R&D Systems, Inc., Minneapolis, MN, USA), 10 μg/L human epidermal growth factor (hEGF, R&D Systems, Inc.), 1 mmol dibutyryl cyclic AMP (dbcAMP, Sigma, St. Louis, MO, USA) and 0.5 mmol isobutylmethylxanthine (IBMX, Sigma, St. Louis, MO, USA) for 14 days. Morphological changes were observed by phase-contrast microscope. Immunofluorescent and Western Blotting were used to analyze the expression level of Microtubule-associated protein 2 (MAP2) and Neurofilament heavy chain (NF-H).
Immunofluorescent staining
After cultured in the induced intermediary for 14 days, the cells were analyzed by fluorescence staining. The neuron oriented induced cells were incubated with primary antibodies to MAP2 (dilution 1:200; Santa Cruz, CA, USA) and to NF-H (dilution 1:200; Santa Cruz, CA, USA), respectively. The endothelium oriented induced cells were incubated with an anti-VWF antibody (dilution 1:200; Santa Cruz, CA, USA) and an anti-CD31 antibody (dilution 1:200; Santa Cruz, CA, USA), respectively, at 4 °C overnight. After we washed out the primary antibodies, fluorescein isothiocyanate (FITC) or TRITC-conjugated fluorescent secondary antibodies (dilution 1:100; Santa Cruz, CA, USA) were incubated, respectively, for 1 h at 37 °C in the dark. At last the nucleoli were stained by DAPI for 7 min. The number of positive cells was divided by the total number of cells to calculate the rate of positivity.
Western Blotting
The cells were washed three times in phosphate-buffered saline (PBS) and then lysed in RIPA buffer containing protease and phosphatase inhibitors (Sigma-Aldrich). Protein concentrations were quantified, and subsequently, separated on a sodium dodecyl sulfate (SDS)—12 % polyacrylamide gels, then electroblotted onto nitrocellulose membranes. Membranes were blocked and probed. The concentrations of the primary antibodies used were for the anti-MAP2 antibody (dilution 1:500), NF-H (dilution 1:500), for the anti-VWF antibody (dilution 1:500), for the anti-CD31 antibody (dilution 1:500), for the anti-PPARγ2 antibody (dilution 1:500) and for the anti-GAPDH antibody (1:2000). BMSCs cultured in normal media after rotated for 0 h, 48 h, 72 h and 120 h were incubated with an anti-OCT4 antibody as the primary antibody (dilution 1:500; Santa Cruz, CA, USA), the primary antibodies overnight at 4 °C. The next day, they were made to hybridized horseradish peroxidase-labeled the goat anti-rabbit secondary antibodies fluorescein isothiocyanate (FITC) or TRITC (dilution 1:100; Santa Cruz, CA, USA) respectively. Membranes were exposed to X-ray film for chemiluminescence detection. The bands were analyzed using the Quantity One software. Data were normalized by GAPDH protein level and the corresponding relative expression ratio was calculated as protein of interest/GAPDH.
Statistical analysis
Data were expressed as mean ±SD and analyzed using SPSS 13.0 (SPSS, Chicago, IL, USA). Statistical analyses were performed using the two-tailed Student’s t test for two groups. The one-way ANOVA was used to analyze the results of more than two groups. When one-way ANOVA showed a significant difference, the significance between the groups was calculated with the Bonferroni test for multiple comparisons. Different levels represented significance at P values <0.05.
Results
Cell culture and cell identification
The 3rd to 6th passage cells which were prepared for this research displayed spindle-shaped or broadened flattened morphology. Flow Cytometry results showed that these cells were CD44 and CD90 positive (the ratios were 94.74 and 93.57 %, respectively) and CD45 and CD34 negative [Fig. 1A(a)]. After the cells have been cultured in SMG condition for 72 h, they were also CD44 and CD90 positive (the ratios were 93.04 and 92.83 %, respectively) and CD45 and CD34 negative [Fig. 1A(b)].
