Abstract
Background
Patients with inflammatory bowel disease (IBD) on certain immunosuppressants have increased herpes zoster (HZ) risk.
Aim
To determine the risk of HZ in IBD and how anti-tumor necrosis factor-alpha (anti-TNF)agents affect this risk.
Methods
We performed a retrospective cohort and nested case-control study using administrative data from IMS LifeLink® Information Assets-Health Plan Claims Database. In the cohort, we identified IBD patients < age 64 by diagnosis codes; matched to 4 individuals without IBD. HZ risk was evaluated by incidence rate ratio (IRR) and adjusted Cox proportional hazards models (HR). In the nested case-control analysis, 2,659 IBD patients with HZ were each matched to 4 IBD patients without HZ. We determined associations between medications and HZ using conditional logistic regression.
Results
The cohort included 50,932 patients with Crohn’s disease (CD), 56,403 patients with ulcerative colitis (UC), and 1,269 with unspecified IBD, matched to 434,416 individuals without IBD. The IBD cohort had increased HZ risk compared to non-IBD (IRR 1.68, 95% CI 1.60-1.76). After adjustment, IBD patients had a higher risk of HZ than non-IBD (HR 1.49, 95% CI 1.42-1.57). In the nested case-control multivariate adjusted analyses, anti-TNF medications (OR 1.81, 95% CI 1.48-2.21), corticosteroids (OR 1.73, 95% CI 1.51-1.99) and thiopurines (OR 1.85, 95% CI 1.61-2.13) were independently associated with HZ. Risk of HZ was highest with combination anti-TNF and thiopurine therapy (OR 3.29, 95% CI 2.33-4.65).
Conclusions
Patients with IBD are at increased risk for HZ. Use of thiopurines, anti-TNF agents, combination therapy, and corticosteroids increases HZ risk.
Keywords: inflammatory bowel disease, complications, infections, zoster
Introduction
Herpes zoster (HZ) infection, also known as shingles, is associated with significant morbidity and substantial costs. There are close to a million incident cases in the United States (US) each year.1 Infection causes a painful, blistering rash usually isolated to one or adjoining dermatomes. In approximately 10-18% of cases, post-herpetic neuralgia (PHN) occurs.2 This pain syndrome can last from months to years after the initial HZ rash, without very effective treatments. Other complications that can arise include bacterial skin infection, ocular complications, motor neuropathy and meningitis. HZ is caused by reactivation of latent varicella zoster virus (VZV), the virus that causes varicella (chicken pox). The incidence of HZ in the United States (US) has been increasing over the past decade, and significantly increases with age.3 It is estimated that the direct medical burden of HZ infection is $1000 US dollars per patient, with costs doubling for those who are immunosuppressed and quadrupling for those with PHN. This equates to greater than 1 billion dollars annually attributed to HZ in the US.4
Risk factors for HZ infection primarily include increasing age and immunosuppression. Patients with inflammatory bowel disease (IBD) are one chronic disease population who routinely use immunosuppressive medications including corticosteroids, thiopurines, biologic anti-tumor necrosis factor alpha (anti-TNF) agents, and calcineurin inhibitors, and therefore may be at increased risk for HZ. In a prior study by US researchers using data from the United Kingdom (UK), Gupta et al showed an increased risk of HZ among individuals with IBD as compared to the general population. Additionally, they found that corticosteroids and thiopurines were risk factors for HZ in the IBD population.5 Data on biologic anti-TNF medications were not available. More recently, Zhang et al showed a 1.2-to 2.0-fold greater risk of HZ associated with corticosteroid use, including those also on therapy with anti-TNF agents, in the older (≥ age 60) population with autoimmune conditions such as rheumatoid arthritis (RA) or IBD.6
Biologic anti-TNF medications, particularly the monoclonal antibodies, have been associated with an increased risk of HZ in the RA population.7-9 To further evaluate these associations in the IBD population, we aimed to determine the risk of HZ in patients with IBD as compared to a non-IBD cohort in the United States. We also aimed to determine whether specific immunosuppressive medications, including biologic anti-TNF agents, increase the risk of HZ in patients with IBD.
Methods
We analyzed the procedural and retail pharmacy claims covered by insurers contained in IMS LifeLink® Information Assets-Health Plan Claims Database (a unit of IMS, Watertown, MA. Copyright 2009, All Rights Reserved), for the period January 1, 1997 through December 31, 2009. This longitudinal, patient-level database has been used in previous epidemiologic studies of IBD.10-12 At the time of the extraction, the source database contained enrollment information on over 60 million persons from over 98 health plans across the U.S. The included health care plans capture a geographically diverse sample from across the United States. Prior studies have reported the IMS Health database to be representative of the national commercially insured population on a variety of demographic measures, including geographic region, age, gender, and health plan type.13
Study Design
We performed a retrospective cohort study to determine the overall risk of herpes zoster in IBD patients compared with a non-IBD cohort and a nested case-control study to determine the independent effects of medication use (immunosuppressive, biologic anti-TNF therapy and corticosteroids) on zoster risk among patients with IBD. A similar design has previously been used by our group10, 12 and also by Gupta et al5 to evaluate the incidence of disorders in patients with IBD and the effects of various medications.
