Abstract
Cadmium is a modern environmental contaminant that is toxic and carcinogenic. Glycyrrhiza glabra is a traditional medicinal herb which grows in the various parts of the World. Recent studies demonstrated that G. glabra has antifungal, antimicrobial, antioxidant, and powerful antiinflammatory features. The purpose of this study was to investigate the genetic safety of extracts from G. glabra and its effects on cadmium (as CdCl2) induced genotoxicity. Therefore we evaluated the capability of G. glabra extract to inhibit the rate of micronucleus (MN), sister chromatid exchange (SCE) formations induced by CdCl2. Moreover, to assess the effects of G. glabra on cell viability and oxidative status, we performed 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and total antioxidant capacity (TAC) assays. Our results showed that there were significant increases (P < 0.05) in both SCE and MN frequencies of cultures treated with CdCl2 (5 ppm) as compared to controls. However, co-application of G. glabra extract (5, 10 and 20 ppm) and CdCl2 resulted in decreases of MN and SCE rates as compared to the group treated with CdCl2 alone. Again, the results of MTT and TAC assays clearly indicated dose dependent ameliorative effects of G. glabra extracts against CdCl2 toxicity. In conclusion, this study demonstrated for the first time that G. glabra extracts provided increased resistance of DNA against CdCl2 induced genetic and oxidative damage in human lymphocytes. So, the risk on target tissues of CdCl2 could be reduced and ensured early recovery from its toxicity.
Keywords: Cadmium, Glycyrrhiza glabra, Genotoxicity, Human lymphocytes, Oxidative stress, Protective effect
Introduction
Glycyrrhiza glabra L. formerly known as licorice or sweetwood, native to the Mediterranean and certain areas of Asia, is a tall shrub of the Leguminosae family (Fenwick et al. 1990; Olukoga and Donaldson 1998). Roots of G. glabra have demulcent, antacid, anti-ulcer (Nadkarni 1998), anti-inflammatory, expectorant, tonic, diuretic, laxative, and sedative properties (Hikino 1985). They also possess antipyretic (Lata et al. 1999), antimicrobial, antiherpes (Ceremelli et al. 1996), and anxiolytic (Ambawade et al. 2001) activities. Glycyrrhizin, a triterpene saponin, possesses antiviral activity (Hirabayashi et al. 1991). Licorice (G. glabra) is one of the most important crude drugs in the world, and its major triterpene saponin, glycyrrhizin, is a well-known natural sweetener and pharmaceutical (Gibson 1978; Shibata 2000). In addition, extracted licorice, containing glycyrrhizin, is used as an additive for flavoring and sweetening tobacco, candies, chewing gum, toothpaste, and beverages all around the world (Baltina et al. 2003; Rauchensteiner et al. 2005). G.glabra and G. uralensis are known to be the major glycyrrhizin-producing species (Shibata 2000). The roots of licorice are rich sources of flavonoids, in particular, prenylated flavonoids, such as glabridin and glabrene. This plant has been long used to treat fevers, liver ailments, dyspepsia, constipation, gastric ulcers, sore throats, spasm, asthma, bronchitis, Addison’s disease and rheumatoid arthritis. Moreover, G. glabra is widely used in Indian Medicine (Schulz et al. 1998; Wang et al. 2000; Anon 2005).
Heavy metals are chemical elements that are commonly found in our environment, which are poisonous at high doses and even at fairly low concentrations cause human health problems. Cadmium (Cd) is one such highly toxic heavy metal known to have impact on human and animals (Loser 1980; Watanabe et al. 1986). Cd is a particularly dangerous pollutant due to its high toxicity and great solubility in water that can cause emphysema, anemia, osteoporosis, chronic rhinitis (Duxbury 1985; Jiang et al. 2001; Waisberg et al. 2003). According to the International Agency for Research on Cancer Cd is suspected as co-mutagen and carcinogen in human (IARC 1993). Moreover, exposures to high Cd concentration have been found to be carcinogenic, mutagenic and teratogenic for a large number of animal species (Degraeve 1981). Numerous experimental studies have shown the genotoxicity of Cd salts (Fojtova and Kovarik 2000; Seoane and Dulout 2001). Several studies, indicated that Cd damaged the nucleolar structure, DNA and RNA in both animal and plant cells (Misra et al. 1998; Jonak et al. 2004). Although Cd is a systemic poison known to affect many cell functions (Berti and Averbeck 2006), the cellular and molecular effects of a prolonged exposure to this metal are not completely understood.