Morphological change of BMSCs
After SMG stimulation, the shape of the cells shifted to roughly round, which obviously contrasted with the spindle cells cultured in NG (Fig. 1B). We measured the width and length of the cells and calculated the ratio to describe the morphology by the MetaMorph 7.1 software (Universal Imaging, Downingtown, USA). The statistical data indicated that the most significant change of the shape occurred after SMG 72 h and differences were found rarely between SMG 72 and 120 h (Fig. 1C).
Apoptotic rate analysis
Flow Cytometry results showed that there was little difference of the quantity of apoptotic BMSCs among different groups. This indicated that SMG did not induce apoptosis of BMSCs (Fig. 2a).
Fig. 2.
a FACS analysis of the apoptotic rate of BMSCs in four groups. b Time-dependent change of cytoskeleton. The scale bar is 20 μm. The changes of filaments bundles orientation are shown in the red rectangles. (Color figure online)
Time-dependent change of cytoskeleton
The cytoskeleton of BMSC was sensitive to SMG. This was especially true for the change in microfilaments that are composed of F-actin. It was observed that F-actin organized clearly into bundles as thick filaments in the NG group. The filaments bundles were oriented along the long axis of the cells, displaying a typical radial array. However, SMG induced transient changes of the cytoskeleton, made microfilaments disturbed and induced the modification of some bundles. The most obvious change was found in SMG 72 h. The bundles were thin, dispersed and lost their long-range orientation that appeared under the NG condition. Further more, some were even observed to be perpendicular to the substratum. However partial restoration of F-actin occurred in SMG 120 h. The bundles showed signs of polymerization and restored the preferential orientation toward the cell periphery (Fig. 2b).
Effect of simulated microgravity on pluripotency marker
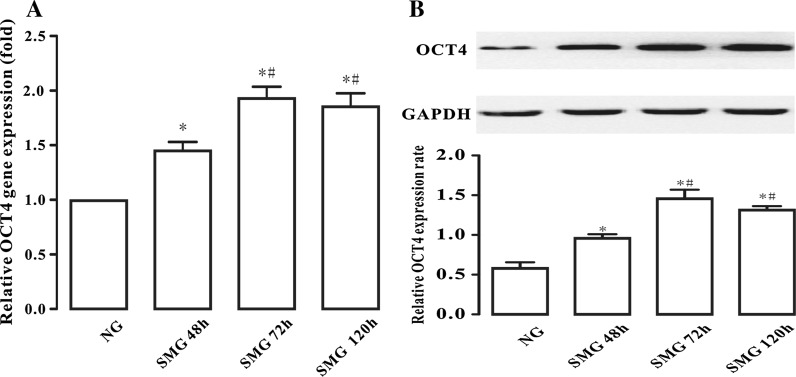
Analysis by Quantitative PCR detected that cells maintained in SMG could up-regulate OCT4 expression. While the expression of OCT4 was changed slightly after 48 h of SMG simulation, more obvious changes were found after SMG 72 h (Fig. 3a). However, this up-regulation had no significance in the SMG 120 h group compared with the 72 h group analyzed by the one-way ANOVA. Furthermore, the results of Western Blotting were very similar to those obtained with Quantitative PCR (Fig. 3b). Therefore, we deduced that 72 h might be the most appropriate SMG duration for the highest pluripotency of BMSCs.
Fig. 3.
a Quantitative PCR analysis of the expression of OCT4. OCT4 expression was normalized to endogenous GAPDH in the same samples. The results represent three independent experiments, where each experiment was repeated three times. b Western Blotting showed the changes of OCT4 expression for different SMG durations. The results represent 4 independent experiments, where each experiment was repeated three times. * Denotes P < 0.05, versus the NG group. # Denotes P < 0.05, versus the SMG 48 h group
Endothelial differentiation of BMSCs
After the cells were induced with endothelium oriented revulsive, we found cell morphology exhibited endothelium-like changes and phase-contrast microscope showed that there were more endothelium-like cells in the SMG group than in the NG group (Fig. 4a). Immunostaining showed that the positivity rate of VWF and CD31 were higher in SMG than in the NG group cells (Fig. 4b). Western Blotting analysis confirmed the above immunostaining observations. As shown in Fig. 4c, the induced cells in the SMG group expressed higher VWF and CD31 levels than that seen in the NG group (P < 0.05). This showed that SMG could enhance the endothelium oriented differentiation of BMSCs.