Cohort Study
Patient selection
All patients aged <64 years with at least 12 months of continuous health plan enrollment were eligible for inclusion in this analysis. We chose 64 as the upper age limit to avoid the possibility of missing data resulting from Medicare dual eligibility (which begins at age 65). Individuals were also required to have ongoing pharmacy coverage with their health plan in order to fully capture medication exposures. We identified cases of Crohn’s disease (CD) and ulcerative colitis (UC) using a previously reported administrative definition expanded to include updated medications recently approved for IBD indications.14 The precise definition included patients with at least 3 health care contacts, on different days, associated with an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9) diagnosis code for CD (555.xx) or UC (556.xx), or patients with at least 1 claim for CD or UC and at least 1 pharmacy claim for any of the following medications: mesalamine, olsalazine, balsalazide, sulfasalazine, 6-mercaptopurine, azathioprine, methotrexate, infliximab, adalimumab, certolizumab pegol, natalizumab and enteral budesonide. We did not include corticosteroid use in the definition, due to widespread use of these medications for other indications. For patients who had claims for both CD and UC, disease assignment was made according to the majority of the last 9 claims or the majority of total claims if there were fewer than 9. If there were equal numbers of claims for CD and UC, the individuals were classified as IBD-unspecified. We matched each IBD patient to 4 non-IBD subjects by age, gender, and U.S. census region. Region of the country was defined by standard regional definitions from the United States Census Bureau. All individuals with any ICD-9 code for human immunodeficiency virus (HIV) (042-044.9) were excluded related to differences in susceptibility to infections from inherent immune dysfunction.
Cohort lead time and follow-up
Each cohort member was required to have a minimum of 6 months of health plan enrollment prior to cohort entry. This “screening period” was used to assess potential confounders of interest, particularly health care utilization and cormorbidities. Each member of cohort was followed until the first diagnosis of zoster, or, if none, until censoring at the earlier of one of two events: discontinuation of primary or pharmacy insurance coverage, or age > 64.
Assessment of outcome (zoster)
Herpes zoster was defined as any 1 ICD-9 code for herpes zoster (053.xx). This definition has been used previously to study zoster infection in IBD.5 Additionally, this definition has been used to determine the incidence of zoster infection in various other administrative databases in the United States.3, 15 This definition has been validated, and has been found to have a sensitivity of 98% and a positive predictive value of 93%.16 In a separate validation, ICD-9 code 053.xx for HZ correlated extremely well with HZ diagnosis from chart review (kappa=0.92).9 No medications were used in the definition of zoster, as antiviral agents are not uniformly prescribed. Anti-viral agents are only recommended when the zoster diagnosis is made within 48-72 hours of the onset of the blistering rash. Prior studies have demonstrated that only 2/3 of individuals with zoster are treated with anti-viral agents.9
Assessment of exposures
All exposure assessment occurred during the 6 month “screening period”prior to cohort entry. Health care utilization was estimated in a continuous fashion by the total of days with at least one health care contact over the time period. Utilization has been measured in this fashion in prior pharmacoepidemiology studies.17 Comorbidities were assessed using the Quan update18 of the validated Deyo comorbidity index for administrative data.19 Medications, including corticosteroids, immunomodulators, biologic anti-TNF agents, and 5-aminosalicylic acid (5-ASA) agents, were assessed in the exposure period in an any/none fashion.
Statistical analysis
We used descriptive statistics to summarize characteristics of patients with and without IBD. Continuous variables are reported as mean +/-standard deviation or median and interquartile range (IQR), and categorical variables are reported as percentages. We then calculated incidence rates of zoster (per 100,000 person-years) and used incidence rate ratios (IRRs) and 95% confidence intervals (CIs) to compare the incidence of zoster in IBD and non-IBD patients. We also performed subsequent analyses, stratifying by age (in decades), and disease type (UC versus CD). We assessed for possible violation of the proportional hazards assumption via log-log plots. As the assumption was not violated, we used Cox proportional hazards models to calculate hazard ratios (HRs) and 95% CIs for the risk of zoster in the IBD cohort as compared to the non-IBD cohort, controlling for health care utilization and comorbidities. Analyses were repeated stratified by CD versus UC.
Nested Case-Control Study
We next conducted a case-control study evaluating the association between corticosteroids, immunosuppressive or biologic anti-TNF medication use and zoster. This study was nested within the previously defined cohort of patients with IBD.
Selection of cases and controls
Cases were those IBD patients who were diagnosed with zoster, and controls were those IBD patients without zoster. Each case patient was matched on gender, age, geographic region, disease type (CD or UC), and duration of follow-up to 4 IBD patients who did not have zoster using incidence density sampling. In this sampling technique, a case patient can also be a control patient, provided that the case patient has not yet been diagnosed with zoster (has not yet become a case) at the time he or she is selected as a control. Once a case is diagnosed with zoster, he or she is no longer eligible to become a control, or a case again. A total of 257 patients were included as both cases and controls.
Assessment of outcome (zoster)
The outcome was herpes zoster infection, as defined within the cohort study by ICD-9 code (053.xx). This definition has been validated in other populations, with appropriate sensitivity, specificity, and concordance with medical records.9, 16
Assessment of exposures
Medication Use
The primary medications evaluated included azathioprine and 6-mercaptopurine (thiopurine class), methotrexate, tacrolimus and cyclosporine (calcineurin class), infliximab, adalimumab, and certolizumab pegol (biologic anti-TNF class) and systemic corticosteroids. Medication exposures were analyzed with respect to the amount of time preceding the diagnosis of zoster in cases or the corresponding index date in controls. All exposures were defined as at least 1 outpatient pharmacy claim occurring during the 120 days prior to zoster diagnosis.
