Abstract
Recovery from tendon injury is based on long periods of rest, which results in sub-optimal repair, often replacing tendon with fibrocartilage scar tissue. Recently, the use of stem cells in equine tendon repair has been attempted with variable success. The objective of this work was to determine the expression of scleraxis (scx) and tenascin C (TnC), two markers of tenocytes, in adipose (AdMSC) and umbilical cord blood (UCB) stem cells during culture on various substrata and in response to fibroblast growth factor (FGF) treatment. Equine UCB and AdMSC were cultured on gelatin-coated plasticware, 30 % matrigel or collagen-coated Cytodex beads and treated with 10 ng/ml FGF2, FGF4 or FGF5 prior to measurement of proliferation, kinase activity and tenocyte gene expression. Supplementation with FGF2 or FGF5 activated the ERK1/2 signaling pathway in AdMSC and UCB; no effect of FGF4 was observed in UCB. FGF2 increased proliferation in AdMSC but not UCB. Conversely, FGF5 stimulated proliferation of UCB. Culture in matrigel increased scx expression in both cell populations and increased TnC in AdMSC. In AdMSC grown in matrigel, supplementation with FGF2 or FGF5 increased TnC expression. Thus, culture conditions (substrata and FGF supplementation) impact markers of tenocytes in AdMSC and UCB stem cells, indicating that careful consideration should be given to culture conditions prior to use of UCB or AdMSC as therapeutic aids. Optimal culture conditions may promote early differentiation of these cells, improving their ability to aid tendon regeneration and facilitating more efficient recovery from tendon injury.
Keywords: Umbilical cord blood, Mesenchymal stem cell, Adipose, Tendon, Scleraxis, Tenascin C
Introduction
In athletes, including man and horse, tendon injuries are slow to repair and often lead to weakened areas that are prone to re-injury (Clegg et al. 2007; Longo et al. 2009). Efforts to improve repair rates and strengthen regenerated tendons include injection of bone marrow and adipose derived mesenchymal stem cells (MSCs) in the horse (Taylor et al. 2007). However, many stem cell populations require in vitro expansion prior to use as a therapeutic aid. Inclusion of growth factors with culture media and/or use of different substrata matrices may influence gene transcription and cell identity.
Distinguishing multipotent stem cells from tenocytes is routinely achieved by examining the expression of several known protein markers. Scleraxis (scx) is a class II basic helix-loop-helix transcription factor expressed early during mouse embryogenesis in the syndetome, a derivative of the somitic sclerotome compartment (Brent et al. 2003; Cserjesi et al. 1995). Expression is associated with connective tissue and skeleton structures during prenatal development and with periodontal ligaments, force generating tendons, brain, lung and Sertoli cells in adult rodents (Liu et al. 1996; Muir et al. 2005; Murchison et al. 2007; Perez et al. 2003; Pryce et al. 2007). Expression of scleraxis is largely confined to tendons early in postnatal development and absent in the structures as they become increasingly acellular (Pryce et al. 2007). Conditional ablation of scleraxis in the developing limb of mice produces an animal with missing flexor tendons; a few tendons are present but small in size (Murchison et al. 2007). An additional marker of tendon cells, tenascin C (TnC), is an extracellular matrix protein present in developing and mature tendons (Kardon 1998); however, it is not specific to tendon cells (Mackie and Ramsey 1996). Tenascin C is physically complexed with fibronectin in the tendon (Swasdison and Mayne 1989; To and Midwood 2011). It is suggested that TnC plays a role in proper alignment and orientation of collagen fibrils within the tendon (Mackie and Ramsey 1996).
Members of fibroblast growth factor (FGF) family regulate transcription of scx and TnC. FGFs produced by the myotome supply a paracrine signal that allows formation of the sclerotome and syndetome (Brent and Tabin 2004). Exogenous FGF4 induces scleraxis and tenascin C mRNA synthesis in the developing chick limb (Edom-Vovard et al. 2002). Ectopic expression of FGF5 in chick embryos inhibited skeletal muscle growth and promoted proliferation of tenascin C immunopositive fibroblasts in the hind limb (Clase et al. 2000). FGFs often signal through the mitogen activated protein kinase (MAPK) signaling cascade. Indeed, expression of scleraxis in somitic progenitor cells was dependent on the phosphorylation of ERK1/2, two prominent kinases of the MAPK signaling cascade (Smith et al. 2005). Further, intracellular signals transmitted in response to FGF4 include increased activity of MEK1, a kinase upstream of ERK1/2.