Fig. 4.
Effect of SMG on the endothelium oriented differentiation of BMSCs. a Phase-contrast microscopic image of the differentiated cells from both groups. The scale bar is 100 μm. b Immunostained images of VWF and CD31. The scale bar is 40 μm. c Western Blotting analysis of the expression of VWF and CD31. The results represent three independent experiments, where each experiment was repeated three times.* Denotes P < 0.05, compared with the NG group
Adipogenic differentiation of BMSCs
After we induced the cells for 7 days, the number of the adipogenic-like cells was higher in the SMG group as observed by phase-contrast microscope (Fig. 5a). Oil Red O staining indicated that the numbers of derived adipocytes in the SMG group were higher than those in the NG group (Fig. 5b). Western Blotting showed that the expression of PPARγ2 increased significantly under SMG condition (Fig. 5c). This indicated that SMG could promote adipogenic differentiation of BMSCs.
Fig. 5.
Effect of SMG on the adipogenic differentiation of BMSCs. a Phase-contrast microscopic images showed that there were more adipocyte -like cells in the SMG group. The scale bar is 100 μm. b Images of Oil Red O staining of the cells from both groups cells. The scale bar is 100 μm. c Western Blotting analysis of the expression of PPARγ2. The results represent three independent experiments, where each experiment was repeated three times.* Denotes P < 0.05, compared with the NG group
Neuron oriented differentiation of BMSCs
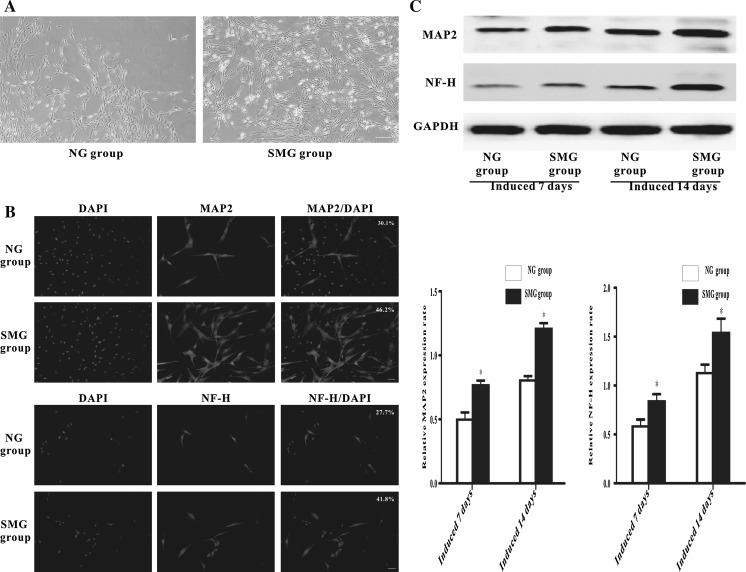
When these cells were placed in neuronal induction medium for 14 days, many of them took on long neuron-like cell processes and these cells developed long branching that contacted with neighboring cells. Phase-contrast microscope showed that there were more neuron-like cells in the SMG group than in the NG group (Fig. 6a). MAP2 and NF-H were shown to have lower expression in the NG group than that in the SMG group, as observed by immunostaining (Fig. 6b). Western Blotting concurred with the immunostaining results either at 7 or 14 days after induction (Fig. 6c). This evidence suggested that SMG was helpful for the neuron oriented differentiation of BMSCs.
Fig. 6.