Recently, extensive efforts were made to investigate therapeutic substances capable of reducing the genotoxicity of various natural and man-made mutagens in human life (Turkez et al. 2005; Turkez and Geyikoglu 2010b). Also, these include antibody, fatty acids, minerals and vitamins (Edenharder et al. 1999; Rao et al. 2001; Yoshida et al. 2010; Turkez et al. 2012a, b, c, d). Concomitant treatment with antioxidants provided protection against oxidative damage by mutagens in experimental animals (Abubakar et al. 2003; Esparza et al. 2003; Geyikoglu et al. 2005; Turkez and Geyikoglu 2010a). So far, antioxidants have attracted much interest with respect to their protective effect against free radical damage that may be the cause of many diseases including cancer (Shon et al. 2004). Since the complete avoidance of exposure to CdCl2 is very difficult, chemoprevention is an attractive strategy for protecting humans and animals from the risk of cancer caused by exposure to this heavy metal. Besides, many efforts are being made to investigate therapeutic substances capable of reducing the toxicity of man-made or natural mutagens in human life. Thus, this study investigated the efficacy of G. glabra extract against CdCl2-induced DNA and oxidative damages.
Materials and methods
Experimental design
Blood samples were obtained by veinpuncture from five healthy non-smoking donors. Human peripheral blood lymphocyte cultures were set up according to the protocol with slight modifications described by Evans and O’Riordan (1975). The heparinized blood (0.5 ml) was cultured in 6 ml culture medium (Chromosome Medium B, Biochrom®, Berlin, Germany) with 5 μg/l of phytohemagglutinin (Biochrom®). CdCl2 was purchased from Sigma (St. Louis, MO, USA; CAS No. 7790-78-5). G. glabra was purchased in ready package from local markets. For water extraction of G. glabra, 20 g roots was mixed with 400 ml distilled and boiling water using magnetic stirrer for 15 min. Then extract was filtered over Whatman No. 1 paper. Then, CdCl2 (5 ppm) and G. glabra extracts (5, 10 and 20 ppm) were added into culture tubes separately and together. After supplementation of CdCl2 and plant extracts, the blood samples were incubated for 72 h at 37 °C to adjust to body conditions. Each individual whole blood culture without CdCl2 or G. glabra extract was used as a control group. Table 1 shows the chemical composition of G. glabra samples according to the analysis of Khalaf et al. (2010) by using HPLC technique.
Table 1.
Chemical composition of G. Glabra extracts
| Compound | Amount (mg/l of extract) |
|---|---|
| Ferulic acid | 5.01 |
| p-coumaric acid | 2.17 |
| Gentisic acid | ND |
| Luteolin | 0.33 |
| Apigenin | 1.05 |
| Caffeic acid | ND |
| Sinapic acid | 2.03 |
ND not detected
Genotoxicity testing
SCE assay
With the aim of providing successive visualization of SCEs, 5-bromo-20-deoxyuridine (Sigma®) was added at culture initiation. The cultures were incubated in complete darkness for 72 h at 37 °C. Exactly 70 h and 30 min after beginning the incubations, demecolcine (N-Deacetyl-N-methylcolchicine, Sigma®) was added to the cultures. After hypotonic treatment (0.075 M KCl), followed by three repetitive cycles of fixation in methanol/acetic acid solution (3:1, v/v), centrifugation, and resuspension, the cell suspension was dropped onto chilled, grease-free microscopic slides, air-dried, aged for 3 days, and then differentially stained for the inspection of the SCE rate according to the fluorescence plus Giemsa (FPG) procedure. For each treatment condition, well-spread 25 s division metaphases containing 42–46 chromosomes in each cell were scored by one observed (E. Dirican), and the values obtained were calculated as SCEs per cell.
MN assay
The MN test was performed by adding cytochalasin B (Sigma®) after 44 h of culture. At the end of the 72 h incubation period, the lymphocytes were fixed with ice-cold methanol/acetic acid (1:1, v/v). The fixed cells were put directly on slides using a cytospin, and stained with Giemsa solution. All slides were coded before scoring. The criteria for scoring MN were as described by Fenech (1993). At least 1,000 binucleated lymphocytes were examined per concentration for the presence of one, two or more MN by one observer (E. Dirican).
TAC analysis
Automated total antioxidant capacity (TAC) assays were carried out in the culture medium by commercially available kits (Rel Assay Diagnostics ®, Gaziantep, Turkey) in plasma samples obtained from blood cultures for 2 h (Erel 2004).
MTT assay
Cytotoxicity was assessed by measuring the formation of formazan from 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) in a spectrophotometrically test, modified after Mosmann (1983). Blood cells were incubated with 0.7 mg/ml MTT for 24 h at 37 °C at the end of the experiment. After washing with PBS the blue formazan was extracted from cells with isopropanol/formic acid (95:5). Cytotoxicity was photometrically determined at 560 nm (Lewerenz et al. 2003).
Statistics
Statistical analysis was performed using SPSS Software (version 18.0, SPSS, Chicago, IL, USA). For statistical analysis of obtained data Duncan’s test was used. Statistical decisions were made with a significance level of 0.05.
