Abstract
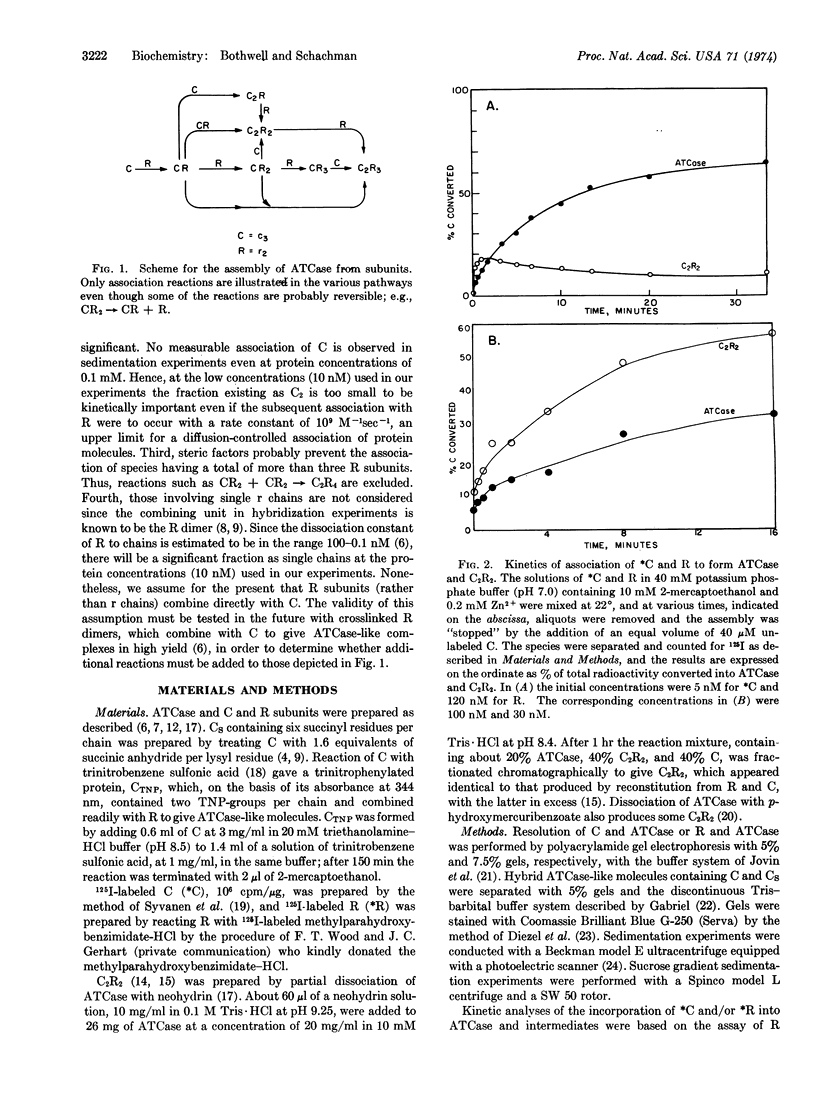
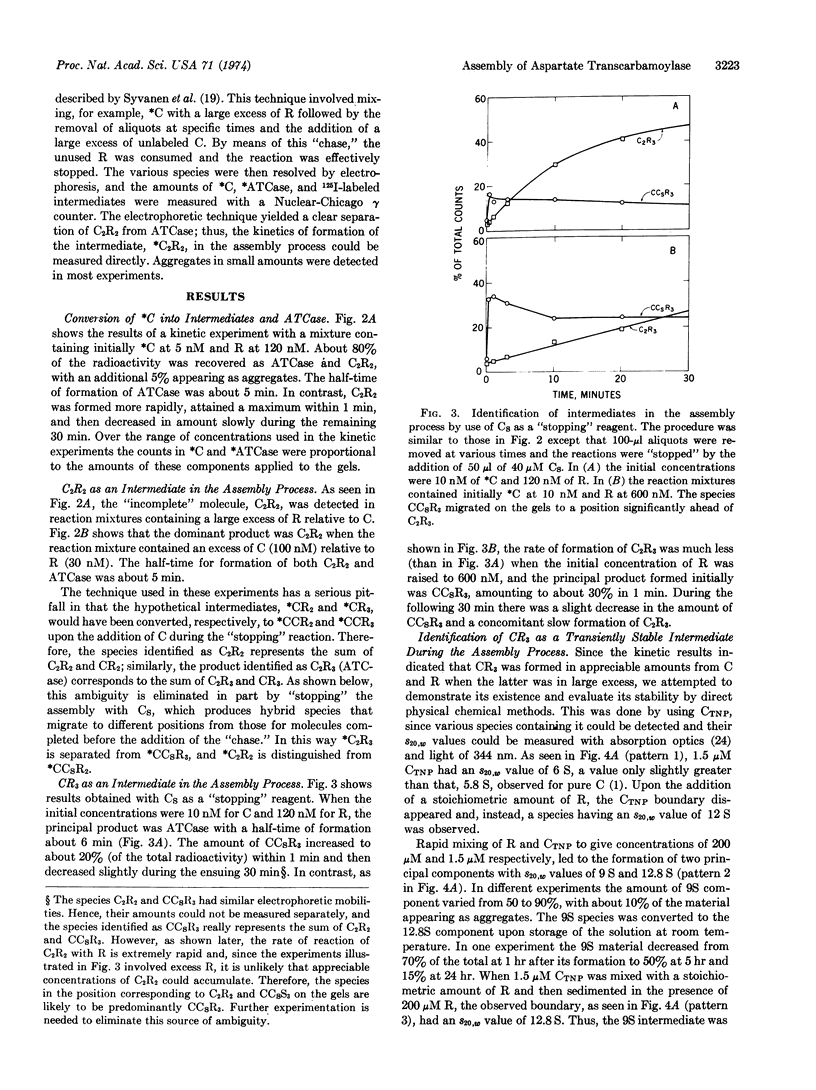
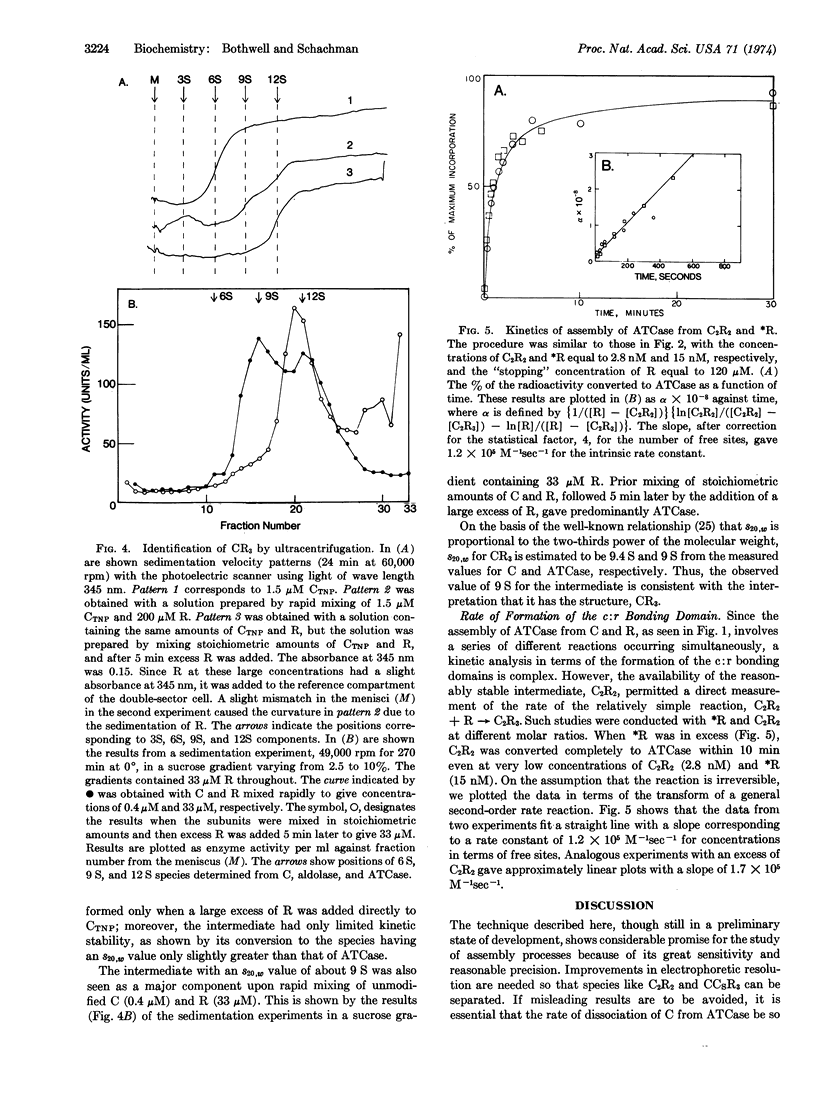
A scheme for the assembly of aspartate transcarbamoylase (EC 2.1.3.2; carbamoylphosphate:L-aspartate carbamoyltransferase) from catalytic and regulatory subunits is presented along with a technique for detecting intermediates and measuring the kinetics of assembly at protein concentrations of about 10 nM. 125I-labeled subunits (106 cpm/μg) were used, and the reaction was “topped”at specific times with an appropriate “chase” followed by electrophoretic separation and measurement of the amounts of the various species. Two intermediates were identified. A stable enzyme complex lacking one regulatory subunit is the principal product when catalytic subunits are in excess. A transiently stable complex lacking one catalytic subunit is the principal species when regulatory subunits are in excess. Measurements with mixtures of the purified regulatory-deficient molecules and free regulatory subunits gave a second-order rate constant of 105 105 M-1 sec-1 for the formation of bonding domains between catalytic and regulatory chains. No data for the rate of rupture of these bonds are available, but the kinetics of the assembly of the enzyme in vitro can be accounted for if this value is about 10-2 sec-1. Assembly from subunits occurs in seconds at concentrations equivalent to those that would exist in vitro even if there were only enzyme molecule per cell.