Effect of SMG on the neuron oriented differentiation of BMSCs. a Phase-contrast microscopic images of the cells from two groups. The scale bar is 100 μm. b Immunostained images of MAP2 and NF-H. The scale bar is 40 μm. c Western Blotting analysis of the expression of MAP2 and NF-H after MSCs cultured in the neuronal differentiation media for 7 and 14 days. The results represent three independent experiments, where each experiment was repeated three times.* Denotes P < 0.05, compared with the NG group
Discussion
Microgravity, as one of the most important elements of space flight, has been reported to cause extensive physiological changes in MSCs, including the maintenance of the undifferentiated state of BMSCs and potentiating the proliferation and differentiation of the cells to a single direction (Yuge et al. 2006, 2011). In this study, we further demonstrated that SMG could enhance the multipotential differentiation capacity of BMSCs which enriches the previous studies.
We used a clinostat for simulating microgravity, which could generate a multidirectional G force that placed the cells in a constant direction changing the gravity environment (Yuge et al. 2003). As a result, the cells had no time to respond to normal gravity. While it is impossible for this device to entirely model a weightless environment, it is a comprehensive and feasible research method for use in the space life science field (Klaus 2001).
This study illustrated that the morphology of BMSCs changed from spindle to round shaped in the SMG condition which was caused by a change of the cytoskeleton. But the BMSCs did not loose their immunophenotype. The idea that the cytoskeletal tension can affect the stem cell fate via RhoA pathway is widely accepted (McBeath et al. 2004). This study found that the cytoskeleton present time-dependent change. The most obvious changes were found after SMG 72 h and partial restoration occurred after SMG 120 h. Consequently, it can be deduced that the duration of rotation for SMG was fundamental in directing the differentiation fate of BMSCs. RhoA is a protein associated with actin cytoskeleton has also been reported to be affected by SMG. Our previous study found that the activation of RhoA in the SMG 72 h group decreased significantly but increased in SMG 10 days. In addition, our study also found that the expression of osteoblastic gene ALP was lower in SMG 72 h group but higher in SMG 10 day group (Zhang et al. 2013), We can therefore speculate that 72 h stimulation might down-regulate the RhoA pathway associated genes to encourage the differentiation to endothelium, adipocyte or neuron. On the other hand, a long duration for SMG might activate the RhoA pathway and reorganized the cytoskeleton to advance BMSCs in differentiating to other cells such as osteogenic progenitor cells. Therefore, SMG can enhance the pluripotency of BMSCs through adjustments in the duration. But still more experiments are needed to confirm this hypothesis.
It is clearly evident that the differentiation potential and functional activities of MSCs were decided by multilevel nature. In addition to the cytoskeleton, the surface markers of BMSCs were also very important. It was interesting to find that not only the cytoskeleton, but also the surface markers of the stem cells had been modified. OCT4 is a key transcription factor that can regulate the pluripotency and self-renewal of stem cells. It has been established that levels of OCT4 expression were crucial for the maintenance of pluripotency and for governing the fate of the stem cell. Both the up-regulation and the down-regulation of the level of OCT4 could affect the differentiation potential of stem cells (Niwa et al. 2000). We found that SMG can lead to up-regulation of the OCT4 gene, especially after 72 h stimulation which was the same duration causing the most obvious changes of the shape and the cytoskeleton of BMSCs. Though the cytoskeleton was re-established and the preferential orientation toward the cell periphery was restored at SMG 120 h, the shape change of BMSCs in the SMG 120 h group was not significant compared with the SMG 72 h group. However, the expression level of OCT4 no longer increased after SMG 72 h. Some experts found that the more complex change of BMSCs occurred after SMG 120 h (Gershkovich et al. 2011). We also agree with that the change of the multipotential differentiation capacity of stem cell may be relevant to the telomerase activity. But, results reported by Yuge et al. (2006) showed that telomere length for the NG group or the SMG group did not change during culturing, and telomerase activity was undetectable in both groups in their study. The specific effect of the telomerase activity on the multipotential differentiation capacity of stem cell is unclear. In addition, there is no evidence that OCT4 expression can be affected by the telomerase activity. So, when we designed this experiment, we did not consider that the influence of telomerase activity could impact the results.