Results
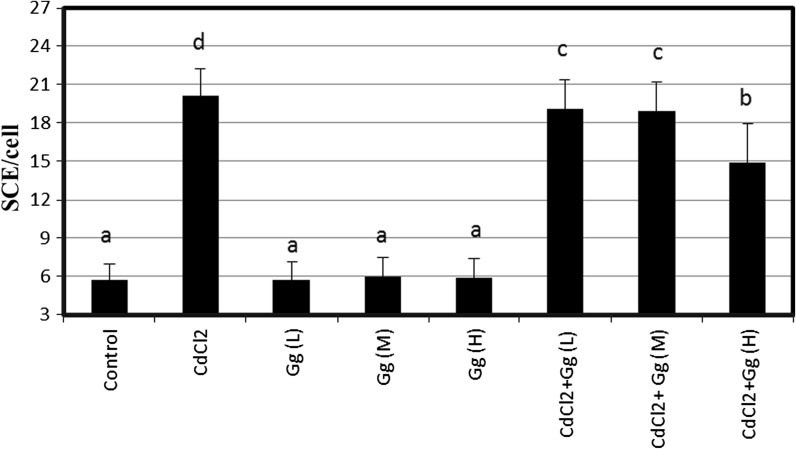
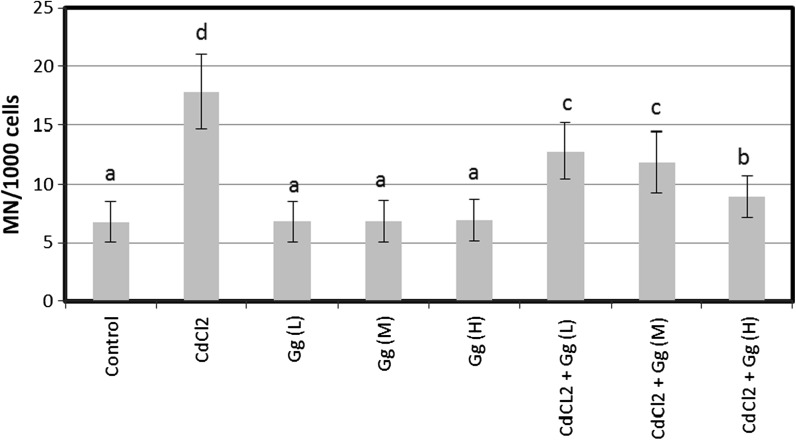
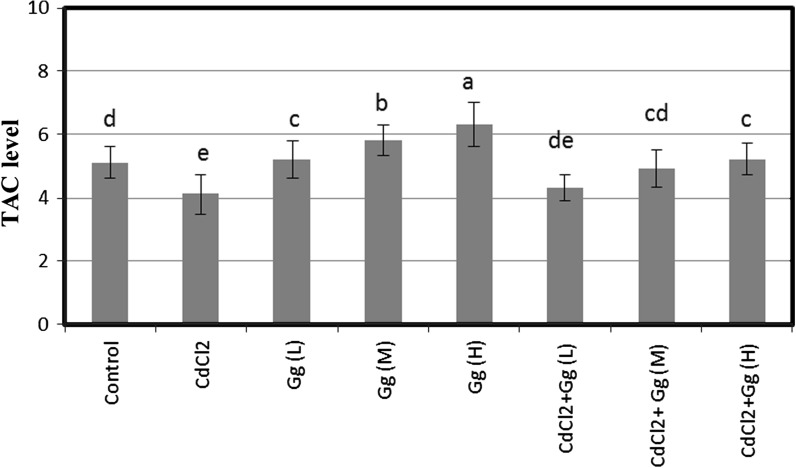
SCE and MN were analyzed in human peripheral lymphocytes treated for 72 h with different concentrations of CdCl2 in presence or absence of G. Glabra extract (Figs. 1, 2). The SCE and MN frequencies observed in cultures treated with CdCl2 (5 ppm) were significantly higher than control values. But, G. glabra extract did not lead to genetic damages at all tested concentrations. Moreover, it was successful against CdCl2-induced genotoxicity in blood cells at the applied dose. When CdCl2 treated cultures were treated with G. glabra extract, SCEs/cell and MN/1000 cell values decreased significantly as compared to CdCl2 treatment alone. Figure 3 shows the results of cytotoxicity measured by MTT assay. When assayed in vitro on human blood cells using the MTT assay, the cell viability was found to be lower in CdCl2 -treated cultures as compared to control group. However, the three doses of G. glabra extract (5, 10 and 20 ppm) improved cellular in vitro activities with respect to the cytotoxicity of CdCl2. Figure 4 shows the effect of G. glabra extract and CdCl2 on TAC levels in human whole blood cultures. As shown in Fig. 4, the TAC value decreased in comparison to the sole addition of CdCl2. In contrast G. glabra extract increased the TAC level when applied alone to the cultures. Moreover, G. glabra extract had dose dependent inhibitory effects on oxidative damage caused by CdCl2 in human blood cells.
Fig. 1.
Effect of G. glabra water extracts on cadmium chloride induced SCE formations in human peripheral lymphocytes. Values are expressed as means of five cultures in each group; means in the figure followed by the different letters present significant differences at the P < 0.05 level; (CdCl2: Cadmium chloride; Gg (L): 5 ppm G. glabra extract; Gg (M): 10 ppm G. glabra extract; Gg (H): 20 ppm G. glabra extract)
Fig. 2.
