Abstract
The association between body composition parameters and toxicity associated with hepatic arterial infusion (HAI) chemotherapy regimens has not been analyzed. We analyzed data from patients with advanced cancer and liver metastases treated on a clinical trial of HAI oxaliplatin combination regimen. Patient characteristics, response and toxicities were analyzed in relevance with body composition data from CT images. Forty-eight of 57 patients (mean age 57 years; 60% women) had available CT scans. The most common diagnosis was colorectal cancer (22/48, 46%); 30/48 patients (63%) had body mass index (BMI) ≥ 25 kg/m2. Twenty of 48 patients were sarcopenic (42%). Grade 3–4 adverse events did not differ among patients with and without sarcopeniaor according to BMI. The median survival (95% confidence interval [CI]) was 167 (128–206) days for sarcopenic and 280 (214–346) days for non-sarcopenic patients (p=0.271). Among patients treated at the maximum tolerated dose, the median survival was 103 days for sarcopenic and 312 days for non-sarcopenic patients (p=0.173). Sarcopenia was present in 30% (6/20) of patients with reduction in tumor size post-treatment, and in 52% (14/27) of patients with increased tumor size (p=0.171). In conclusion, body composition was not associated with toxicities. Sarcopenia might be associated with shorter survival.
Keywords: neoplasms, antineoplastic combined chemotherapy protocols, adverse effects, treatment outcome, survival, sarcopenia
INTRODUCTION
Body composition features have been associated with various aspects of cancer that relate to prevention, etiology, and therapy. Obesity is an etiologic and factor in various cancers (1). Weight loss is frequent among cancer patients, especially in advanced disease (2), and is a feature of cancer cachexia, which is characterized by loss of fat and muscle mass, decreased muscle strength, low fat-free mass, fatigue, anorexia, elevated inflammatory markers, anemia, and low serum albumin level (3). Cachexia, which occurs in up to 80% of cancer patients (4), is a marker of poor prognosis (5–7), negatively impacts patients’ quality of life (8, 9), and impairs normal physical function (10). Sarcopenia, which denotes severe muscle depletion, has received special attention recently in the cancer literature because it is associated with reduced physical ability and increased mortality in patients without cancer (11–16). Sarcopenia was linked to dose-limiting toxicities in a phase I study of sorafenib in renal cell carcinoma (17).
Hepatic arterial infusion (HAI) is used in the treatment of hepatic metastases from colorectal cancer, on the basis that malignant lesions derive most of their blood supply from the hepatic artery, in contrast to normal hepatocytes (portal venous circulation) (18, 19). HAI of cytotoxic drugs is thought to be associated with higher antitumor activity in liver metastases and fewer toxicities than systemic administration (18).
We previously published results of a phase I clinical trial of HAI oxaliplatin combined with systemic 5-fluorouracil, leucovorin, and bevacizumab in patients with advanced solid tumors predominantly metastatic to the liver (20). This regimen had promising antitumor activity and 58% of patients had no toxicity > Grade 1 (20).
However, many other HAI studies have failed to show an advantage over usual treatments, although selected trials demonstrated a survival advantage (18, 19), including high response rates and improved survival of patients with colorectal cancer treated with HAI after hepatic resection (19–21).
To our knowledge, the association between body composition parameters and toxicity associated with HAI chemotherapy regimens has not been systematically analyzed. We hypothesized that there is no relationship between body composition and HAI toxicities, due to the very fact that body composition would most likely play a minor role when drug delivery is less dependent on tissue distribution. The objectives of this study were to assess body composition in patients with advanced solid tumors metastatic to the liver treated on a study of HAI oxaliplatin combined with systemic 5-fluorouracil/leucovorin and bevacizumab; determine the association, if any, between body composition and toxicities; and determine the association between clinical outcomes and body composition and other pretreatment characteristics.
PATIENTS AND METHODS
We conducted a retrospective analysis of data from patients who were treated on protocol, as previously published (20). Briefly, a hepatic intraarterial catheter was placed by an interventional radiologist before oxaliplatin administration. Cohorts of patients were treated with escalating doses of HAI oxaliplatin 60–175 mg/m2 intraarterially over 2 hours on day 1 and 3000 units of heparin intraarterially on day 1; followed by leucovorin at 200 mg/m2 intravenously (IV) on days 1 and 2 and a 5-fluorouracil 300 mg/m2 bolus plus 600 mg/m2 in 250 mL of Dextrose 5% as a continuous IV infusion over 22 hours on days 1 and 2 and by bevacizumab at 10 mg/kg IV over 60 minutes on day 3. Oxaliplatin dose escalation was as follows: 60, 80, 100, 120, 140, 150, 160 and 175 mg/m2. Cycles were repeated every 3 weeks. There was no intrapatient dose escalation. All procedures were performed in accordance with the guidelines of The University of Texas MD Anderson Cancer Center Institutional Review Board.
Study Design
The study was designed using a conventional “3 + 3” design, followed by an expansion phase, with patients treated at the maximum tolerated dose (MTD).