A number of studies show that CD31 and VWF play important roles in endothelium maturation and angiogenesis. CD31 is also known as platelet-endothelial cell adhesion molecule-1 (PECAM-1) which is the only known member of the CAM family on platelets. CD31 proteins which are expressed by endothelial cells, mediate homotypic or heterotypic cell adhesion and thus play a role in cardiovascular development (Jin et al. 2012). Vascular endothelial cells synthesize and secrete VWF. VWF is only expressed in normal endothelial cells (Ruggeri 2003). Thus, they are regarded as endothelial-specific markers. MAP2 and NF-H are involved in the formation of the cytoskeleton of nerve cells. MAP2 can participate in composing the structure of different parts of nerve cells and can be found mostly in dendrites and axon. Neurofilaments usually contain three intermediate filament proteins: L, M, and H which are involved in the maintenance of neuronal caliber. NF-H has an important function in mature axons. So according to the previous article by Yuge et al. (2011) we have chosen MAP2 and NF-H as the neural differentiation markers. Peroxisome proliferator-activated receptor gamma 2 (PPARγ2) is an adipocyte-specific nuclear hormone receptor that has recently been identified as a critical transcription factor involved in adipogenic differentiation in MSCs. Previous research suggested that the physiologic role of PPARγ2 is to regulate development of the adipose lineage in response to endogenous lipid activators and that this factor may serve to link the process of adipocyte differentiation to systemic lipid metabolism (Muruganandan et al. 2011). So we have chosen the PPARγ2 as an adipogenic differentiation marker of BMSCs. After induction, we found that the positivity expression rate of VWF, CD31, MAP2, NF-H and Oil Red O staining increased indeed in the SMG 72 h group, which demonstrated that 72 h SMG could promote BMSCs to differentiate into many kinds of cells. As we all known that endothelium, adipocytes and neuron cells were all force-insensitive cells, and previous report showed that the force-insensitive cells were easily affected by short time SMG. However, the osteoblasts are force-sensitive cells and they were easily affected by long term SMG. So we have chosen BMSCs differentiation into endothelium, adipocytes and neuron-like cells to research the multipotential differentiation capacity of BMSCs in short time SMG condition and we indeed demonstrated that SMG could promote BMSCs to differentiate into these kinds of cells.
A previous study reported that SMG boosted their survival rate by maintaining BMSCs in an undifferentiated state, thus enhancing the efficiency of differentiation (Yuge et al. 2011). In accordance with our observation, we predicted that the up-regulated OCT4 gene and cytoskeleton were relevant to the possible mechanisms of SMG affecting BMSCs. But in our study, we only little discussed the modifications of other capabilities of BMSCs under the SMG condition. We will further study further effects of SMG on BMSCs such as the proliferation and migration capacities in our future work.
In this study, we used as base for induction condition normal gravity (for unification). The cells were first treated in the “micro-gravity” conditions and then treated with various differentiation cocktails under normal gravity conditions. In the previous study, we found that most of the BMSCs did not differentiate into the corresponding cells when they were induced in the SMG condition, but the stem cell surface markers were up-regulated (Yuge et al. 2011). We suspected that SMG condition was helpful for maintaining the undifferentiated state of BMSCs and consequently can improve the induction efficiency of BMSCs in the NG condition. This may be another reason of the SMG enhancing multipotential differentiation capacity of BMSCs. However, more experimental studies are needed to confirm this in our future work.
We demonstrated that the morphology of BMSCs was changed under the SMG condition and thus had larger capability to differentiate to other cell types. This result further demonstrated that there was some interplay between the geometric cues of the cells and their functions (Kilian et al. 2010). While previous report illustrated that SMG was helpful to expand stem cell populations in vitro (Yuge et al. 2006), our work further demonstrated that SMG enabled cells to maintain pluripotency and therefore enhance multipotential differentiation capacity.
Conclusion
In this study, we demonstrated that BMSCs exposed to microgravity became morphologically modified from a spindle to a round shape. This subsequently increased the differentiation potential of BMSCs through cytoskeletal changes and up-regulation in the multipotential mark OCT4. We also highlighted 72 h as the most appropriate SMG duration for enhancing the pluripotency of BMSCs. Therefore, in the light of not inducing apoptosis, SMG can be expected to provide a novel method for obtaining higher-quality stem cells with an improved capacity for differentiation and self-renewal. This suggests great potential for use in clinical applications and developmental biology.