Manipulation of culture conditions can affect gene expression and influence cell identity. FGFs induce tenocytic gene expression during embryonic development, but their effect on multipotent cells in vitro is unclear. The influence of the matrix substrata on tenocyte gene expression is also currently unknown. Thus, the objective of this work was to culture two mesenchymal stem cell populations (equine umbilical cord blood and adipose derived stem cells) on various substrata and in the presence of fibroblast growth factors to determine the effects on tendon gene expression. We hypothesized that maintenance in a three dimensional environment and FGF supplementation would increase expression of scx and TnC in both cell types. We show here that UCB and AdMSC respond to FGF2 and FGF5 stimulation by increasing phosphorylation of ERK1/2. Further, culture on matrigel increases scleraxis expression in both UCB and AdMSC. However, the regulation of tenocytic gene expression in response to fibroblast growth factor stimulation is very different in the two cell types.
Materials and methods
Stem cell culture
Umbilical cord blood stem cells (UCB) were isolated from thoroughbred foals at birth and cryopreserved as previously described (Reed and Johnson 2008). All procedures were approved by the University of Florida Institutional Animal Care and Use Committee. UCB stem cells were thawed and immediately placed into culture on plastic tissue culture plates coated with 0.1 % gelatin in Dulbecco’s Modified Eagle Medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum (FBS) and 5 μg/ml Plasmocin (InVivoGen, San Diego, CA, USA) for expansion. Equine adipose derived cells (AdMSC) were purchased commercially (Sciencell, Carlsbad,CA, USA). Cells were cultured on 0.1 % gelatin coated tissue culture plates in Mesenchymal Stem Cell Medium (Sciencell) according to manufacturer’s recommendations. Cells were passaged at 70 % confluency using 0.025 % trypsin–EDTA. Three dimensional cultures were established by growing UCB or AdMSC on collagen coated Cytodex3 beads (Invitrogen) or allowing cells to infiltrate into 30 % reduced growth factor Matrigel (diluted in serum free media, BD Biosciences, San Jose, CA, USA). All experiments were run concurrently using UCB from three horses (passages 3–4) or in triplicate (AdMSC; passages 3–5).
Confocal microscopy
UCB stem cells and AdMSC cultured on glass-bottom tissue culture plates coated with gelatin or matrigel, or collagen beads were fixed in 4 % paraformaldehyde in phosphate buffered saline (PBS) for 15 min. Fixed cells were permeabilized with 0.1 % Triton X-100 in PBS containing 5 % FBS. Polymerized actin filaments were visualized using fluorescein conjugated phalloidin (1:50) and nuclei were stained with Hoechst 33342 (1:1,000). Confocal microscopy was performed on a Leica TCS SP5 Laser Scanning Confocal Microscope running Leica LAS-AF software for instrument control and image analysis (Leica, Buffalo Grove, IL, USA). Images were adjusted for brightness and contrast in Adobe Photoshop CS (Adobe Systems Inc., San Jose, CA, USA).
Protein isolation
UCB stem cells and AdMSC were treated with 10 μg/ml protamine sulfate in PBS for 10 min to remove growth factors from the extracellular matrix. Cells were placed in serum free media for 1 h prior to stimulation with 10 ng/ml FGF2, FGF4, or FGF5. Cells were lysed directly into SDS PAGE sample buffer. Protein was loaded based on equal cell number and electrophoresed through a 10 % denaturing polyacrylamide gel. ERK1/2 activity was assessed by Western blot using phospho- and total-ERK1/2 antibodies (Cell Signaling Technologies, Danvers, MA, USA). Briefly, proteins were transferred to nitrocellulose and non-specific binding sites were blocked with 10 % non-fat dry milk in TRIS-buffered saline supplemented with 0.1 % Tween 20. Blots were incubated overnight at 4 °C with primary antibody (1:1,000) in blocking solution. Following extensive washing, blots were incubated with secondary antibody (1:2,000). Equal protein loading was ensured by probing membranes with anti-tubulin for 1 h (1:2,000) followed by incubation in secondary antibody for 1 h (1:5,000). Immune complexes were visualized by chemiluminescence and autoradiography.
Assessment of proliferation
UCB and AdMSC were seeded at 2,000 cells/cm2 on gelatin coated tissue culture plates and cultured in low serum medium (2 % FBS) supplemented with 10 ng/ml FGF2, FGF4 or FGF5. After 46 h of supplementation, cells were pulsed with 10 μM bromodeoxyuridine (BrdU) for 2 h and fixed. Proliferation index was determined as the proportion of cells expressing BrdU:total cell number.
RNA isolation, reverse transcription, and real time PCR
Total RNA was isolated by lysis in STAT60 (Iso-Tex Diagnostics, Friendswood, TX, USA) and captured with RNeasy Mini columns (Qiagen, Valencia, CA, USA). The RNA was digested with DNase to remove genomic DNA contaminants. One microgram of total RNA was reverse transcribed in 20 μl reaction volume. The resulting cDNA was amplified with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and the appropriate forward and reverse primers (Table 1; 20 pM) in an ABI 7300 Real-Time PCR System (Applied Biosystems). Thermal cycling parameters included a denature step of 95 °C for 10 min and 50 cycles of 15 s at 95.0 °C and 1 min at 60.0 °C. A final dissociation step included 95 °C for 15 s, 55 °C for 30 s, and 95 °C for 15 s. Serial dilutions of pooled samples were used to generate standard curves to ensure generation of cycle threshold values that were within the linear range of amplification (Castellani et al. 2004). Fold change was calculated using the ΔΔCt method. Expression was normalized to ribosomal 18S, which did not differ between any treatment groups.