The frequencies of MNs (%) in human lymphocytes treated with different concentrations of CdCl2 and Gg. Abbreviations are as in Fig. 1
Fig. 3.
3-(4,5-Dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction in human blood cultures maintained for 24 h in the presence of CdCl2, Gg and their combinations. Abbreviations are as in Fig. 1
Fig. 4.
Extracellular total antioxidant capacity (TAC) levels (expressed as mmol Trolox Equiv./L) in human blood cells maintained for 2 h in the presence of CdCl2, Gg and their combinations. Abbreviations are as in Fig. 1
Discussion
Heavy metals accumulate in the soft tissues, resulting in stress conditions, manifested by lower energy levels and damage to blood composition, kidney, lungs, liver and other vital organs. Cd compounds inhibit the repair of DNA damaged by other agents, thereby enhancing their genotoxicity (Rossman et al. 1992). Cd as the chloride salt is immediately bound to plasma proteins and subsequently accumulates primarily in liver and in other tissues; these processes do not exclude the possibility that after some time Cd accumulation was a factor in the induction of DNA damage observed in kidney (Foulkes 1994). In accordance with previous data we determined increased SCE and MN rates after exposure to CdCl2 in cultured human lymphocytes. The cytotoxicity as well as genotoxicity of CdCl2 is well studied. It appears that its toxicity is mainly due to oxidative deterioration of biomolecules including DNA, proteins and lipids (Fotakis et al. 2005; Turkez 2011; Turkez et al. 2012b). Moreover, according to our results CdCl2 caused oxidative stress in human blood lymphocytes, because decreased TAC levels were found in cultures only treated with CdCl2 as compared to the control group. In addition, cytotoxicity, the degree to which a chemical can cause cell damage, is assessed in this study by the means of MTT assay. As shown in Fig. 3, the MTT assay results revealed that CdCl2 was cytotoxic to human blood cells. Overall, CdCl2 caused significant decreases of % viability of blood cells. Consistent with our finding, MTT assay demonstrated that the viabilities of hepatocyte were significantly decreased after CdCl2 treatment (Wang et al. 2007).
CdCl2 promotes formation of ROS such as hydrogen peroxide, which may cause cell membrane damage and DNA strand breaks (Watjen and Beyersmann 2004). Cells are normally able to defend themselves against ROS damage through the use of enzymes such as GSH, GST, SOD and catalase. CdCl2 has high affinities for glutathione (GSH), which is the primary intracellular antioxidant and conjugating agent (Kidd 1997). Under normal conditions, the inherent defense system, including glutathione and the antioxidant enzymes, protects against oxidative damage. Besides, enhanced production of free radicals and inhibition of antioxidant enzymes have been suggested as possible mechanisms to explain CdCl2-induced oxidative damages (Nzengue et al. 2008). ROS impairs cell membrane stability and causes cell death by lipid peroxidation (Sun 1990). Cd was found to decrease the mitotic index (MI) and also induced chromosomal abberations and micronucleus (MN) formation in plant root cells (Zhang and Yang 1994; Zhang and Xiao 1998). In several studies, it is indicated that Cd damaged the nucleolar structure, DNA and RNA in both animal and plant cells (Misra et al. 1998; Jonak et al. 2004). Some researchers reported that the Cd salts are not directly genotoxic in rodent cell lines. According to the International Agency for Research on Cancer Cd is suspected as co-mutagen in human carcinogenesis (IARC 1993). Also, Cd, a potent immunotoxic metal, induces DNA strand breaks, sister chromatid exchanges and chromosomal aberrations in human cells; on the other hand, lead is considered a potential mutagen by inducing direct DNA damage, clastogenicity and inhibition of DNA synthesis or interfering with DNA repair (Donma and Donma 2005).
Our results clearly reveal that G. glabra extract exhibits antigenotoxic properties at concentrations of 5, 10 and 20 ppm. Moreover, we suggest that G. glabra extract can be a new source of therapeutics against oxidative DNA damages as recognized in this study. G. glabra extract is beneficial in cases of CdCl2 to inhibit blood cell damage by correcting the disturbance of oxidant/antioxidant balance system and ensures early recovery from CdCl2 toxicity. Therefore, G. glabra extract decreases incidences of DNA damages. The root extract of G. glabra has antioxidant activity reportedly due to the presence of a variety of phenolic compounds including flavonoids, isoflavonoids, chalcones, and bibenzyls (Fuhrman et al. 1997). In addition, antioxidants (both enzymatic and non-enzymatic) play a central role in cellular oxidant defense systems that protect cells against damage induced by free radicals, such as superoxide anion and hydrogen peroxide (H2O2) (Cerutti et al. 1994; Valko et al. 2005; Turkez and Geyikoglu 2010a).