Toxicity and adverse event monitoring
Patients were monitored approximately every three weeks by physical examination, hematology and chemistry laboratory studies, measurement of vital signs, and assessment of adverse events. Patients were evaluated for adverse events > Grade 3 according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0 (22), and for the current study, all hematology and chemistry results of laboratory tests (independent of CTCAE grade) were obtained by chart review. Patients were restaged after every two cycles of therapy (1 cycle = 3 weeks).
Body composition assessment
Computed tomography (CT) imaging studies performed within four weeks before initiation of therapy were used to compute skeletal muscle, visceral adipose tissue, subcutaneous adipose tissue and intramuscular adipose tissue mass.
Body mass index (BMI) was calculated by dividing the patient’s weight in kilograms by height in meters squared, as previously described (23). Patients with BMI ≤ 18.5 kg/m2 are considered to be underweight, those with BMI > 18.5 kg/m2 and ≤ 25 kg/m2 were considered to have normal weights, and those with BMI > 25 kg/m2 are classified as overweight. Lean body mass and muscularity were calculated using the validated method described below (24). Abdominal images at the level of the 3rd lumbar vertebra were identified and saved for body composition analysis. The 3rd lumbar vertebra CT cross-sectional image was chosen for analysis because it contains muscles that are optimal for estimating lean body mass. The use of the 3rd lumbar vertebra as the landmark for body composition analysis has been described previously and validated against dual x-ray absorptiometry and bioimpedance analysis in healthy populations and in patients with advanced cancer (24–26). Muscles, subcutaneous fat, and visceral fat were identified by an operator trained in musculoskeletal anatomy using previously described Hounsfield unit thresholds (27–29) and quantified with SliceOMatic software, version 4.3 (Tomovision, Montreal, QC, Canada). Whole body composition as well as lean and fat body mass were estimated by applying the values obtained for muscularity (LBM=lean body mass) and adiposity (FM=fat mass) at the L3 level to the Mourtzakis et al. formulas ( ) with demonstrated reliability (r=0.94, p<0.0001 and r=0.88, p<0.0001, respectively) (24). Patients were considered to be sarcopenic if they had a lumbar skeletal muscle index lower than 38.5 cm2/m2 for women and less than 52.4 cm2/m2 for men, as previously described (15).
Clinical outcomes
The clinical outcomes of this study were response and overall survival. Best response was assessed by an MD Anderson radiologist and a physician team in the departmental RECIST assessment clinic starting after 2 cycles of therapy, and then after every subsequent 2 cycles (1 cycle = 3 weeks) using RECIST (30) guidelines. Survival was measured from start of treatment on protocol until death from any cause or last follow-up. Patients still alive at the last follow up were censored at that time point.
Statistical methods
Descriptive statistics analysis was performed first. Lean body mass – normalized oxaliplatin doses (mg/kg of lean body mass) were obtained by dividing the oxaliplatin dose administered by the estimated amount of total lean body mass. Grade 3/4 adverse events were described using contingency tables. Continuously scaled measures including body composition variables were summarized with mean and standard deviation. Waterfall plot analysis was used to illustrate antitumor activity (31). A t-test or Mann-Whitney test was used to assess the body composition variables between different groups (grade 3/4 toxicity versus no grade 3/4 toxicity, for example). Chi-square or Fisher’s exact tests (where appropriate) were used to assess association between two categorical variables. We estimated the overall survival distributions using Kaplan-Meier curves and compare these distributions among groups using the log rank test. A p-value <0.05 was considered statistically significant. Spearman coefficients were calculated to assess correlations between body composition variables. Statistical analyses were performed using SPSS v.16 (SPSS Inc., Chicago, IL) and S-Plus, version 7.0 (Insightful Corp., Seattle, WA) software.
RESULTS
Of the 57 patients who participated in the original phase I trial (20), data for determination of body composition by CT scans were available for 48 patients. Of the remaining 9 patients, 8 had images that were of too poor quality to assess and another patient who was 11 years old had no CT analyzed because the technique has not been validated for patients younger than 18 years.
Of 48 patients, the mean age was 56 years (range 32–76 years), and 29 (60%) participants were women. The most common diagnosis was colorectal cancer (22 patients, 46%), followed by melanoma (7 patients, 15%). Detailed demographic information is shown in Table 1. No statistically significant differences were found between men’s and women’s demographic data. In addition, when comparing patients in the expansion cohort with those in other cohorts, the only statistically significant difference (p=0.003) was in the number of patients with melanoma, which was none in the expansion cohort and seven in the other cohorts.
Table 1.