Table 1.
Real-time PCR primers
| Primer | Sequence | Expected product size, bp | Standard curve slope | Primer efficiency, % |
|---|---|---|---|---|
| TnCa | F-GGGCGGCCTGGAAATG R-CAGGCTCTAACTCCTGGATGATG |
70 | −3.34 | 99.25 |
| ScxBa | F-TCTGCCTCAGCAACCAGAGA R-TCCGAATCGCCGTCTTTC |
59 | −3.35 | 98.84 |
| 18S | F-GTAACCCGTTGAACCCCATT R-CCATCCAATCGGTAGTAGCG |
151 | −3.35 | 98.84 |
aTaylor et al. (2009)
Statistical analysis
Data were analyzed by ANOVA (GraphPad Software Inc., San Diego, CA, USA). When the results of the ANOVA were significant, differences between means were analyzed using a Bonferroni post hoc test. Data are presented as mean ± SEM. Statistical significance for all experiments was set at p < 0.05.
Results
Fibroblast growth factors elicit differing ERK1/2 responses in UCB and AdMSC
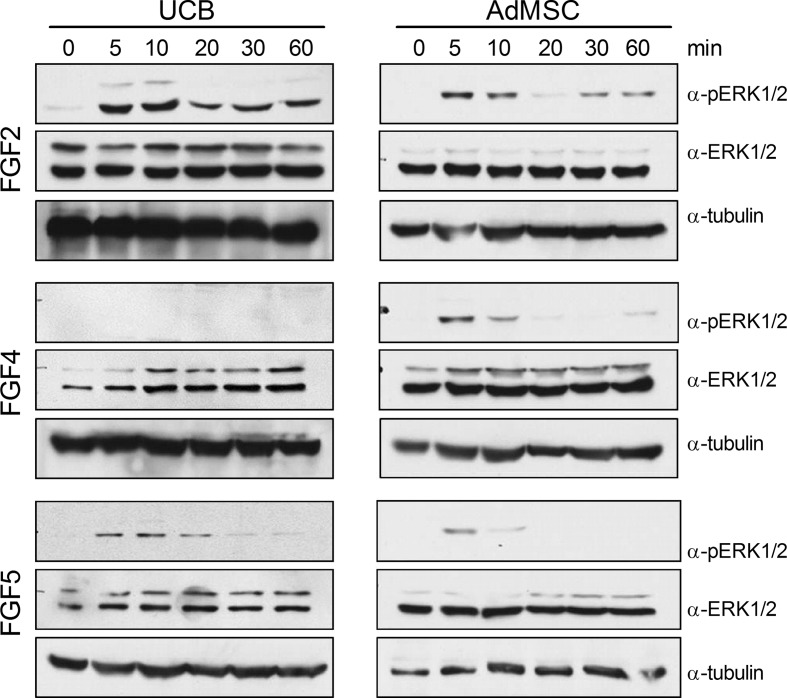
Not only can FGF stimulation promote mitogenesis in stem cells, but FGF signaling through the ERK1/2 pathway can lead to downstream transcription of scleraxis (Smith et al. 2005). Thus, the ERK1/2 response was examined in UCB and AdMSC following stimulation by FGF2, FGF4, or FGF5. FGF2 elicited ERK1/2 phosphorylation in both cell types, albeit with differing activation kinetics (Fig. 1). In AdMSC, FGF2 appears to preferentially phosphorylate ERK2, as ERK1 is not phosphorylated. FGF4 did not result in ERK1/2 phosphorylation in UCB, however the growth factor was responsible for a slight increase in phosphorylated ERK2 in AdMSC. Further experiments with FGF4 were discontinued due to lack of an ERK-signaling response in UCB. Stimulation with FGF5 elicited a transient increase in phosphoERK2 in both AdMSC and UCB, with phosphorylation decreasing after 20 min in UCB and 5 min in AdMSC. Levels of total ERK1/2 were not affected by any FGF, however the ratio of ERK1:ERK2 appears to differ between AdMSC and UCB. Akt activity was not affected by supplementation of FGF2, FGF4, or FGF5 (data not shown).
Fig. 1.