Many chemical studies revealed that licorice roots contain many saponins and flavonoids, along with glycyrrhizin (GL) (Nomura and Fukai 1998; Shibata 2000). In addition, many species-specific flavonoids, such as glabridin (GB) for G. glabra and glycycoumarin (GC) for G. uralensis, were detected in the underground parts of the respective Glycyrrhiza species (Zeng et al. 1991; Nomura and Fukai 1998). Variation in flavonoid contents in leaves of G. glabra was also reported (Hayashi et al. 1996; Shibano et al. 1996). Pinocembrin (PN) and licoflavanone (LF) were also isolated from the leaves of G. glabra (Fukui et al. 1988). The primary active constituent of Glycyrrhiza, as it relates to hepatic disorders, is the triterpene glycoside glycyrrhizin (also known as glycyrrhizic acid or glycyrrhetinic acid). Other constituents of Glycyrrhiza include flavonoids (liquiritin and isoliquiritin), isoflavonoids (isoflavonol, kumatakenin, licoricone, and glabrol), chalcones, coumarins (umbelliferone, herniarin), triterpenoids, and phytosterols. Besides, it is known that licorice contains bioactive polyphenols. G. glabra is a major component of many antihepatotoxic polyherbal formulations (Rajesh et al. 2000). Isoflavan derivatives glabridin, hisplaglabridin A, hisplaglabridin B and 4′- O-methyl glabridin have been isolated from G. glabra. These chemicals were reported to provide protection against oxidative stress (Haraguchi et al. 2000).
In parallel to our present findings, Lee et al. (2007) reported that the association between oxidative stress and the inflammatory responses in the hepatoprotective effect of glycyrrhizin against CCl4-induced hepatotoxicity. Chandrasekaran et al. (2011) reported that G. glabra is not mutagenic in a battery of genotoxicity tests. In search for lead molecules in cancer chemoprevention from natural products, a fraction ‘Rlicca’ isolated from G. glabra was studied for modulatory effects against hydrogen peroxide and 4-nitroquinoline-N-oxide induced genotoxicity in Escherichia coli PQ37 using the SOS chromotest and in human peripheral blood lymphocytes using the Comet assay (Kaur et al. 2009). The traditional medicinal properties of Glycyrrhiza include demulcent, expectorant, antitussive, and mild laxative activity (Leung 1980). Recent studies have brought to light the ability of Glycyrrhiza to enhance the detoxification of medications and toxins. In a Russian study, hepatotoxicity reactions in patients being treated for tuberculosis were significantly reduced in patients who received herbal liver support, including a combination of Glycyrrhiza, nettle (Urtica), tansy (Tanacetum), and mint (Mentha) (Galitskií et al. 1997).
In conclusion, the findings of this research clearly indicated that G. glabra extract modulated CdCl2-induced genetic damage in human blood cultures due to its antioxidant and detoxifying nature. Again, G. glabra can be a new resource of therapeutics against oxidative DNA damages as recognized in this study.
References
- Abubakar MG, Taylor A, Ferns GAA. Aluminium administration is associated with enhanced hepatic oxidant stress that may be offset by dietary vitamin E in the rat. Int J Exp Pathol. 2003;84:49–54. doi: 10.1046/j.1365-2613.2003.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambawade SD, Kasture VS, Kasture SB. Anxiolytic activity of Glycyrrhiza glabra L. J Nat Rem. 2001;2:130–134. [Google Scholar]
- Anon (2005) Glycyrrhiza glabra. Monograph. Altern Med Rev 10: 230–237 [PubMed]
- Baltina LA, Flekhter OB, Nigmatullina LR, Boreko EI, Pavlova NI, Nikolaeva SN, Savinova OV, Tolstikov GA. Lupane triterpenes and derivatives with antiviral activity. Bioorg Med Chem Lett. 2003;13:3549–3552. doi: 10.1016/S0960-894X(03)00714-5. [DOI] [PubMed] [Google Scholar]
- Berti G, Averbeck D. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review) Biochimie. 2006;88:1549–1559. doi: 10.1016/j.biochi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Ceremelli C, Portolani M, Cotombari B, Castelli M, Baggio G, Galatulas I, et al. Activity of glycyrrhizin and its diasterioisomers against two new human herpes virus: HHV-6 and HHV-7. Phyto Res. 1996;10:527–528. [Google Scholar]
- Cerutti P, Ghosh R, Oya Y, Amstad P. The role of the cellular antioxidant defense in oxidant carcinogenesis. Environ Health Perspect. 1994;102:123–129. doi: 10.1289/ehp.94102s10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran CV, Sundarajan K, Gupta A, Srikanth HS, Edwin J, Agarwal A. Evaluation of the genotoxic potential of standardized extract of Glycyrrhiza glabra (GutGard™) Regul Toxicol Pharmacol. 2011;61:373–380. doi: 10.1016/j.yrtph.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Degraeve N. Carcinogenic, teratogenic and mutagenic effects of cadmium. Mutat Res. 1981;86:115–135. doi: 10.1016/0165-1110(81)90035-X. [DOI] [PubMed] [Google Scholar]