Electronic supplementary material
Supplementary material 1 (TIFF 16991 kb)
Acknowledgments
This work was carried out in the Physiology laboratory and State Key Laboratory of Aerospace Biodynamics at Fourth Military Medical University. The project was supported by NSFC Grant 30973808.
Abbreviations
- SMG
Simulated microgravity
- NG
Normal gravity
- MSC
Mesenchymal stem cell
- BMSC
Bone marrow mesenchymal stem cell
- VWF
Von Willebrand factor
- MAP2
Microtubule-associated protein 2
- NF-H
Neurofilament heavy chain
- OCT4
Octamer-binding transcription factor 4
Footnotes
N. Wang, H. Wang and J. Chen are the co-first authors.
Contributor Information
Wen Wang, Phone: +86-02984771347, Email: jinzhou@fmmu.edu.cn.
Zongren Wang, Phone: +86-02984771347, Email: zongren@fmmu.edu.cn.
References
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Basso N, Bellows CG, Heersche JN. Effect of simulated weightlessness on osteoprogenitor cell number and proliferation in young and adult rats. Bone. 2005;36:173–183. doi: 10.1016/j.bone.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Chen J, Liu R, Yang Y, Li J, Zhang X, Li J, Wang Z, Ma J. The simulated microgravity enhances the differentiation of mesenchymal stem cells into neurons. Neurosci Lett. 2011;505:171–175. doi: 10.1016/j.neulet.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.97.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisostomo PR, Wang M, Wairiuko GM, Morrell ED, Terrell AM, Seshadri P, Nam UH, Meldrum DR. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26:575–580. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
- Gershkovich PM, Gershkovich I, Buravkova LB (2011) Expression of cytoskeleton genes in culture of human mesenchymal stromal cells in different periods of simulating the effects of microgravity. Aviakosm Ekolog Med 45(4):39–41 [PubMed]
- Graziano A, D’Aquino R, Cusella-De AM, Laino G, Piattelli A, Pacifici M, De Rosa A, Papaccio G. Concave pit-containing scaffold surfaces improve stem cell-derived osteoblast performance and lead to significant bone tissue formation. PLoS One. 2007;2:e496. doi: 10.1371/journal.pone.0000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosu BG, Mullen SF, Critser JK, Forgacs G. Reversible disassembly of the actin cytoskeleton improves the survival rate and developmental competence of cryopreserved mouse oocytes. PLoS One. 2008;3:e2787. doi: 10.1371/journal.pone.0002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Liu Y, Antonyak M, Peng X. Isolation and characterization of vascular endothelial cells from murine heart and lung. Methods Mol Biol. 2012;843:147–154. doi: 10.1007/978-1-61779-523-7_14. [DOI] [PubMed] [Google Scholar]
- Kasper G, Dankert N, Tuischer J, Hoeft M, Gaber T, Glaeser JD, Zander D, Tschirschmann M, Thompson M, Matziolis G, Duda GN. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 2007;25:903–910. doi: 10.1634/stemcells.2006-0432. [DOI] [PubMed] [Google Scholar]
- Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus DM. Clinostats and bioreactors. Gravit Space Biol Bull. 2001;14:55–64. [PubMed] [Google Scholar]
- Kodama H, Inoue T, Watanabe R, Yasuoka H, Kawakami Y, Ogawa S, Ikeda Y, Mikoshiba K, Kuwana M. Cardiomyogenic potential of mesenchymal progenitors derived from human circulating CD14+ monocytes. Stem Cells Dev. 2005;14:676–686. doi: 10.1089/scd.2005.14.676. [DOI] [PubMed] [Google Scholar]
- Kodama H, Inoue T, Watanabe R, Yasutomi D, Kawakami Y, Ogawa S, Mikoshiba K, Ikeda Y, Kuwana M. Neurogenic potential of progenitors derived from human circulating CD14+ monocytes. Immunol Cell Biol. 2006;84:209–217. doi: 10.1111/j.1440-1711.2006.01424.x. [DOI] [PubMed] [Google Scholar]