UCB and AdMSC respond differently to FGF stimulation. UCB and AdMSC were stimulated with 10 ng/ml FGF2, FGF4, or FGF5 for the times shown. Protein extracts were probed with antibodies specific to phosphorylated ERK1/2, total ERK1/2, and tubulin
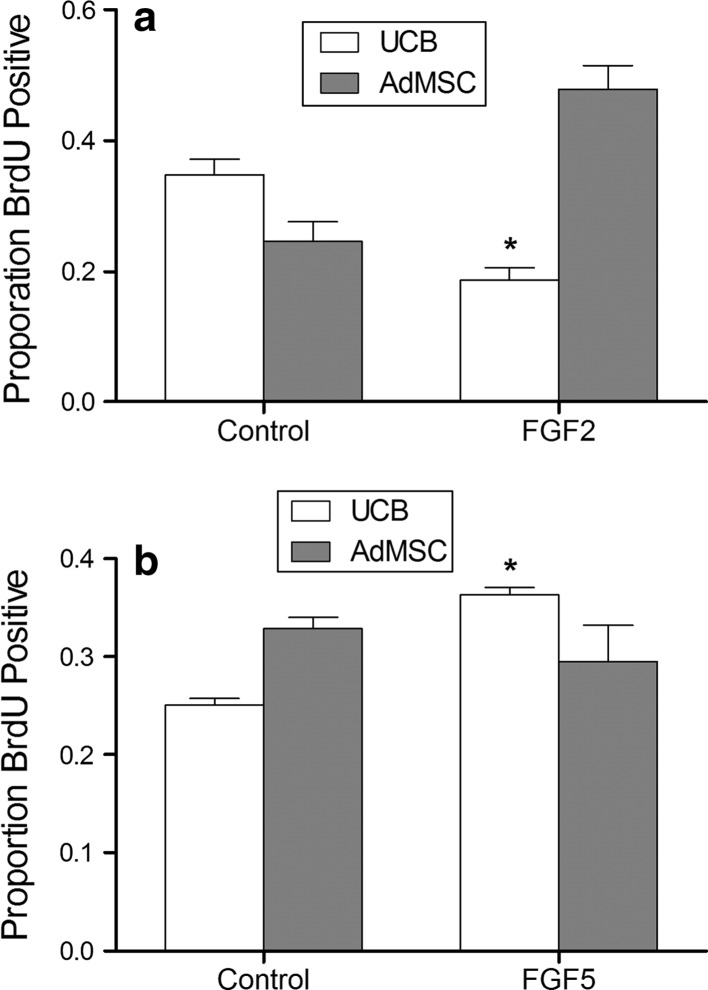
The effect of FGF2 and FGF5 on UCB and AdMSC mitosis was examined after 48 h in treatment media (Fig. 2). Similar to bone marrow derived MSCs, AdMSC showed a tendency of increased proliferation with culture in the presence of FGF2 (p = 0.06). By contrast, supplementation of FGF2 to UCB retarded BrdU incorporation (p < 0.01). Supplementation of AdMSC with FGF5 did not affect proliferation, contrasting with the increased proliferation of UCB (AdMSC Con vs. FGF5: p = 0.45; UCB Con vs. FGF5 p < 0.01). These results support divergent FGF-ERK signaling events in the two sources of mesenchymal stem cells.
Fig. 2.
Fibroblast growth factors stimulate proliferation of AdMSC and UCB stem cells. AdMSC and UCB stem cells were cultured in low serum media supplemented with 10 ng/ml FGF2 or FGF5 for 48 h. Cells were pulsed with BrdU for 2 h prior to fixation. FGF2 inhibited proliferation of UCB (a). FGF5 increased proliferation of UCB stem cells (b). * = p < 0.05
Substratum matrix affects AdMSC and UCB morphology
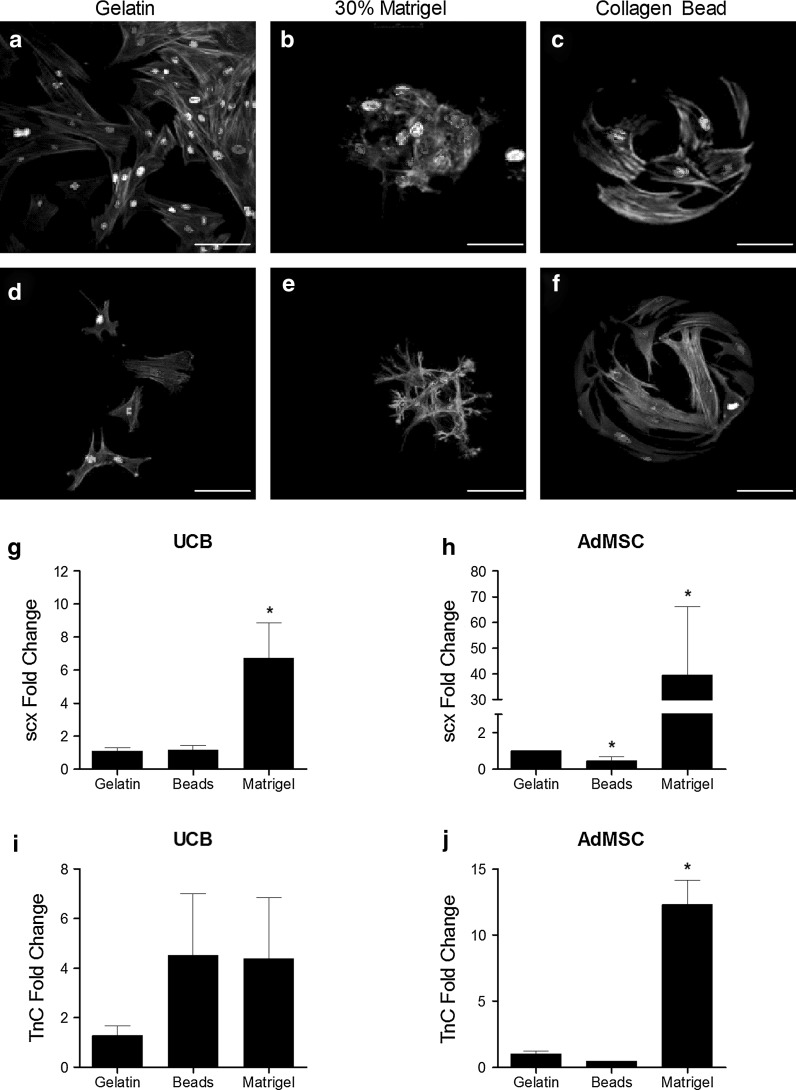
To investigate the effects of various extracellular protein matrices, UCB and AdMSC were cultivated on gelatin coated plasticware, collagen coated beads, or allowed to infiltrate into 30 % Matrigel for 48 h (Fig. 3a–f). MSCs cultivated on gelatin remained as a monolayer and exhibited morphology typical of fibroblasts with visible actin filaments. Incorporation into a 30 % Matrigel resulted in the formation of colonies with compact