- Donma O, Donma M. Cadmium, lead and phytochemicals. Med Hypotheses. 2005;65:699–702. doi: 10.1016/j.mehy.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Duxbury T. Ecological aspects of heavy metal responses in microorganisms. Adv Microb Ecol. 1985;8:185–235. [Google Scholar]
- Edenharder R, Worf-Wandelburg A, Decker M, Platt KL. Antimutagenic effects and possible mechanisms of action of vitamins and related compounds against genotoxic heterocyclic amines from cooked food. Mutat Res. 1999;444:235–248. doi: 10.1016/S1383-5718(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Esparza L, Gomez M, Romeu M, Mulero M, Sanchez DJ, Mallol J, Domingo JL. Aluminum-induced pro-oxidant effects in rats: protective role of exogenous melatonin. J Pineal Res. 2003;35:32–39. doi: 10.1034/j.1600-079X.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- Evans HJ, O’Riordan ML. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat Res. 1975;31:135–148. doi: 10.1016/0165-1161(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Fenech M. The cytokinesis blocks micronucleus technique: a detailed description on the method and its application to genotoxicity studies in human population. Mutat Res. 1993;285:35–44. doi: 10.1016/0027-5107(93)90049-L. [DOI] [PubMed] [Google Scholar]
- Fenwick GR, Lutomski J, Nieman C. Liquorice, Glycyrrhiza glabra L. Composition, uses and analysis. Food Chem. 1990;38:119–143. doi: 10.1016/0308-8146(90)90159-2. [DOI] [Google Scholar]
- Fojtova M, Kovarik A. Genotoxic effect of cadmium is associated with apoptotic changes in tobacco cells. Plant Cell Environ. 2000;23:531–537. doi: 10.1046/j.1365-3040.2000.00573.x. [DOI] [Google Scholar]
- Fotakis G, Cemeli E, Anderson D, Timbrell JA. Cadmium chloride-induced DNA and lysosomal damage in a hepatoma cell line. Toxicol In Vitro. 2005;19:481–489. doi: 10.1016/j.tiv.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Foulkes EC. Epithelial transport pf heavy metals. Adv Comp Eviron Physiol. 1994;20:55–84. doi: 10.1007/978-3-642-78598-6_2. [DOI] [Google Scholar]
- Fuhrman B, Buch S, Vaya J, Belinky PA, Coleman R, Hayek T, Aviram M. Licorice extract and its major polyphenol glabridin protect low-density lipoprotein against lipid peroxidation: in vitro and ex vivo studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 1997;66:267–275. doi: 10.1093/ajcn/66.2.267. [DOI] [PubMed] [Google Scholar]
- Fukui H, Goto K, Tabata M. Two antimicrobial flavanones from the leaves of Glycyrrhiza glabra. Chem Pharm Bull. 1988;36:4174–4176. doi: 10.1248/cpb.36.4174. [DOI] [PubMed] [Google Scholar]
- Galitskií LA, Barnaulov OD, Zaretskií BV, Malkov MI, Konenkov SI, Gol’m NP, Tomakov VS, Ogarkov PI, Batskov SS. Effect of phytotherapy on the prevention and elimination of hepatotoxic responses in patients with pulmonary tuberculosis, carriers of hepatitis B virus markers. Probl Tuberk. 1997;4:35–38. [PubMed] [Google Scholar]
- Geyikoglu F, Turkez H, Keles MS. The role of fruit juices in the prevention of aluminum sulphate toxicity in human blood in vitro. Fres Environ Bull. 2005;14:878–883. [Google Scholar]
- Gibson MR. Glycyrrhiza in old and new perspectives. Lloydia. 1978;41:348–354. [PubMed] [Google Scholar]
- Haraguchi H, Yoshida N, Ishikawa H, Tamura Y, Mizutani K, Kinoshita T. Protection of mitochondrial functions against oxidative stresses by isoflavans from Glycyrrhiza glabra. J Pharm Pharmacol. 2000;52:219–223. doi: 10.1211/0022357001773724. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Yasuma M, Hiraoka N, Ikeshiro Y, Yamamoto H, Yesilada E, Sezik E, Honda G, Tabata M. Flavonoid variation in the leaves of Glycyrrhiza glabra. Phytochemistry. 1996;42:701–704. doi: 10.1016/0031-9422(96)89776-7. [DOI] [Google Scholar]
- Hikino H (1985). Recent Research on Oriental Medicinal Plants. In: Wagner H, Hikino H, and Farnsworth NR, editors. Economic and Medicinal Plant Research. London: Academic Press. pp 53–58
- Hirabayashi K, Iwata S, Matsumoto H, Mori T, Shibata S, Baba M, et al. Antiviral activity of glycyrrhizin and its modified compounds against human immunodeficiency virus type 1 and herpes simplex type 1 in vitro. Chem Pharm Bull. 1991;39:112–115. doi: 10.1248/cpb.39.112. [DOI] [PubMed] [Google Scholar]