Keywords: protein interactions, bonding domains, allosteric enzymes, kinetics
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan W. W., Mort J. S. A complex between the catalytic and regulatory subunits of aspartate transcarbamylase. J Biol Chem. 1973 Nov 10;248(21):7614–7616. [PubMed] [Google Scholar]
- Cohlberg J. A., Pigiet V. P., Jr, Schachman H. K. Structure and arrangement of the regulatory subunits in aspartate transcarbamylase. Biochemistry. 1972 Aug 29;11(18):3396–3411. doi: 10.1021/bi00768a013. [DOI] [PubMed] [Google Scholar]
- Collins K. D., Stark G. R. Aspartate transcarbamylase. Interaction with the transition state analogue N-(phosphonacetyl)-L-aspartate. J Biol Chem. 1971 Nov;246(21):6599–6605. [PubMed] [Google Scholar]
- Diezel W., Kopperschläger G., Hofmann E. An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue. Anal Biochem. 1972 Aug;48(2):617–620. doi: 10.1016/0003-2697(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Evans D. R., Pastra-Landis S. C., Lipscomb W. N. An intermediate complex in the dissociation of aspartate transcarbamylase. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1351–1355. doi: 10.1073/pnas.71.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J. C., Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967 Jun 25;242(12):2886–2892. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Goldfarb A. R. A kinetic study of the reactions of amino acids and peptides with trinitrobenzenesulfonic acid. Biochemistry. 1966 Aug;5(8):2570–2574. doi: 10.1021/bi00872a013. [DOI] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Stark G. R. Aspartate transcarbamylase. A study of possible roles for the sulfhydryl group at the active site. J Biol Chem. 1973 Dec 10;248(23):8003–8014. [PubMed] [Google Scholar]
- Meighen E. A., Pigiet V., Schachman H. K. Hybridization of native and chemically modified enzymes. 3. The catalytic subunits of aspartate transcarbamylase. Proc Natl Acad Sci U S A. 1970 Jan;65(1):234–241. doi: 10.1073/pnas.65.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards K. E., Williams R. C. Electron microscopy of aspartate transcarbamylase and its catalytic subunit. Biochemistry. 1972 Aug 29;11(18):3393–3395. doi: 10.1021/bi00768a012. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P., Weber K. Subunit structure of aspartate transcarbamylase from Escherichia coli. J Biol Chem. 1971 Mar 25;246(6):1644–1657. [PubMed] [Google Scholar]
- Schachman H. K., Edelstein S. J. Ultracentrifuge studies with absorption optics. IV. Molecular weight determinations at the microgram level. Biochemistry. 1966 Aug;5(8):2681–2705. doi: 10.1021/bi00872a029. [DOI] [PubMed] [Google Scholar]
- Syvanen J. M., Yang Y. R., Kirschiner M. W. Preparation of 125 I-Catalytic subunit of asparatate transcarbamylase and its use in studies of the regulatory subunit. J Biol Chem. 1973 Jun 10;248(11):3762–3768. [PubMed] [Google Scholar]
- Warren S. G., Edwards B. F., Evans D. R., Wiley D. C., Lipscomb W. N. Aspartate transcarbamoylase from Escherichia coli: electron density at 5.5 A resolution. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1117–1121. doi: 10.1073/pnas.70.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K. New structural model of E. coli aspartate transcarbamylase and the amino-acid sequence of the regulatory polypeptide chain. Nature. 1968 Jun 22;218(5147):1116–1119. doi: 10.1038/2181116a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Lipscomb W. N. Crystallographic determination of symmetry of aspartate transcarbamylase. Nature. 1968 Jun 22;218(5147):1119–1121. doi: 10.1038/2181119a0. [DOI] [PubMed] [Google Scholar]
- Yang Y. R., Syvanen J. M., Nagel G. M., Schachman H. K. Aspartate transcarbamoylase molecules lacking one regulatory subunit. Proc Natl Acad Sci U S A. 1974 Mar;71(3):918–922. doi: 10.1073/pnas.71.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]