structure. Cells show fewer stress fibers but maintain filopodia that extend into the surrounding matrix. Culture on collagen beads results in cells that resemble those on gelatin, albeit with apparently smaller amounts of cytoplasmic volume. No differences in morphology were observed between AdMSCs or UCB MSCs.
Fig. 3.
Substrata affect UCB and AdMSC morphology and tenocytic gene expression. UCB (a–c) and AdMSC (d–f) were cultured on gelatin coated plasticware, 30 % Matrigel, or on collagen coated beads for 48 h prior to fixation and immunostaining with fluorescein conjugated phalloidin and Hoechst 33342 dye. AdMSC and UCB stem cells were cultured on gelatin coated plasticware, collagen coated beads, or 30 % matrigel for 48 h prior to RNA isolation and real-time PCR with gene-specific primers. Culture on matrigel increased scleraxis expression in UCB and AdMSC (g and h, respectively). Tenascin C mRNA was also increased by culture on matrigel in AdMSC (j) but not UCB (i). * p < 0.05 compared to gelatin. Scale bar = 50 μm
Culture in matrigel increases tenocyte gene expression
To evaluate the effects of different culture matrices on early tenocyte gene expression, AdMSC and UCB were cultured for 48 h on gelatin, collagen coated beads, or matrigel prior to RNA extraction. Real-time PCR revealed increases in scleraxis mRNA in both UCB and AdMSC maintained on matrigel (6.73 ± 2.12 and 39.48 ± 26.63 fold, respectively, p < 0.0001; Fig. 3g, h, respectively). Scleraxis transcripts were fewer in AdMSC grown on collagen-coated beads while scx mRNA remained unchanged in UCBs (p < 0.05). Culture on matrigel increased tenascin C expression 12.32 ± 1.85 fold in AdMSC (p < 0.0001; Fig. 3i, j). No increase in tenascin C occurred in UCB cultured on matrigel.