Potential confounders
Data on utilization and comorbidities were also included as exposure variables in the case-control study. These variables were assessed during the 6 month exposure period prior to zoster diagnosis. Utilization was defined in a continuous fashion as total number of days with at least one health care contact. Comorbidities were assessed through the Quan update18 of the validated Deyo comorbidity index19 for administrative claims data.
Statistical analysis
Characteristics of cases and controls were described using descriptive statistics. We then used conditional logistic regression to calculate odds ratios (ORs) and 95% CIs for each medication exposure related to the outcome of zoster. We constructed a full model by using all potential confounders and eliminated these confounders via a backwards elimination strategy by using a change in estimate approach (threshold of <10% change). Analyses were performed for patients with IBD and also stratified by UC and CD diagnosis.
For all analyses, p values were two-sided, and a p value of .05 or less was considered statistically significant. All statistical analyses were performed using Stata version 11.0 (College Station, TX). The study protocol was granted exemption from review by the Institutional Review Board at University of North Carolina because it involved the use of existing, de-identified data.
Results
The cohort study population included 108,604 patients with IBD. Of these, 50,932 had CD, 56403 had UC and 1,269 had IBD with unknown type. The patients with IBD contributed a total of 364,533 person-years of observation time to the cohort. There were a total of 434,416 individuals in the non-IBD comparison cohort. The non-IBD patients contributed a total of 992,273 person-years of observation time to the cohort. The median length of follow up within the non-IBD cohort was 24 months (IQR 12-42) with a range from 1-138 months after the 6 month “screening” period. Length of follow-up was similar for CD (34 months, IQR 19-51) and UC populations (36 months, IQR 21-54). Duration of follow-up was significantly less in the non-IBD comparison cohort (21 months, IQR 10-38). Table 1 shows the characteristics of the IBD cohort as compared to the non-IBD cohort. The IBD cohort had increased health care utilization, and immunosuppressive medication use as compared to the matched non-IBD cohort, as expected. These same factors were increased in those with CD as compared to those with UC.
Table 1.
Characteristics of the Population by Inflammatory Bowel Disease overall, and Crohn’s disease or Ulcerative Colitis
| Characteristic | Non-IBD Cohort (n=434,416) | IBD Cohort (n=108,604) | Crohn’s disease (n=50932) | Ulcerative Coltiis (n=56403) | ||||
|---|---|---|---|---|---|---|---|---|
| n | median (IQR) or % | n | median (IQR) or % | n | median (IQR) or % | n | median (IQR) or % | |
| Age | 43 (31-52) | 43 (31-52) | 41 (29-51) | 44 (33-53) | ||||
| Gender (% female) | 238812 | 55.0 | 59703 | 55.0 | 28723 | 56.4 | 30272 | 53.7 |
| Region of the country | ||||||||
| East | 91923 | 21.2 | 23069 | 21.2 | 10686 | 21.0 | 12061 | 21.4 |
| Midwest | 169043 | 38.9 | 46232 | 42.6 | 22155 | 43.5 | 23614 | 41.9 |
| South | 97829 | 22.5 | 20350 | 18.7 | 9621 | 18.9 | 10479 | 18.6 |
| West | 75621 | 17.4 | 18953 | 17.5 | 8470 | 16.7 | 10249 | 18.2 |
| Medications* | ||||||||
| 5ASA** | 552 | 0.1 | 37609 | 34.6 | 15974 | 31.4 | 21195 | 37.6 |
| Thiopurine% | 257 | 0.1 | 11155 | 10.3 | 7558 | 18.8 | 3540 | 6.3 |
| Biologicˆ | 200 | 0.1 | 3013 | 2.8 | 2607 | 5.1 | 396 | 0.7 |
| Calcineurin inhibitor& | 136 | 0.0 | 255 | 0.2 | 79 | 0.2 | 173 | 0.3 |
| Health Care Utilizationπ | 1 (0-4) | 4 (2-9) | 5 (2-10) | 4 (1-8) | ||||
| Comorbidities | ||||||||
| Cardiac | 2935 | 0.7 | 1076 | 1.0 | 475 | 0.9 | 584 | 1.0 |
| Diabetes mellitus | 16888 | 3.9 | 4824 | 4.4 | 2055 | 4.0 | 2705 | 4.8 |
| Liver disease | 2603 | 0.6 | 2112 | 1.9 | 982 | 1.9 | 1104 | 2.0 |
| Renal | 1181 | 0.3 | 845 | 0.8 | 399 | 0.8 | 440 | 0.8 |
| COPD | 17191 | 4.0 | 6654 | 6.1 | 3233 | 6.4 | 3338 | 5.9 |
| Total # comorbidities | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | ||||
Defined as any use during screening period, any # of days supply
Defined as mesalamine, sulfasalazine, balsalazide, olsalazine
Defined as mercaptopurine or azathioprine
Defined as infliximab, adalimumab or certolizumab pegol
Defined as cyclosporine or tacrolimus
Any proton pump inhibitor
Defined as total number of days (continuous) in screening period with at least 1 billed health care contact
Total # of comorbidities from the Deyo comorbidity index for administrative data
Source: IMS LifeLink® Information Assets-Health Plan Claims Database, January 1997 through December 2009, IMS Health Incorporated. All Rights Reserved
In the IBD population, there were a total of 2677 cases of zoster. In the non-IBD population, there were a total of 4340 cases of zoster. For patients with IBD, the overall annual incidence of HZ was 734/100,000 (95% CI 707/100,000-763/100,000), compared to 437/100,000 (95% CI 424/100,000-451/100,000) in the non-IBD cohort (incidence rate ratio [IRR] 1.68, 95% CI 1.60-1.76). The incidence of zoster in CD was somewhat higher than that in UC (figure 1). The IBD cohort had an increased zoster risk when compared to non-IBD (IRR 1.68, 95% CI 1.60-1.76), as did CD versus non-CD (IRR 1.91, 95% CI 1.78-2.05) and UC versus non-UC (IRR 1.50, 95% CI 1.40-1.61). The incidence of HZ was then evaluated in strata of age, with increasing incidence of zoster within each strata of age, for both IBD and non-IBD populations. The highest incidence was in the 60+ age strata for those with CD (1502/100,000, 95% CI 1236/100,000-1809/100,000), as expected (figure 2).