Patient characteristics
| Expansion cohort (N=26) | Escalation cohorts (N=22) | All patients (N=48) | |
|---|---|---|---|
|
| |||
| n (%) | n (%) | n (%) | |
| Age, years [mean (SD)] | 57 (11) | 54 (10) | 56 (11) |
| Gender | |||
| Female | 16 (62%) | 13 (59%) | 29 (60%) |
| Male | 10 (38%) | 9 (41%) | 19 (40%) |
| Diagnosis | |||
| Colorectal | 14 (54%) | 7 (32%) | 21 (44%) |
| Melanoma | 0 (0%) | 7 (32%)* | 7 (15%) |
| Ovarian | 1 (4%) | 2 (9%) | 3 (6%) |
| Breast | 1 (4%) | 1 (5%) | 2 (4%) |
| Cholangiocarcinoma | 2 (8%) | 0 - | 2 (4%) |
| Other** | 8 (32%) | 5 (25%) | 13 (27%) |
| ECOG PS | |||
| 0 | 7 (27%) | 10 (45%) | 17 (35%) |
| 1 | 18 (69%) | 12 (55%) | 30 (63%) |
| 2 | 1 (4%) | 0 - | 1 (2%) |
| Number of prior therapies [mean (SD)] | 4 (3) | 3 (2) | 4 (2) |
expansion versus escalation cohort, p=0.003
“Other” includes adenocarcinoma of the duodenum, cervical cancer, cholangiocarcinoma combined with hepatocellular carcinoma, hepatocellular carcinoma, gastric cancer, hemangioperycitoma, neuroendocrine cancer, pancreatic cancer, prostate cancer, small cell lung cancer, and unknown primary.
SD = standard deviation; ECOG = Eastern Cooperative Oncology Group; PS = performance status
Sarcopenia was present in 20 (42%) of 48 patients. Thirty (63%) of 48 patients had a BMI higher than 25 kg/m2. Of 48 patients, six (13%) presented concurrently with a BMI higher than 25 kg/m2 and sarcopenia. All body composition variables are described in Table 2. No differences were found for patients on the expansion cohort compared to patients on the other cohorts. Compared to men, women had significantly lower weights (mean ± SEM, 69.5 ± 2.9 kg versus 89.4 ± 4.7 kg, p=0.002), skeletal muscle area at L3 (mean ± SEM, 108.8 ± 3.6 cm2 versus 168.3 ± 7.0 cm2, p<0.001), visceral adipose area at L3 (mean ± SEM, 76.9 ± 11.5 cm2 versus 152.2 ± 27.4 cm2, p=0.03), muscle index (mean ± SEM, 40.1 ± 1.2 cm2/m2 versus 52.9 ± 2.1 cm2/m2, p<0.001), and total estimated lean body mass (mean ± SEM, 38.7 ± 1.1 kg versus 52.9 ± 2.1 kg, p<0.001).
Table 2.
Body composition variables
| Expansion cohort (N=26) | Escalation cohorts (N=22) | All patients (N=48) | |
|---|---|---|---|
|
| |||
| Average (SEM) | Average (SEM) | Average (SEM) | |
| Female (n=29) | |||
| Weight (kg) | 72.8 (4.1) | 65.5 (3.8) | 69.5 (2.9) |
| Skeletal muscle area at L3 (cm2) | 108.2 (5.1) | 109.6 (5.3) | 108.8 (3.6) |
| Intramuscular adipose area at L3 (cm2) | 13.5 (1.9) | 11.0 (2.2) | 12.4 (1.4) |
| Visceral adipose area at L3 (cm2) | 84.9 (17.6) | 67.0 (13.9) | 76.9 (11.5) |
| Subcutaneous adipose area at L3 (cm2) | 184.0 (27.4) | 173.7 (32.2) | 179.4 (20.5) |