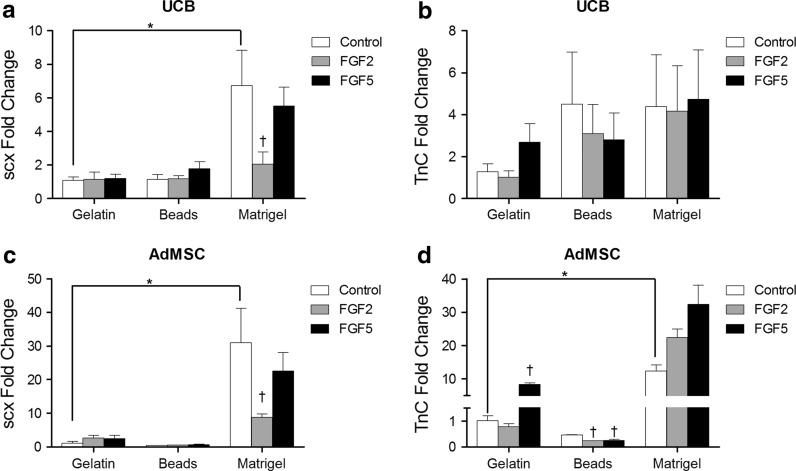
To determine the combined effects of ECM and FGFs on tenocytic gene expression, UCB and AdMSC were cultured on gelatin, collagen beads and matrigel and stimulated with FGF2 or FGF5 for 48 h. Real-time PCR was used to quantify changes in scleraxis and tenascin C expression. UCB cultured on matrigel express increased levels of scx (p < 0.0001; Fig. 4a), which was suppressed by supplementation with FGF2. Levels of TnC mRNA are unaffected by culture on different matrices or the inclusion of FGF2 or FGF5 (Fig. 4b). In AdMSC, scx expression was increased by culture in matrigel but decreased by culture on collagen beads (p < 0.0001; Fig. 4c). When cultured on gelatin, FGF5 supplementation increased TnC transcription in AdMSC (Fig. 4d; p < 0.05). Inclusion of FGF2 or FGF5 in the culture media of cells grown on collagen beads decreased TnC transcription. Culture on matrigel significantly increased TnC transcription when compared to cells culture on gelatin, however supplementation with FGF2 or FGF5 had no significant effect on transcript levels.
Fig. 4.
Culture conditions affect tenocyte gene expression in AdMSC and UCB. AdMSC and UCB stem cells were cultured on gelatin coated plasticware, collagen coated beads, or 30 % matrigel for 48 h in low serum media containing 10 ng/ml FGF2 or FGF5. Total RNA was isolated and subjected to real-time PCR with gene specific primers for scleraxis and tenascin C. Culture on matrigel increased expression of scleraxis in UCB stem cells, which was inhibited by supplementation with FGF2 (a). There was no effect of matrix or FGF supplementation on tenascin C expression in UCB (b). Scleraxis expression in AdMSC was increased by culture on matrigel which was inhibited by supplementation with FGF2 (c). FGF5 supplementation increased tenascin C mRNA expression in AdMSC cultured on gelatin plasticware (d). FGF2 and FGF5 supplementation decreased tenascin C mRNA expression in AdMSC when cultured on collagen coated beads. Matrigel increased tenascin C mRNA in AdMSC which was further increased by supplementation with FGF2 or FGF5. * p < 0.05 between untreated cells grown on different substrata; † p < 0.05 between treatments on cells grown on the same substrata
Discussion
Previously, we have shown that equine AdMSC express fewer stem cell markers and possess a more limited ability to differentiate compared to equine UCB (Reed and Johnson 2008). AdMSC proliferate more rapidly than UCB regardless of the surface substrate (Reed and Johnson 2012), suggesting a difference in regenerative capabilities as more plastic stem cells often have longer population doubling times than more differentiated cells (Stojkovic et al. 2004). Adipose derived mononuclear cells were capable of improving tendon architecture but not biomechanical properties in a collagenase induced lesion of the SDFT (Nixon et al. 2008). Transplantation of bone marrow derived stem cells into tendon lesions results in decreased lesion size and greater tendon density (Crovace et al. 2007; Pacini et al. 2007).
The beneficial effects of stem cells in tendon injury may be due to the population of cells expressing scleraxis. Bone marrow, adipose and umbilical cord blood derived stem cells express this transcription factor prior to any in vitro manipulation (Kuo and Tuan 2008, Fig. 3). In AdMSC and UCB, upregulation of scleraxis mRNA occurred in response to culture on matrigel. Culture in a three-dimensional gelatin environment also upregulated scx in BM-MSC (Kuo and Tuan 2008). Expression of scleraxis precedes that of tenascin C and collagen 1a2 in the developing embryo (Kardon 1998; Schweitzer et al. 2001). Overexpression of scleraxis increased the transcription of col1a2 in NIH-3T3 fibroblasts (Espira et al. 2009; Lejard et al. 2007). Scleraxis appears to bind to the proximal promoter region of col1a2 as a heterodimer with E47 (Lejard et al. 2007). These data suggest that the upregulation of scleraxis is consistent with the induction of an early tendon-like cell that expresses tenascin C and collagen1a2 as it matures. Scleraxis may, in fact, drive the expression of the more mature markers of tendon development.
Fibroblast growth factors are required for proper syndetome formation and induction of tenocytic lineage (Brent and Tabin 2004; Edom-Vovard et al. 2002). As such, the response of adipose and UCB derived stem cells to FGFs was investigated. While stimulation of AdMSC and UCB with FGF2 or FGF5 resulted in ERK1/2 activation in each cell type, opposing effects on proliferation and scleraxis expression were found. In UCB, scx expression was decreased by treatment with FGF2 when cultured on matrigel while supplementation with FGF5 had no effect irrespective of substratum. Stimulation with FGF2 or FGF5 had no effect on scleraxis expression in AdMSC when cultured on gelatin and in fact, reduced scx expression when cells were cultured in matrigel. The mitogenic effects of FGFs on adipose and UCB stem cells are also cell type specific. These differences are likely due to distinct cellular contexts and possibly different FGF receptor expression. While FGF2 can signal through a number of FGF receptor isoforms, FGF5 is more limited and can only signal through FGFR1c and FGFR2 (Reviewed in Clements et al. 1993; Eswarakumar et al. 2005). It is possible that the differences in response to the fibroblast growth factors may also occur because of variations in ERK1 and ERK2 ratios, priming the cells toward proliferation or differentiation.