Figure 1.
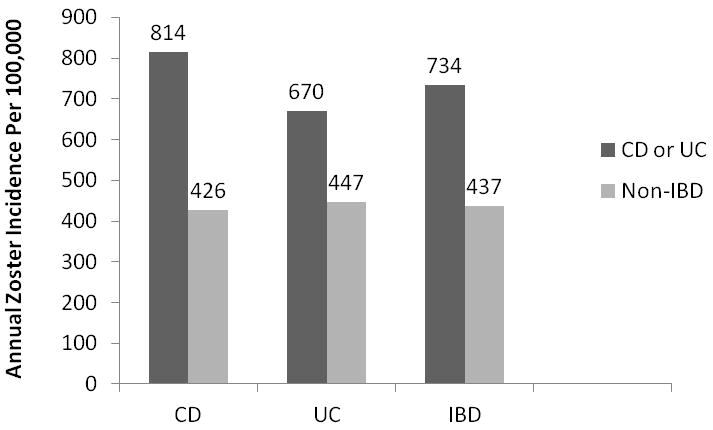
Annual Zoster Incidence (per 100,000) in Inflammatory Bowel Disease (IBD) (n=108,604) and non-IBD Populations (n=434,416), Stratified by Crohn’s disease (CD) (n=50,932) as Compared to non-CD (n=203,728) and Ulcerative Colitis (UC) (n=56,403) as Compared to non-UC (n=225,612) Populations.
Footnote: Data from IMS LifeLink® Information Assets-Health Plan Claims Database (1997-2009), IMS Health Incorporated.
Figure 2.
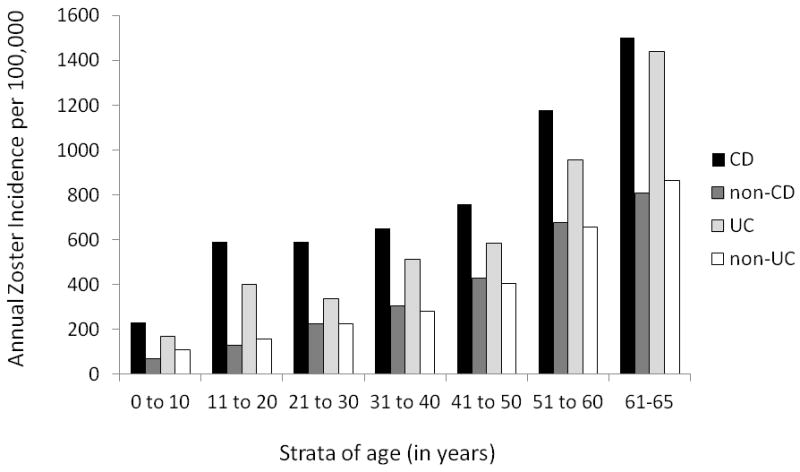
Annual Zoster Incidence (per 100,000) in Crohn’s disease (CD) (n=50,932) as Compared to non-CD Populations (n=203,728) and Ulcerative Colitis (UC) (n=56,403) as Compared to non-UC Populations (n=225,612), within 10 Year Strata of Age
Footnote: Data from IMS LifeLink® Information Assets-Health Plan Claims Database (1997-2009), IMS Health Incorporated.
After adjusting for comorbidities and health care utilization on Cox analysis, zoster risk remained increased in the IBD versus non-IBD cohort (HR 1.49, 95% CI 1.42-1.57). Risk was particularly increased risk for those with CD as compared to non-CD (HR 1.69, 95% CI 1.58-1.82). For UC, the adjusted risk compared to non-UC was HR 1.34, 95% CI 1.25-1.44.
In the nested case control study, 2,659 IBD patients with HZ were matched to 10,470 IBD patients without HZ. A total of 18 IBD patients with zoster were unable to be matched to a comparison IBD patient without zoster. The characteristics of the populations are shown in table 2. Patients with HZ had significantly more comorbidities, including cardiac conditions, diabetes, liver disease, renal disease and chronic pulmonary disease. Those with HZ also had significantly higher health care utilization and immunosuppressive medication use. In adjusted analyses, 5-ASA use was not associated with HZ in the overall IBD population (OR 1.08, 95% CI 0.97-1.19). However, thiopurine use (OR 1.85, 95% CI 1.61-2.13), corticosteroid use (OR 1.73, 95% CI 1.51-1.99), and biologic anti-TNF use (OR 1.81, 95% CI 1.48-2.21) were independently associated with HZ in the overall IBD population. Similar risk estimates were seen when stratified by CD or UC. Crude and adjusted analyses are shown in table 3 for specific medication use in the overall population, and by IBD subtype. In a sub-analysis, we then investigated combination use of anti-TNF and thiopurine and monotherapy of either agent as compared to no immunosuppressive medication use. Those on combination therapy had the highest risk of zoster, even after controlling for corticosteroid use, 5-ASA use, utilization and comorbidities (OR 3.29, 95% CI 2.33-4.65) (table 4).