| BMI (kg/m2) | 26.5 (1.4) | 24.7 (1.6) | 25.7 (1.0) |
| Muscle index (cm2/m2) | 39.4 (1.6) | 41.0 (1.9) | 40.1 (1.2) |
| Total estimated lean body mass (kg) | 38.5 (1.5) | 38.9 (1.6) | 38.7 (1.1) |
| Total estimated fat body mass (kg) | 18.9 (1.2) | 18.5 (1.4) | 18.7 (0.9) |
| Sarcopenic [n (%)] | 4 (30%) | 7 (40%) | 11 (38%) |
| BMI categories [n (%)] | |||
| <18.5 kg/m2 | 0 (0%) | 2 (15%) | 2 (7%) |
| 18.5–24.9 kg/m2 | 7 (44%) | 4 (31%) | 11 (38%) |
| 25–29.9 kg/m2 | 5 (31%) | 5 (39%) | 10 (34%) |
| ≥30 kg/m2 | 4 (25%) | 2 (15%) | 6 (21%) |
| Male (n=19) | |||
| Weight (kg) | 86.7 (5.6) | 92.4 (8.1) | 89.4 (4.7) |
| Skeletal muscle area at L3 (cm2) | 167.8 (10.7) | 168.9 (9.4) | 168.3 (7.0) |
| Intramuscular adipose area at L3 (cm2) | 14.0 (3.6) | 13.3 (2.7) | 13.7 (2.2) |
| Visceral adipose area at L3 (cm2) | 124.8 (43.4) | 182.6 (31.5) | 152.2 (27.4) |
| Subcutaneous adipose area at L3 (cm2) | 159.7 (26.8) | 230.6 (49.6) | 193.3 (27.9) |
| BMI (kg/m2) | 26.8 (1.5) | 29.2 (1.8) | 27.9 (1.2) |
| Muscle index (cm2/m2) | 51.9 (3.1) | 54.1 (2.9) | 52.9 (2.1) |
| Total estimated lean body mass (kg) | 56.4 (3.2) | 56.7 (2.8) | 56.6 (2.1) |
| Total estimated fat body mass (kg) | 17.9 (1.1) | 20.9 (2.1) | 19.3 (1.2) |
| Sarcopenic [n (%)] | 5 (50%) | 5 (55%) | 10 (53%) |
| BMI categories [n (%)] | |||
| <18.5 kg/m2 | 0 (0%) | 0 (0%) | 0 (0%) |
| 18.5–24.9 kg/m2 | 4 (40%) | 1 (11%) | 5 (26%) |
| 25–29.9 kg/m2 | 3 (30%) | 4 (44%) | 7 (37%) |
| ≥30 kg/m2 | 3 (30%) | 4 (44%) | 7 (37%) |
Toxicity
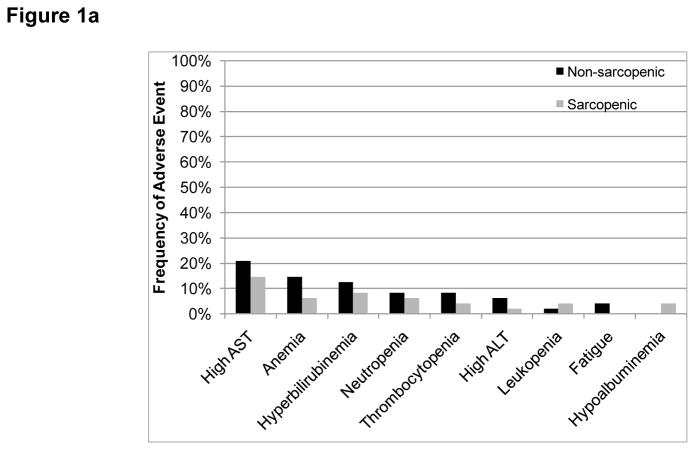
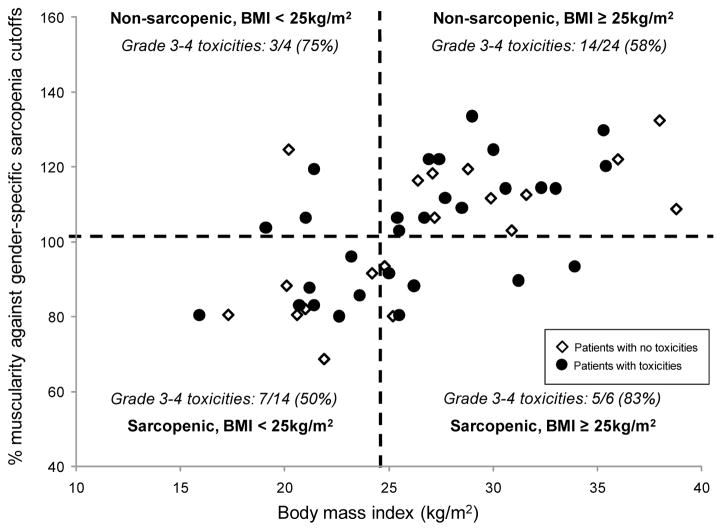
Grade 3–4 (G3–4) adverse events are summarized in Table 3 and Figure 1. No statistically significant differences were found in the frequencies of toxicities between patients with and without sarcopenia. No differences were found when comparing normalized oxaliplatin doses between patients with and without grade 3–4 toxicities (p=0.237 for the difference between normalized oxaliplatin doses for patients with or without any grade 3–4 toxicity). No differences were found with regards to the frequency of toxicities when comparing patients in the first and fourth quartiles of the normalized oxaliplatin doses distribution. The combined effects of muscularity and body mass index on toxicity are summarized in Figure 2. Most patients with G3–4 toxicities were classified as having BMI ≥ 25 kg/m2 without sarcopenia (14/29 patients with toxicities, 48%). Nevertheless, no statistically significant differences were found among the four combinations of BMI (cutoff 25 kg/m2) and presence of sarcopenia (normalized gender-specific cutoff).
Table 3.