The use of stem cells as a therapy for tendon lesions may be enhanced by the use of a delivery agent. Thus, we cultured UCB and AdMSC on collagen beads and in matrigel, both of which have the potential to retain the stem cell population at the site of injury. When cultured on matrigel, scx expression increases drastically in both UCB and AdMSC. Matrigel is composed of a mixture of growth factors (including IGF-1, PDGF, TGF-β, and EGF) and extracellular matrix components laminin, collagen IV and entactin, which form a complex three dimensional matrix at 37 °C. Entactin enables the binding of laminin to collagen IV and the formation of a complex structural matrix (Reviewed in Chung and Durkin 1990). Both AdMSC and UCB embedded into the matrigel and formed colonies with distinct cellular morphology compared to cells on gelatin or collagen coated beads. The differences in cell:cell contact or contact with a complex ECM may be responsible for the upregulation of tenocytic genes. Integrin signaling activated by changes in ECM can result in differentiation of a number of stem cell types. Culture of hES cells on laminin activated integrin signaling which led to an increase in ERK1/2 activation and subsequent decrease in Nanog and SSEA1 expression (Hayashi et al. 2007). The growth factors contained in the matrigel may also affect tenocytic gene expression in UCB and AdMSC. Indeed, adipose derived stem cells treated with a combination of IGF-1 and TGF-β1 produced a well-organized extracellular matrix reminiscent of that produced by cultured tenocytes (Schneider et al. 2011). Further, scleraxis, type I and III collagen, tenomodulin, and phosphorylation of ERK1/2 was increased following IGF-1/TGF-β1 treatment in these cells. The influence of these growth factors on tenocytic differentiation in UCB and AdMSC requires further investigation.
In conclusion, we have further delineated the differences between adipose and umbilical cord blood derived stem cells. While both cell types upregulate scleraxis expression in response to culture on matrigel, they respond very differently to stimulation with fibroblast growth factors. To promote differentiation into a tenocyte-like cell, the appropriate culture conditions for UCB appear to be culture on matrigel in the absence of any FGF supplementation. Culture of adipose derived stem cells on matrigel in culture medium supplemented with FGF2 promotes proliferation of an early tenocyte-like phenotype. Further work should clarify the mechanisms behind such changes.
Acknowledgments
This work was supported by a grant to S.E.J. from the Florida Pari-Mutuel Racing Trust Fund.
References
- Brent AE, Tabin CJ. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development. 2004;131:3885–3896. doi: 10.1242/dev.01275. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/S0092-8674(03)00268-X. [DOI] [PubMed] [Google Scholar]
- Castellani LW, Gargalovic P, Febbraio M, Charugundla S, Jien ML, Lusis AJ. Mechanisms mediating insulin resistance in transgenic mice overexpressing mouse apolipoprotein A-II. J Lipid Res. 2004;45:2377–2387. doi: 10.1194/jlr.M400345-JLR200. [DOI] [PubMed] [Google Scholar]
- Chung AE, Durkin ME. Entactin: structure and function. Am J Respir Cell Mol Biol. 1990;3:275–282. doi: 10.1165/ajrcmb/3.4.275. [DOI] [PubMed] [Google Scholar]
- Clase KL, Mitchell PJ, Ward PJ, Dorman CM, Johnson SE, Hannon K. FGF5 stimulates expansion of connective tissue fibroblasts and inhibits skeletal muscle development in the limb. Dev Dyn. 2000;219:368–380. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1056>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Clegg PD, Strassburg S, Smith RK. Cell phenotypic variation in normal and damaged tendons. Int J Exp Pathol. 2007;88:227–235. doi: 10.1111/j.1365-2613.2007.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements DA, Wang JK, Dionne CA, Goldfarb M. Activation of fibroblast growth factor (FGF) receptors by recombinant human FGF-5. Oncogene. 1993;8:1311–1316. [PubMed] [Google Scholar]
- Crovace A, Lacitignola L, De Siena R, Rossi G, Francioso E. Cell therapy for tendon repair in horses: an experimental study. Vet Res Commun. 2007;31:281–283. doi: 10.1007/s11259-007-0047-y. [DOI] [PubMed] [Google Scholar]
- Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–1110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F, Schuler B, Bonnin MA, Teillet MA, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev Biol. 2002;247:351–366. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- Espira L, Lamoureux L, Jones SC, Gerard RD, Dixon IM, Czubryt MP. The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. J Mol Cell Cardiol. 2009;47:188–195. doi: 10.1016/j.yjmcc.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Furue MK, Okamoto T, Ohnuma K, Myoishi Y, Fukuhara Y, Abe T, Sato JD, Hata R, Asashima M. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells. 2007;25:3005–3015. doi: 10.1634/stemcells.2007-0103. [DOI] [PubMed] [Google Scholar]