Table 2.
Characteristics of the IBD Population by Zoster (matched characteristics include age, IBD type, gender, region)
| Characteristics | No Zoster (n=10470) | Zoster (n=2659) | |||
|---|---|---|---|---|---|
| n | % or median (IQR) | n | % or median (IQR) | p value* | |
| Age** | 10470 | 49 (37-56) | 2659 | 49 (38-56) | 0.11 |
| Gender (% male) | 3883 | 37.1 | 991 | 37.3 | 0.86 |
| IBD type | |||||
| Crohn’s disease | 5335 | 51.0 | 1353 | 50.9 | 0.34 |
| Ulcerative colitis | 5095 | 48.7 | 1290 | 48.5 | |
| IBD unspecified | 38 | 0.4 | 15 | 0.6 | |
| Utilizationˆ | 10470 | 4 (1-9) | 2659 | 9 (5-16) | <0.01 |
| Region | |||||
| East | 1741 | 16.6 | 443 | 16.7 | 0.99 |
| Midwest | 4753 | 45.4 | 1207 | 45.4 | |
| South | 2100 | 20.1 | 532 | 20.0 | |
| West | 1876 | 17.9 | 477 | 17.9 | |
| Comorbidities | |||||
| Cardiac | 199 | 1.9 | 78 | 2.9 | <0.01 |
| Diabetes mellitus | 774 | 7.4 | 256 | 9.6 | <0.01 |
| Liver disease | 324 | 3.1 | 116 | 4.4 | <0.01 |
| Renal | 185 | 1.8 | 73 | 2.8 | <0.01 |
| COPD | 896 | 8.6 | 335 | 12.6 | <0.01 |
| # Comorbidities= | 10470 | 0 (0-0) | 2659 | 0 (0-1) | <0.01 |
| Medications% | |||||
| Biologic+ | 327 | 3.1 | 196 | 7.4 | <0.01 |
| Thiopurine& | 806 | 7.7 | 419 | 15.8 | <0.01 |
| Corticosteroid | 728 | 7.0 | 425 | 16.0 | <0.01 |
| 5-ASA | 2563 | 24.5 | 744 | 28.0 | <0.01 |
p value by Pearson’s chi square for categorical variables, Wilcoxon rank sum for continuous variables
Age at time of zoster (case) or index date (control)
Defined as total number of days (continuous) with any health care contact over observation period
Number of comorbities from Charlson comorbidity index
Any use over observation period
Infliximab, adalimumab or certolizumab pegol
Mercaptopurine or azathioprine
Source: IMS LifeLink® Information Assets-Health Plan Claims Database, January 1997 through December 2009, IMS Health Incorporated. All Rights Reserved
Table 3.
Crude and multivariate adjusted¶ estimates (odds ratios and 95% CI) of medication use and zoster in patients with IBD, overall and by CD or UC
| IBD Overall (n=13,129) | Crohns disease (n=6688) | Ulcerative Colitis (n=6381) | |||||
|---|---|---|---|---|---|---|---|
| Medication | Crude OR, 95% CI | Adjusted OR, 95% CI | Crude OR, 95% CI | Adjusted OR, 95% CI | Crude OR, 95% CI | Adjusted OR, 95% CI | |
| Any use in prior 120 days | |||||||
| 5-ASA | 1.20 (1.09-1.32) | 1.08 (0.97-1.19) | 1.27 (1.10-1.46) | 1.09 (0.94-1.27) | 1.14 (1.00-1.31) | 1.07 (0.93-1.24) | |
| Biologic | 2.57 (2.13-3.10) | 1.81 (1.48-2.21) | 2.37 (1.92-2.92) | 1.72 (1.38-2.15) | 3.85 (2.47-6.00) | 2.36 (1.47-3.79) | |
| Thiopurine | 2.28 (2.00-2.60) | 1.85 (1.61-2.13) | 2.31 (1.98-2.70) | 1.93 (1.63-2.28) | 2.20 (1.73-2.78) | 1.68 (1.30-2.16) | |
| Corticosteroid | 2.53 (2.22-2.87) | 1.73 (1.51-1.99) | 2.28 (1.91-2.72) | 1.54 (1.27-1.86) | 2.83 (2.34-3.43) | 1.96 (1.60-2.40) | |
Biologic defined as infliximab, adalimumab, or certolizumab pegol, thiopurine defined as mercaptopurine or azathioprine 5-ASA defined as mesalamine, olsalazine, balsalazide, sulfasalazine,
adjusted for health care utilization, comorbidities, 5-ASA, biologic, thiopurine, corticosteroid use as appropriate (see methods)
Source: IMS LifeLink® Information Assets-Health Plan Claims Database, January 1997 through December 2009, IMS Health Incorporated. All Rights Reserved
Table 4.