Grade 3–4 adverse events
| a. Frequency of grade 3–4 adverse events
| ||||||
|---|---|---|---|---|---|---|
| Escalation cohorts (N=22) | Expansion cohort (N=26) | All patients (N=48) | ||||
|
| ||||||
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Fatigue | 1 | 1 | 2 | |||
| Leukopenia | 1 | 2 | 3 | |||
| Anemia | 2 | 7 | 1 | 9 | 1 | |
| Thrombocytopenia | 3 | 3 | 6 | |||
| Neutropenia | 4 | 1 | 2 | 6 | 1 | |
| AST | 5 | 2 | 7 | 3 | 12 | 5 |
| ALT | 1 | 1 | 1 | 1 | 2 | 2 |
| Hyperbilirubinemia | 3 | 5 | 2 | 8 | 2 | |
| Hypoalbuminemia | 2 | 2 | ||||
| b. Normalized oxaliplatin dose/lean body mass according to presence of grade 3–4 toxicities
| |||
|---|---|---|---|
| Grade 3–4 toxicity | |||
| Absent | Present | ||
|
| |||
| Median oxaliplatin/LBM dose (mg/kg) (IQR) | Median oxaliplatin/LBM dose (mg/kg) (IQR) | P* | |
| Any grade 3–4 | 10.32 (7.52–12.49) | 12.15 (9.3–14.22) | 0.237 |
| Any hematological | 10.18 (7.3–12.54) | 10.77 (9.47–12.54) | 0.317 |
| Any hepatic | 10.24 (7.25–12.54) | 11.07 (9.05–12.55) | 0.503 |
| Fatigue | 10.36 (8.92–12.54) | 10.93 (9.3–12.56) | 0.819 |
| Leukopenia | 10.32 (8.92–12.54) | 10.76 (9.47–11.09) | 1.000 |
| Anemia | 10.31 (7.3–12.54) | 10.82 (9.63–12.54) | 0.334 |
| Thrombocytopenia | 10.31 (8.97–12.54) | 10.72 (6.97–12.56) | 0.867 |
| Neutropenia | 10.4 (8.97–12.54) | 9.47 (6.97–10.77) | 0.510 |
| AST | 10.32 (7.3–12.54) | 10.77 (8.98–12.61) | 0.788 |
| ALT | 10.36 (8.95–12.51) | 10.98 (7.16–13) | 0.957 |
| Hyperbilirubinemia | 10.31 (7.52–12.54) | 11.16 (9.11–12.15) | 0.755 |
| Hypoalbuminemia | 10.31 (8.92–12.54) | 13.16 (12.1–14.22) | 0.195 |
Note: Nausea, diarrhea, emesis, abdominal pain, and creatinine were reviewed and did not appear as Grade 3–4 adverse events. Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; LBM, lean body mass; IQR, interquartile range.
Mann-Whitney test
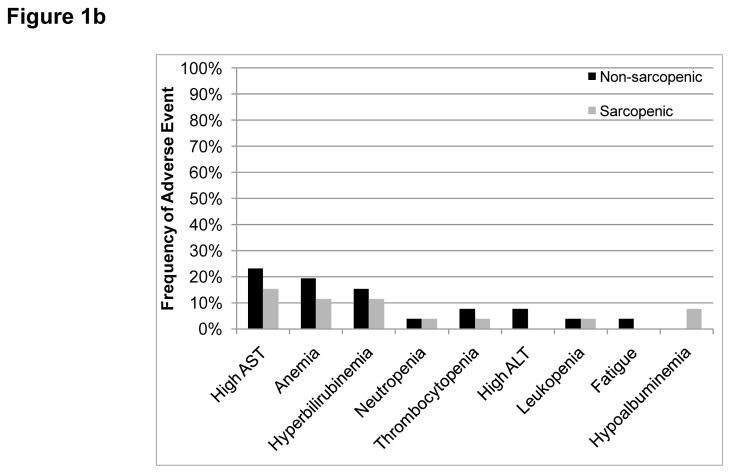
Figure 1.
Frequency of grade 3–4 adverse events according to sarcopenia status (a) All participants (b) Only participants in the expansion cohort. No statistically significant differences were found.
Figure 2. Frequency of patients with grade 3–4 adverse events according to BMI and presence of sarcopenia.
Patients with grade 3–4 adverse events (black circles) are depicted along with patients without such events (empty diamonds). Muscle index cutoffs for sarcopenia were normalized according to gender on a scale in which 100% matches the gender-specific cutoff. The vertical dashed line marks the cutoff of 24.9 kg/m2 for BMI, and the horizontal line depicts the 100% cutoff for sarcopenia. No statistically significant differences in frequency of grade 3–4 adverse events were found among the 4 quadrants (p=0.381)
Survival
Median overall survival for the sarcopenic patients was 167 days (95% CI = 128 – 206), whereas for the non-sarcopenic group it was 280 days (95% CI = 214 – 346, p = 0.271). Median overall survival for patients with a BMI < 25 kg/m2 was 179 days (95% CI = 138 – 377), and it was 267 days for patients with a BMI ≥ 25 kg/m2 (95% CI = 173 –360, p = 0.778) (Figure 3a). Patients with concurrent BMI ≥ 25 kg/m2 and sarcopenia had a median overall survival of 267 days (95% CI = 176 – 358) versus 240 days (95% CI = 140 – 465) for all other patients (p = 0.541). Overall survival of patients treated at the MTD did not differ from that of patients treated on the dose escalation cohorts (251 days [95%CI = 174 – 327] versus 220 days [95%CI = 39 – 401], respectively, p = 0.402). Among the patients treated at the MTD, the 14 patients who were not sarcopenic seemed to have much longer median survival (312 days [95% CI = 231 – 393]) compared to the 12 patients with sarcopenia (103 days [95% CI = 100–358]) (Figure 3b); however, this apparent difference was not statistically significant (p = 0.173) due to the small sample size. Patients with a BMI < 25 kg/m2 and concurrent sarcopenia had no statistically significant differences in survival compared to all other patients (median survival 167 days [95% CI = 26 – 308] versus 267 days [95%CI = 188 – 346], p=0.5). Survival of patients with BMI < 25 kg/m2 and concurrent sarcopenia was not statistically different from the survival of patients with BMI ≥ 25 kg/m2 without sarcopenia (167 days [95% CI = 26–308] versus 280 days [95% CI = 194–366], respectively, p = 0.476).