- Koike M, Shimokawa H, Kanno Z, Ohya K, Soma K. Effects of mechanical strain on proliferation and differentiation of bone marrow stromal cell line ST2. J Bone Miner Metab. 2005;23:219–225. doi: 10.1007/s00774-004-0587-y. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang S, Chen J, Du T, Wang Y, Wang Z. Modeled microgravity causes changes in the cytoskeleton and focal adhesions, and decreases in migration in malignant human MCF-7 cells. Protoplasma. 2009;238:23–33. doi: 10.1007/s00709-009-0068-1. [DOI] [PubMed] [Google Scholar]
- Mauney JR, Sjostorm S, Blumberg J, Horan R, O’Leary JP, Vunjak-Novakovic G, Volloch V, Kaplan DL. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-d partially demineralized bone scaffolds in vitro. Calcif Tissue Int. 2004;74:458–468. doi: 10.1007/s00223-003-0104-7. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- McBride SH, Falls T, Knothe TM. Modulation of stem cell shape and fate b: mechanical modulation of cell shape and gene expression. Tissue Eng Part A. 2008;14:1573–1580. doi: 10.1089/ten.tea.2008.0113. [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Parlee SD, Rourke JL, Ernst MC, Goralski KB, Sinal CJ. Chemerin, a novel peroxisome proliferator-activated receptor gamma (PPARgamma) target gene that promotes mesenchymal stem cell adipogenesis. J Biol Chem. 2011;286:23982–23995. doi: 10.1074/jbc.M111.220491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. 2003;1:1335–1342. doi: 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:686–695. doi: 10.1634/stemcells.2005-0176. [DOI] [PubMed] [Google Scholar]
- Tamama K, Sen CK, Wells A. Differentiation of bone marrow mesenchymal stem cells into the smooth muscle lineage by blocking ERK/MAPK signaling pathway. Stem Cells Dev. 2008;17:897–908. doi: 10.1089/scd.2007.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Zheng Q, Jia B, Huang G, Xie C, Pan J, Wang J. Ex vivo expansion, adipogenesis and neurogenesis of cryopreserved human bone marrow mesenchymal stem cells. Cell Biol Int. 2007;31:444–450. doi: 10.1016/j.cellbi.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Peel SA, Gladstone JR, Davies JE. Biochemical analysis of the response in rat bone marrow cell cultures to mechanical stimulation. Biomed Mater Eng. 1997;7:369–377. [PubMed] [Google Scholar]
- Yuge L, Hide I, Kumagai T, Kumei Y, Takeda S, Kanno M, Sugiyama M, Kataoka K. Cell differentiation and p38(MAPK) cascade are inhibited in human osteoblasts cultured in a three-dimensional clinostat. In Vitro Cell Dev Biol Anim. 2003;39:89–97. doi: 10.1290/1543-706X(2003)039<0089:CDAPCA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Yuge L, Kajiume T, Tahara H, Kawahara Y, Umeda C, Yoshimoto R, Wu SL, Yamaoka K, Asashima M, Kataoka K, Ide T. Microgravity potentiates stem cell proliferation while sustaining the capability of differentiation. Stem Cells Dev. 2006;15:921–929. doi: 10.1089/scd.2006.15.921. [DOI] [PubMed] [Google Scholar]
- Yuge L, Sasaki A, Kawahara Y, Wu SL, Matsumoto M, Manabe T, Kajiume T, Takeda M, Magaki T, Takahashi T, Kurisu K, Matsumoto M. Simulated microgravity maintains the undifferentiated state and enhances the neural repair potential of bone marrow stromal cells. Stem Cells Dev. 2011;20:893–900. doi: 10.1089/scd.2010.0294. [DOI] [PubMed] [Google Scholar]
- Zhang X, Nan Y, Wang H, Chen J, Wang N, Xie J, Ma J, Wang Z. Model microgravity enhances endothelium differentiation of mesenchymal stem cells. Naturwissenschaften. 2013;100:125–133. doi: 10.1007/s00114-012-1002-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (TIFF 16991 kb)