Multivariate adjusted¶ estimates (odds ratios and 95% CI) of immunosuppressive medication combinations and zoster in patients with IBD, overall and by CD or UC
| IBD Overall (n=13,129) | Crohns disease (n=6688) | Ulcerative Colitis (n=6381) | ||
|---|---|---|---|---|
| Medication | Adjusted OR, 95% CI | Adjusted OR, 95% CI | Adjusted OR, 95% CI | |
| Any use in prior 120 days | ||||
| Thiopurine* | 1.86 (1.61-2.15) | 1.96 (1.64-2.34) | 1.65 (1.27-2.14) | |
| Biologic** | 1.83 (1.44-2.31) | 1.78 (1.37-2.32) | 2.22 (1.30-3.80) | |
| Combination*** | 3.29 (2.33-4.65) | 3.13 (2.16-4.55) | 4.79 (1.84-12.45) |
Thiopurine defined as mercaptopurine or azathioprine
Biologic defined as infliximab, adalimumab, or certolizumab pegol
Combination therapy defined as both thiopurine and biologic
adjusted for health care utilization, comorbidities, 5-ASA and corticosteroid use as appropriate
Source: IMS LifeLink® Information Assets-Health Plan Claims Database, January 1997 through December 2009, IMS Health Incorporated. All Rights Reserved
Discussion
Herpes zoster is an important cause of increased morbidity and mortality in the United States, with approximately 1 million cases annually. Incidence of HZ in the general population is 340/100,000, but is strongly affected by age, with the highest incidence in those >80 years at 1100/100,000.20
The lifetime risk of developing HZ is approximately 30%. The course of the unilateral, painful rash is generally 2-4 weeks. There can be significant complications from HZ including: post-herpetic neuralgia, ophthalmologic, neurologic (encephalitis) and dermatologic (secondary skin infection) involvement or complications. Understanding the incidence and risk factors for HZ in an IBD population is important to guide prevention efforts. As HZ is associated with substantial morbidity and costs,4 this emphasis upon prevention is warranted.
In our large administrative study of HZ infection in IBD patients, we demonstrated a significantly increased risk of HZ when compared to the general population; with particularly increased risk among those on immunosuppression. As with the general population, we showed increasing HZ risk with advancing age. The etiology of the increased HZ risk in IBD is likely multifactorial; immunosuppressive medications, age-related changes in immune function and the innate immune dysregulation associated with IBD itself.
Other populations receiving immunosuppression are also at increased risk for HZ. For example, in individuals with RA, the incidence of HZ has been found to be 996 per 100,000 patient-years. Risk factors for HZ in this population of Veterans included advancing age, corticosteroid use, immunosuppressive medications used in moderate RA (such as thiopurines, calcineurin inhibitors or methotrexate) and severe RA (biologic anti-TNF agents), malignancy, and comorbid conditions such as chronic lung disease, renal failure or liver disease.9 A separate study of anti-TNF use in RA patients found increased risk associated with the monoclonal anti-TNF antibodies (HR 1.82, 95% CI, 1.05-3.15).7 Increased risk has also been demonstrated in the solid organ transplant (SOT) population. The incidence of HZ is 2222 per 100,000 person-years in the SOT population, with increasing risk associated with increasing levels of immunosuppression (the highest absolute risk is among heart transplant recipients) and increasing age.21
The IBD population has been previously shown to have an increased risk of HZ. Gupta et al evaluated data from the UK and found an increased relative risk of HZ for both CD and UC (IRR 1.61, 95% CI 1.35-1.92 and IRR 1.21, 95% CI 1.05-1.40 respectively).5 We found similar risks in our population, with unadjusted IRRs of 1.9 for CD and 1.5 for UC. Similar to Gupta et al, we found increasing risk with advancing age. The overall incidence in our population was 734/100,000 (95% CI 707/100,000-763/100,000) person-years, and overall age-specific risks in 10 year strata were similar to those risks found by Gupta et al. We also found an increased risk of HZ associated with increasing numbers of comorbidities, as has been reported in the other populations.9 In a recent study by Zhang et al of older individuals (age ≥60 years) with autoimmune conditions such as RA or IBD, the incidence of HZ in those with prior shingles vaccination was 780/100,000 and 1160/100,000 in those not previously receiving vaccination.6 These effect estimates are slightly higher than overall estimates found in our study; but similar to those in our older age strata.
In our nested case-control study, we investigated the independent effects of various classes of medications used in the treatment of IBD. We found that immunosuppressive medications, including anti-TNF agents (OR 1.81, 95% CI 1.48-2.21), corticosteroids (OR 1.73, 95% CI 1.51-1.99) and thiopurines (OR 1.85, 95% CI 1.61-2.13) were each independently associated with increased HZ risk. The study by Gupta et al pre-dated use of anti-TNF agents, but did estimate the risk of corticosteroids and thiopurines in IBD. Their adjusted odds ratio for corticosteroid use was OR 1.5, 95% CI, 1.1–2.2; whereas for thiopurines, the risk was greater (adjusted odds ratio, 3.1; 95% CI, 1.7–5.6). We found similar levels of increased risk, albeit somewhat lower for thiopurines. We found no risk associated with 5-ASA use, which served as a negative control, demonstrating that the mechanism of increased risk is driven by immunosuppression and/or severity of underlying IBD. Finally, we investigated the risk associated with combination therapy (anti-TNF and thiopurine), controlling for comorbidities, utilization and other medication use, and found the highest overall odds ratio of 3.3. This demonstrates there are potentially additive risks of HZ with >1 immunosuppressive agent.