Figure 3. Survival analyses.
Survival analyses were performed using Kaplan-Meier curves and log-rank tests to detect differences in survival. (a) Overall survival of all patients (n=48) according to sarcopenia status (p=0.778). (b) Overall survival for patients in the expansion cohort (n=26) according to sarcopenia status, p=0.173.
Treatment response
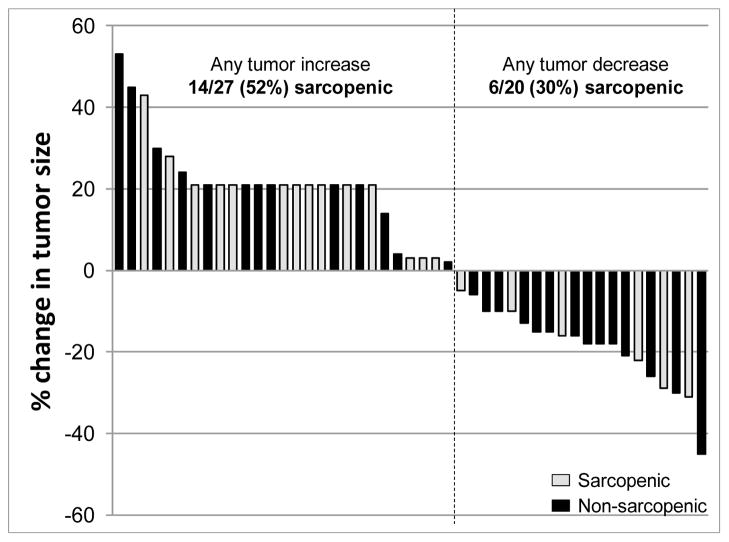
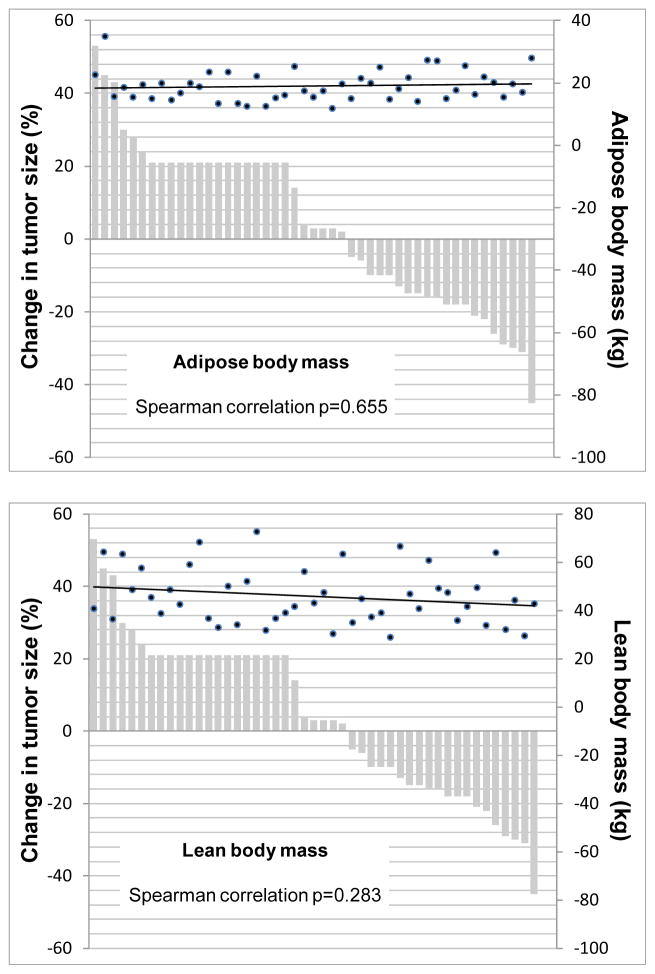
Treatment response data were available for 47 patients, and there was no association between response and the presence of sarcopenia. Among the 27 patients without sarcopenia, 14 (52%) had a reduction in tumor size, whereas only six of 20 (30%) patients with sarcopenia achieved such a reduction (p=0.152) (Figure 4). In addition, treatment response was not correlated with specific body composition variables (Figure 5) or with lean body mass - normalized oxaliplatin doses (median 10.84 mg/kg [interquartile range 9.14–13] for patients with any tumor decrease and 10.24 [interquartile range 8.22–12.48] for patients who any tumor increase, p=0.38).