- IARC (1993) Cadmium and cadmium compounds (group 1). In: International agency for research on cancer monographs on the evaluation of carcinogenic risks to humans, vol 58. Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. Summary of data reported and evalution, Lyon, pp 119–237 [PMC free article] [PubMed]
- Jiang W, Liu D, Hou W. Hyperaccumulation of cadmium by roots, bulbs and shoots of garlic (Allium sativum L.) Bioresour Technol. 2001;76:9–13. doi: 10.1016/S0960-8524(00)00086-9. [DOI] [PubMed] [Google Scholar]
- Jonak C, Nakagami H, Hirt H. Heavy metal stress. Activation of distinct mitogen- activated protein kinase pathways by copper and cadmium. Plant Physiol. 2004;136:3276–3283. doi: 10.1104/pp.104.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Kaur S, Kumar N, Singh B, Kumar S. Evaluation of antigenotoxic activity of isoliquiritin apioside from Glycyrrhiza glabra L. Toxicol In Vitro. 2009;23:680–686. doi: 10.1016/j.tiv.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Khalaf I, Vlase L, Lazar D, Corciova A, Ivanescu B, Lazar MI. Hplc-uv-ms study of polyphenolsfrom Glycyrrhıza glabra. Farmacia. 2010;58:416–421. [Google Scholar]
- Kidd P. Glutathione: systemic protectant against oxidative and free radical damage. Altern Med Rev. 1997;2:155–176. [Google Scholar]
- Lata S, Saxena RS, Kumar A, Kakkar S, Srivastava VK, Saxena KK. Comparative antipyretic activity of Ocimum sanctum, Glycyrrhiza glabra and aspirin in experimentally induced pyrexia in rats. Indian J Pharmacol. 1999;31:71–75. [Google Scholar]
- Lee CH, Park SW, Kım YS, Kang SS, Kım JA, Lee SH, Lee SM. Protective mechanism of glycyrrhizin on acute liver injury induced by carbon tetrachloride in mice. Biol Pharm Bull. 2007;30:1898–1904. doi: 10.1248/bpb.30.1898. [DOI] [PubMed] [Google Scholar]
- Leung A. Encyclopedia of common natural ingredients used in food drugs and cosmetics. New York: Wiley; 1980. pp. 220–223. [Google Scholar]
- Lewerenz V, Hanelt S, Nastevska C, El-Bahay C, Ro¨hrdanz E, Kahl R. Antioxidants protect primary rat hepatocyte cultures against acetaminophen-induced DNA strand breaks but not against acetaminophen-induced cytotoxicity. Toxicol. 2003;191:179–187. doi: 10.1016/S0300-483X(03)00256-7. [DOI] [PubMed] [Google Scholar]
- Loser E. A 2 year oral carcinogenicity study with cadmium on rats. Cancer Lett. 1980;9:191–198. doi: 10.1016/0304-3835(80)90086-5. [DOI] [PubMed] [Google Scholar]
- Misra RR, Smith GT, Waalkes MP. Evaluation or the direct genotoxic potential of cadmium in four different rodent cell lines. Toxicol. 1998;126:103–114. doi: 10.1016/S0300-483X(98)00003-1. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nadkarni AK. Indian materia medica. Bombay: Popular Prakashan; 1998. [Google Scholar]
- Nomura T, Fukai T (1998) Progress in the Chemistry of Organic Natural Products. In Herz W, Kirby GW, Moore RE, Steglich W, Tamm C, (eds) Springer, Wien-New York, 73:1–140
- Nzengue Y, Steiman R, Garrel C, Lefèbvre E, Guiraud P. Oxidative stress and DNA damage induced by cadmium in the human keratinocyte HaCaT cell line: role of glutathione in the resistance to cadmium. Toxicol. 2008;243:193–206. doi: 10.1016/j.tox.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Olukoga A, Donaldson D. Historical perspectives on health. The history of liquorice: the plant, its extract, cultivation, commercialisation and etymology. J R Soc Health. 1998;118:300–304. doi: 10.1177/146642409811800517. [DOI] [PubMed] [Google Scholar]
- Rajesh MG, Paul Beena, Latha MS. Efficacy of Kamilari in alcoholic liver cirrhosis. Antiseptic. 2000;97:320–321. [Google Scholar]
- Rao MV, Chinoy NJ, Suthar MB, Rajvanshi MI. Role of ascorbic acid on mercuric chloride-induced genotoxicity in human blood cultures. Toxicol In Vitro. 2001;15:649–654. doi: 10.1016/S0887-2333(01)00081-9. [DOI] [PubMed] [Google Scholar]
- Rauchensteiner F, Matsumura Y, Yamamoto Y, Yamaji S, Tani T. Analysis and comparison of Radix glycyrrhizae (licorice) from Europe and China by capillary- zone electrophoresis (CZE) J Pharm Biomed Anal. 2005;38:594–600. doi: 10.1016/j.jpba.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Roy NK, Lin WC (1992) Is cadmium genotoxic? In: GF Nordberg, Alessio L, Herber RFM (eds.) Cadmium in the human environment: toxicity and carcinogenicity. Lyon: IARC Scientific Publication No. 118, pp 367–373 [PubMed]
- Schulz V, Hänsel R, Tyler VE (1998) Rational Phytotherapy. A Physicians’ Guide to Herbal Medicine. Springer-Verlag: Berlin pp 160–187
- Seoane AI, Dulout FN. Gentoxicity ability of cadmium, chromium and nickel salts studied by kinetochore staining in the cytogenesisblocked micronucleus assay. Mutat Res. 2001;490:99–106. doi: 10.1016/S1383-5718(00)00145-5. [DOI] [PubMed] [Google Scholar]