There are strengths to this large study of HZ incidence and risk factors. Primarily, we studied a very large and geographically diverse population from throughout the United States. Due to this diversity, the sample is broadly generalizable to the commercially insured US population. Secondly, we utilized complete data on all billed outpatient prescriptions in order to capture medication exposures; without relying on patient recall. Third, were also able to account for both health care utilization and potentially confounding comorbidities. To do so, we used a validated comorbidity index for administrative data.18,19
There are also several limitations to this study that utilized administrative claims data. As with all studies of claims data, there is the possibility of misclassification of exposure and outcome related to the lack of clinical detail. However, we used an established IBD exposure definition 10, 11 that requires multiple health contacts and/or IBD related prescriptions. A similar, but even less specific, administrative case definition has been validated by Herrinton et al with a sensitivity exceeding 90% and a PPV exceeding 80% for overall IBD.22 We used an outcome definition of HZ consisting of any ICD-9 code (053.xx). This administrative definition has been validated, with a sensitivity of 98% and a positive predictive value of 93%.16 In a separate validation, ICD-9 code 053.xx for HZ correlated extremely well with HZ diagnosis from chart review (kappa=0.92).9 Additionally, as risk of HZ is known to increase with age, it is a limitation that the elderly (>age 65) were not represented in our population. This lack of older persons in our study population does not affect the validity of the relative risks we presented in the context of the under 65 population. In spite of controlling for both health care utilization and comorbidities (including malignancy) within our study, and excluding those with HIV, there are other potential risk factors associated with HZ that may not have been accounted for. For example, we were unable to capture race, and there are some data to suggest that age at diagnosis of HZ may be associated with race.23 However, in order to be a confounder, a factor must be associated with both the exposure and the outcome. Race may or may not be associated with our exposures of IBD (in the cohort study) and immunosuppressive medication use (in the nested case-control study). In fact, the incidence of IBD among African Americans now approaches that of Caucasians in the US. Among studies that have shown differences in immunosuppressive medication use by race,24 many quote access to care and socioeconomic factors as a potential cause. In our study, all patients have health insurance, thereby alleviating many of the socioeconomic factors. Finally, we could not utilize the potentially ideal study design of a cohort study with time varying medication exposures due to the inability to account for the precise timing of initiation and discontinuation of medications. This is particularly true for corticosteroids which are often written for use “as directed .” For this reason, we designed the nested-case control study and accounted for any medication use over a 120 day window. We were also unable to assess the role of the shingles vaccination in our population, as this vaccination is often not covered by insurers in those <age 60, and our data do not extend beyond age 64 due to medicare dual eligibility.
In conclusion, understanding the medication-specific risks of HZ is important, particularly as practice patterns in IBD management change. For example, there is now an emphasis upon early, aggressive management of CD. As biologic anti-TNF and other immunosuppressant use in IBD becomes more prevalent, this increased utilization may account for a further increase in the incidence of HZ. A new vaccine against varicella zoster virus (shingles), Zostavax®, became commercially available in 2006. The vaccine contains the same strain used in the varicella (chicken pox) vaccine, but is 14 times more potent. The shingles vaccine is effective, decreasing zoster incidence by >50%. 25 This vaccine is currently recommended by the Advisory Committee on Immunization Practices (ACIP) in individuals ≥ age 60 without contraindications. 26 As the vaccine is live, it is contraindicated in individuals already on immunosuppression (corticosteroids (≥20 mg/day for greater than 2 weeks), biologic anti-TNF agents, chemotherapy). In its most recent Guide to Vaccine Contraindications and Precautions, the Centers for Disease Control (CDC) determined that the vaccine could be offered to individuals on low doses of immunosuppression (defined as methotrexate (≤0.4 mg/Kg/week), azathioprine (≤3.0 mg/Kg/day), or 6-mercaptopurine (≤1.5 mg/Kg/day)).27 However, the ideal time to dose this vaccine may be prior to initiation of immunosuppression. The vaccine has not specifically been tested in younger populations or in populations with immune dysfunction such as IBD. Zhang et al recently found a reduced incidence of HZ in older individuals (≥ age 60) with autoimmune conditions and prior receipt of vaccination as compared to a similar group with no prior vaccination; including those exposed to biologics.6 Importantly, overall rates of HZ vaccination in older individuals with autoimmune conditions remain quite low; 1.2% in one US population.28 Therefore, this represents a potential missed opportunity for prevention. Future studies will need to determine the role, safety and timing of this vaccination in patients of all ages with IBD. As HZ and its complications are potentially preventable, optimizing the delivery and timing of vaccination in IBD patients will be important in years to come.
Acknowledgments
Grant support: This work was supported by a Career Development Award from the Crohn’s and Colitis Foundation of America (MDL), NIH P30 DK34987 (RSS), and NIH 1K08DK088957-01 (MDK). The study sponsors supported the time of the investigators; without any involvement in study design, collection, analysis, or interpretation of data.
Footnotes
Author contributions: ML participated in all aspects of the study including: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; study supervision. CM participated in study design, data programming, analysis and interpretation of data, critical revision of the manuscript. RSS participated in study design, critical revision of the manuscript for important intellectual content, study supervision. MDK participated in study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content and study supervision. All authors approved the final version of the manuscript.
The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health Incorporated information service(s): IMS LifeLink® Information Assets-Health Plan Claims Database (1997-2009), IMS Health Incorporated. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities.
Disclosures: No conflicts of interest exist for any author.