Figure 4. Waterfall plot depicting best response by RECIST.
Changes in tumor size from baseline are shown. Change in the tumors of patients who progressed clinically but had no RECIST measurements available is arbitrarily shown as an increase of 21% from the baseline. Black bars represent patients without sarcopenia, while light grey bars represent patients with sarcopenia (p=0.171).
Figure 5. Waterfall plots depicting best response by RECIST and its correlation with body composition variables.
Changes in tumor size from baseline are shown. Change in the tumors of patients who progressed clinically but had no RECIST measurements available is arbitrarily shown as an increase of 21% from the baseline. Black dots depict the body composition variables (estimated amounts of lean body mass and adipose body mass) for each patient. The black lines represent a fitted regression line for the body composition variables in each graph. Changes in tumor size are depicted in the left vertical axis, while the estimated amounts of lean body mass and adipose body mass are shown in the secondary (right) vertical axis. The Spearman correlations between each body composition variable and RECIST responses were not statistically significant (p=0.655 and p=0.283 for adipose and lean body mass, respectively).
DISCUSSION
To our knowledge, this is the first study to explore the role of body composition variables in adverse events and response to HAI therapy. Sarcopenia was not directly associated with the side effect profile of HAI therapy or with response to therapy. In addition, we have shown that other body composition estimates such as total lean body mass and total adipose body mass were also not correlated with response to therapy.
All patients on the current study had advanced cancer and had been treated with an average of four prior therapies before participation on the protocol of HAI oxaliplatin treatment combined with systemic IV infusion of bevacizumab, leucovorin and fluorouracil (20).
We found an overall frequency of sarcopenia of 42%. This is similar to previous reports, such as 56% sarcopenia in a sample of 111 patients with advanced pancreatic cancer (p=0.12 versus our population) (32), and 51% in a group of 104 patients with diverse solid tumors referred to our Phase I Clinical Trials Program (p=0.3 versus the patient population in the current study) (33).
A wide range of adverse events was observed in our study patients (Table 3), and sarcopenia was not associated with their presence, as depicted in Figure 1. Oxaliplatin is known to cause several side effects, such as peripheral neuropathy, nausea, vomiting, diarrhea, mucositis, and myelosupression (34). However, here we found no association between toxicities and oxaliplatin administration, and also no differences between lean body mass-normalized oxaliplatin doses and toxicities. We hypothesize that this is because the direct HAI route of administration at least partially eliminates distribution to other body tissues, reducing the systemic impact of the drug. Lower plasma concentrations of platinum were previously reported in animal models receiving oxaliplatin by HAI compared to standard IV administration (35). With respect to 5-fluorouracil and leucovorin, Prado et al. showed that lower lean body mass predicted a higher frequency of toxicities in a study of 62 patients with colorectal cancers treated with a 5-fluorouracil-based regimen (36). The Prado et al. report, however, considered toxicities reported only during the first cycle of therapy, whereas our study assessed all adverse events in all treatment cycles. In addition, patients on the Prado et al. study received a higher total dose of 5-fluorouracil (2125 mg/m2 versus 1800 mg/m2 for patients on the current study), which might also have increased the frequency of side effects. Although patients with a combination of sarcopenia and overweight seemed to have a high frequency of G3–4 adverse events (83%) in our limited sample, as shown in Figure 2, there is no statistically significant difference on adverse events among the 4 subgroups by sarcopenia and BMI (< 25 versus ≥ 25).
Patients with normal muscularity had an overall survival duration 68% longer (approximately 3.5 months) than patients with sarcopenia (280 days [95%CI=214–346] versus 167 days [95%CI=128–206]), as shown in Figure 3. Even though this difference had practical importance, it did not attain statistical significance. Previously, it has been shown that the combination of sarcopenia and BMI ≥ 25 kg/m2 was predictive of a shorter overall survival (15, 32). In the current study, however, this was not the case as the overall survival of patients with concurrent sarcopenia and BMI ≥ 25 kg/m2 was almost equal to that of the other body composition groups (267 days [95%CI = 176 –358] versus 240 days [95% CI = 14–465], respectively, p=0.541). We hypothesize that this is due to the small number of patients with concurrent sarcopenia and BMI ≥ 25 kg/m2 (n = 6 patients), which did not allow meaningful statistical comparisons to be made. Further research in larger samples is needed to fully evaluate this finding. Patients without sarcopenia who were treated at the MTD and evaluated independently demonstrated a survival advantage of more than 6 months compared to patients with sarcopenia, but this was not statistically significant, perhaps because of the small number of patients in this subgroup. Even though sarcopenia might play a role in reducing survival, the survival differences might, in fact, be due to the differences in underlying diseases. Some patients had shorter survival because of the greater severity of their cancers, with sarcopenia being a consequence of more severe disease. Causality cannot be proven by this small retrospective study.