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019–4032. doi: 10.1242/dev.125.20.4019. [DOI] [PubMed] [Google Scholar]
- Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- Lejard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MH, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007;282:17665–17675. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cserjesi P, Nifuji A, Olson EN, Noda M. Sclerotome-related helix-loop-helix type transcription factor (scleraxis) mRNA is expressed in osteoblasts and its level is enhanced by type-beta transforming growth factor. J Endocrinol. 1996;151:491–499. doi: 10.1677/joe.0.1510491. [DOI] [PubMed] [Google Scholar]
- Longo UG, Ronga M, Maffulli N. Achilles tendinopathy. Sports Med Arthrosc. 2009;17:112–126. doi: 10.1097/JSA.0b013e3181a3d625. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Ramsey S. Expression of tenascin in joint-associated tissues during development and postnatal growth. J Anat. 1996;188:157–165. [PMC free article] [PubMed] [Google Scholar]
- Muir T, Sadler-Riggleman I, Skinner MK. Role of the basic helix-loop-helix transcription factor, scleraxis, in the regulation of Sertoli cell function and differentiation. Mol Endocrinol. 2005;19:2164–2174. doi: 10.1210/me.2004-0473. [DOI] [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am J Vet Res. 2008;69:928–937. doi: 10.2460/ajvr.69.7.928. [DOI] [PubMed] [Google Scholar]
- Pacini S, Spinabella S, Trombi L, Fazzi R, Galimberti S, Dini F, Carlucci F, Petrini M. Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 2007;13:2949–2955. doi: 10.1089/ten.2007.0108. [DOI] [PubMed] [Google Scholar]
- Perez AV, Perrine M, Brainard N, Vogel KG. Scleraxis (Scx) directs lacZ expression in tendon of transgenic mice. Mech Dev. 2003;120:1153–1163. doi: 10.1016/j.mod.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Pryce BA, Brent AE, Murchison ND, Tabin CJ, Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn. 2007;236:1677–1682. doi: 10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- Reed SA, Johnson SE. Equine umbilical cord blood contains a population of stem cells that express Oct4 and differentiate into mesodermal and endodermal cell types. J Cell Physiol. 2008;215:329–336. doi: 10.1002/jcp.21312. [DOI] [PubMed] [Google Scholar]
- Reed SA, Johnson SE. Refinement of culture conditions for maintenance of undifferentiated equine umbilical cord blood stem cells. J Equine Vet Sci. 2012;32:360–366. doi: 10.1016/j.jevs.2011.12.004. [DOI] [Google Scholar]
- Schneider PR, Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Three-dimensional high-density co-culture with primary tenocytes induces tenogenic differentiation in mesenchymal stem cells. J Orthop Res. 2011;29:1351–1360. doi: 10.1002/jor.21400. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Smith TG, Sweetman D, Patterson M, Keyse SM, Munsterberg A. Feedback interactions between MKP3 and ERK MAP kinase control scleraxis expression and the specification of rib progenitors in the developing chick somite. Development. 2005;132:1305–1314. doi: 10.1242/dev.01699. [DOI] [PubMed] [Google Scholar]
- Stojkovic M, Lako M, Strachan T, Murdoch A. Derivation, growth and applications of human embryonic stem cells. Reproduction. 2004;128:259–267. doi: 10.1530/rep.1.00243. [DOI] [PubMed] [Google Scholar]
- Swasdison S, Mayne R. Location of the integrin complex and extracellular matrix molecules at the chicken myotendinous junction. Cell Tissue Res. 1989;257:537–543. doi: 10.1007/BF00221463. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Smith RK, Clegg PD. Mesenchymal stem cell therapy in equine musculoskeletal disease: scientific fact or clinical fiction? Equine Vet J. 2007;39:172–180. doi: 10.2746/042516407X180868. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Vaughan-Thomas A, Clements DN, Pinchbeck G, Macrory LC, Smith RK, Clegg PD. Gene expression markers of tendon fibroblasts in normal and diseased tissue compared to monolayer and three dimensional culture systems. BMC Musculoskelet Disord. 2009;10:27. doi: 10.1186/1471-2474-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To WS, Midwood KS. Identification of novel and distinct binding sites within tenascin-C for soluble and fibrillar fibronectin. J Biol Chem. 2011;286:14881–14891. doi: 10.1074/jbc.M110.189019. [DOI] [PMC free article] [PubMed] [Google Scholar]