References
- 1.Insinga RP, Itzler RF, Pellissier JM. Acute/subacute herpes zoster: healthcare resource utilisation and costs in a group of US health plans. Pharmacoeconomics. 2007;25:155–69. doi: 10.2165/00019053-200725020-00007. [DOI] [PubMed] [Google Scholar]
- 2.Straus SE, Ostrove JM, Inchauspe G, Felser JM, Freifeld A, Croen KD, Sawyer MH. NIH conference. Varicella-zoster virus infections. Biology, natural history, treatment, and prevention. Ann Intern Med. 1988;108:221–37. doi: 10.7326/0003-4819-108-2-221. [DOI] [PubMed] [Google Scholar]
- 3.Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52:332–40. doi: 10.1093/cid/ciq077. [DOI] [PubMed] [Google Scholar]
- 4.White RR, Lenhart G, Singhal PK, Insinga RP, Itzler RF, Pellissier JM, Segraves AW. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics. 2009;27:781–92. doi: 10.2165/11317560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:1483–90. doi: 10.1016/j.cgh.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Xie F, Delzell E, Chen L, Winthrop KL, Lewis JD, Saag KG, Baddley JW, Curtis JR. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA. 2012;308:43–9. doi: 10.1001/jama.2012.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, Zink A. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA. 2009;301:737–44. doi: 10.1001/jama.2009.146. [DOI] [PubMed] [Google Scholar]
- 8.Bongartz T, Orenstein R. Therapy: The risk of herpes zoster: another cost of anti-TNF therapy? Nat Rev Rheumatol. 2009;5:361–3. doi: 10.1038/nrrheum.2009.120. [DOI] [PubMed] [Google Scholar]
- 9.McDonald JR, Zeringue AL, Caplan L, Ranganathan P, Xian H, Burroughs TE, Fraser VJ, Cunningham F, Eisen SA. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis. 2009;48:1364–71. doi: 10.1086/598331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long MD, Herfarth HH, Pipkin CA, Porter CQ, Sandler RS, Kappelman MD. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2010;8:268–74. doi: 10.1016/j.cgh.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long MD, Porter CQ, Sandler RS, Kappelman MD. Suboptimal rates of cervical testing among women with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2009;7:549–53. doi: 10.1016/j.cgh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long MD, Martin C, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of Melanoma and Non-Melanoma Skin Cancer among Patients with Inflammatory Bowel Disease. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stempel DA, Mauskopf J, McLaughlin T, Yazdani C, Stanford RH. Comparison of asthma costs in patients starting fluticasone propionate compared to patients starting montelukast. Respir Med. 2001;95:227–34. doi: 10.1053/rmed.2000.1027. [DOI] [PubMed] [Google Scholar]
- 14.Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, Finkelstein JA. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–9. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748–53. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klompas M, Kulldorff M, Vilk Y, Bialek SR, Harpaz R. Herpes zoster and postherpetic neuralgia surveillance using structured electronic data. Mayo Clin Proc. 2011;86:1146–53. doi: 10.4065/mcp.2011.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund JL, Sturmer T, Porter CQ, Sandler RS, Kappelman MD. Thiazolidinedione use and ulcerative colitis-related flares: an exploratory analysis of administrative data. Inflamm Bowel Dis. 2011;17:787–94. doi: 10.1002/ibd.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571–5. [PMC free article] [PubMed] [Google Scholar]
- 21.Pergam SA, Forsberg CW, Boeckh MJ, Maynard C, Limaye AP, Wald A, Smith NL, Young BA. Herpes zoster incidence in a multicenter cohort of solid organ transplant recipients. Transpl Infect Dis. 2011;13:15–23. doi: 10.1111/j.1399-3062.2010.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrinton LJ, Liu L, Lafata JE, Allison JE, Andrade SE, Korner EJ, Chan KA, Platt R, Hiatt D, O’Connor S. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflamm Bowel Dis. 2007;13:451–61. doi: 10.1002/ibd.20021. [DOI] [PubMed] [Google Scholar]
- 23.Nagasako EM, Johnson RW, Griffin DR, Elpern DJ, Dworkin RH. Geographic and racial aspects of herpes zoster. J Med Virol. 2003;70(Suppl 1):S20–3. doi: 10.1002/jmv.10315. [DOI] [PubMed] [Google Scholar]
- 24.Flasar MH, Johnson T, Roghmann MC, Cross RK. Disparities in the use of immunomodulators and biologics for the treatment of inflammatory bowel disease: a retrospective cohort study. Inflamm Bowel Dis. 2008;14:13–9. doi: 10.1002/ibd.20298. [DOI] [PubMed] [Google Scholar]
- 25.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 26.Hendriksz T, Malouf P, Foy JE. Vaccines for measles, mumps, rubella, varicella, and herpes zoster: immunization guidelines for adults. J Am Osteopath Assoc. 2011;111:S10–2. [PubMed] [Google Scholar]
- 27. [8-14-2012]; http://www.cdc.gov/vaccines/recs/vac-admin/downloads/contraindications-guide-508.pdf.
- 28.Zhang J, Delzell E, Xie F, Baddley JW, Spettell C, McMahan RM, Fernandes J, Chen L, Winthrop K, Curtis JR. The use, safety, and effectiveness of herpes zoster vaccination in individuals with inflammatory and autoimmune diseases: a longitudinal observational study. Arthritis Res Ther. 2011;13:R174. doi: 10.1186/ar3497. [DOI] [PMC free article] [PubMed] [Google Scholar]