- Shibano M, Matsumoto Y, Kusano G, Shibata T. Nat Med. 1996;50:273–283. [Google Scholar]
- Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology licorice. Yakugaku Zasshi. 2000;120:849–862. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- Shon MY, Choi SD, Kahng GG, Nam SH, Sung NJ. Antimutagenic, antioxidant and free radical scavenging activity of ethyl acetate extracts from white, yellow and red onions. Food Chem Toxicol. 2004;42:659–666. doi: 10.1016/j.fct.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8:583–599. doi: 10.1016/0891-5849(90)90156-D. [DOI] [PubMed] [Google Scholar]
- Turkez H. The role of ascorbic acid on titanium dioxide-induced genetic damage assessed by the comet assay and cytogenetic tests. Exp Toxicol Pathol. 2011;63:453–457. doi: 10.1016/j.etp.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F. The anti-genotoxic effect of taurine on aluminum sulphate induced DNA damage in human peripheral lymphocytes. IUFS J Biol. 2010;69:25–32. [Google Scholar]
- Turkez H, Geyikoglu F. Boric acid: a potential chemoprotective agent against aflatoxin b(1) toxicity in human blood. Cytotechnology. 2010;62:157–165. doi: 10.1007/s10616-010-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Keles MS. Biochemical response to colloidal bismuth subcitrate: dose-time effect. Biol Trace Elem Res. 2005;105:151–158. doi: 10.1385/BTER:105:1-3:151. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Mokhtar YI, Togar B. Eicosapentaenoic acid protects against 2,3,7,8-tetrachlorodibenzop-dioxin-induced hepatic toxicity in cultured rat hepatocytes. Cytotechnology. 2012;64:15–25. doi: 10.1007/s10616-011-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Tatar A, Keles MS, Kaplan I. The effects of some boron compounds against heavy metal toxicity in human blood. Exp Toxicol Pathol. 2012;64:93–101. doi: 10.1016/j.etp.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoğlu F, Dirican E, Tatar A (2012c) In vitro studies on chemoprotective effect of borax against aflatoxin B1-induced genetic damage in human lymphocytes. Cytotechnology 64:607–612 [DOI] [PMC free article] [PubMed]
- Turkez H, Geyikoglu F, Yousef MI, Celik K, Bakir TO (2012d) Ameliorative effect of supplementation with L: -glutamine on oxidative stress, DNA damage, cell viability and hepatotoxicity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat hepatocyte culture. Cytotechnology 64:687–699 [DOI] [PMC free article] [PubMed]
- Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Waisberg M, Joseph P, Hale B, Beyermann D. Molecular and celular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/S0300-483X(03)00305-6. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Athar M, Bickers DR. Licorice in foods and herbal drugs: chemistry, pharmacology, toxicology and uses. In: Mazza G, Oomah BD, editors. Herbs, Botanicals & Teas. Lancaster: Technomic Publishing Co. Inc; 2000. pp. 321–353. [Google Scholar]
- Wang SS, Chen L, Xıa SK. Cadmium is acutely toxic for murine hepatocytes: effects on intracellular free Ca 2 + homeostasis. Physiol Res. 2007;56:193–201. doi: 10.33549/physiolres.930954. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Shiroishi K, Nishino H, Shinmura T, Murase H, Shoji T, Naruse Y, Kagamimori S. An experimental study on the longterm effect of cadmium in mice fed cadmium-polluted rice with special reference to the effect of repeated reproductive cycles. Environ Res. 1986;40:25–46. doi: 10.1016/S0013-9351(86)80079-2. [DOI] [PubMed] [Google Scholar]
- Watjen W, Beyersmann D. Cadmium-induced apoptosis in C6 glioma cells: influence of oxidative stress. Biometals. 2004;17:65–78. doi: 10.1023/A:1024405119018. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Watanabe K, Takahashi S, Ichikawa K. Protective effects of HFE7A, mouse anti-human/mouse Fas monoclonal antibody against acute and lethal hepatic injury induced by Jo2. Cytotechnology. 2010;62:313–323. doi: 10.1007/s10616-009-9244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Lou ZC, Zhang RY. Quality evaluation of Chinese licorice. Yao Xue Xue Bao. 1991;26:788–793. [PubMed] [Google Scholar]
- Zhang Y, Xiao H. Antagonistic effect of calcium, zinc and selenium against cadmium induced chromosomal aberrations and micronuclei in root cells of Hordeum vulgare. Mutat Res. 1998;420:1–6. doi: 10.1016/S1383-5718(98)00133-8. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Yang XL. The toxic effects of cadmium on cell division and chromosomal morphology of Hordeum vulgare. Mutat Res. 1994;312:121–126. doi: 10.1016/0165-1161(94)90016-7. [DOI] [PubMed] [Google Scholar]