There was no statistically significant difference on frequent reduction in tumor size by RECIST between patients with and without sarcopenia (6/20, 30% versus 14/27, 52%, respectively, p=0.152) (Figure 4). Several factors could be causal, including differences in disease severity, with patients suffering from more aggressive diseases being more likely to have sarcopenia, and the altered distribution of drugs across body tissues in patients with sarcopenia. Further investigation is needed to determine the effects of sarcopenia on survival and response in this treatment setting.
Other continuous body composition variables (total body adipose tissue and total body lean tissue) did not correlate with response to treatment, as shown in Figure 5.
In conclusion, the present study suggests that sarcopenia is likely not associated with treatment toxicities when treatment is given via the HAI route. Although it has been recently proposed that more precise body composition variables should be used to determine drug dosing for cancer treatment (15, 16, 36), such factors might not have significant clinical importance for patients receiving HAI-based treatments. Because systemic effects are attenuated when drugs are administered by the HAI route, variables such as sarcopenia, which might contribute to toxicity after IV or oral drug administration, might assume less importance when treatment is given via hepatic infusion. Indeed, a considerable amount of literature suggests that repeated infusion is associated with less intense toxicities (18, 20, 21) while maintaining an increasing concentration of chemotherapy at the tumor bed in the liver. Our current data further suggest that patients with sarcopenia are not at higher risk for toxicity after HAI treatment, which broadens the utility of HAI for the treatment of patients with advanced cancer.
Acknowledgments
FUNDING
Supported in part by Grant Number RR024148 from the National Center for Research Resources, a component of the NIH Roadmap for Medical Research, and by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
References
- 1.Murthy NS, Mukherjee S, Ray G, Ray A. Dietary factors and cancer chemo prevention: An overview of obesity-related malignancies. J Post grad Med. 2009;55:45–54. doi: 10.4103/0022-3859.43549. [DOI] [PubMed] [Google Scholar]
- 2.Sarhill N, Mahmoud F, Walsh D, Nelson KA, Komurcu S, et al. Evaluation of nutritional status in advanced metastatic cancer. Support Care Cancer. 2003;11:652–9. doi: 10.1007/s00520-003-0486-0. [DOI] [PubMed] [Google Scholar]
- 3.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, et al. Cachexia: A new definition. Clin Nutr. 2008;27:793–9. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Ma G, Alexander HR. Prevalence and pathophysiology of cancer cachexia. In: Bruera E, Portenoy R, editors. Topics in palliative care. New York: Oxford University Press; 1998. pp. 91–129. [Google Scholar]
- 5.Deans DA, Wigmore SJ, de Beaux AC, Paterson-Brown S, Garden OJ, et al. Clinical prognostic scoring system to aid decision-making in gastro-oesophageal cancer. Br J Surg. 2007;94:1501–8. doi: 10.1002/bjs.5849. [DOI] [PubMed] [Google Scholar]
- 6.Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345–50. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
- 7.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern cooperative oncology group. Am J Med. 1980;69:491–7. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 8.Fouladiun M, Korner U, Gunnebo L, Sixt-Ammilon P, Bosaeus I, et al. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin Cancer Res. 2007;13:6379–85. doi: 10.1158/1078-0432.CCR-07-1147. [DOI] [PubMed] [Google Scholar]
- 9.Hinsley R, Hughes R. ‘the reflections you get’: An exploration of body image and cachexia. Int J Palliat Nurs. 2007;13:84–9. doi: 10.12968/ijpn.2007.13.2.23068. [DOI] [PubMed] [Google Scholar]
- 10.Dahele M, Skipworth RJ, Wall L, Voss A, Preston T, et al. Objective physical activity and self-reported quality of life in patients receiving palliative chemotherapy. J Pain Symptom Manage. 2007;33:676–85. doi: 10.1016/j.jpainsymman.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS, Sarcopenia J Lab Clin Med. 2001;137:231–43. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 12.Roubenoff R. Excess baggage: Sarcopenia. obesity, and cancer outcomes. Lancet Oncol. 2008;9:605–7. doi: 10.1016/S1470-2045(08)70160-8. [DOI] [PubMed] [Google Scholar]
- 13.Roubenoff R. Sarcopenia: Effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58:1012–7. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- 14.Roubenoff R. Exercise, sarcopenia, cognition, and mood. Nestle Nutr Workshop Ser Clin Perform Programme. 2002;6:151–9. doi: 10.1159/000061864. discussion 160–2. [DOI] [PubMed] [Google Scholar]
- 15.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008;9:629–35. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 16.Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–6. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- 17.Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol. 2010;21:1594–1598. doi: 10.1093/annonc/mdp605. [DOI] [PubMed] [Google Scholar]
- 18.Barber FD, Mavligit G, Kurzrock R. Hepatic arterial infusion chemotherapy for metastatic colorectal cancer: A concise overview. Cancer Treat Rev. 2004;30:425–36. doi: 10.1016/j.ctrv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–48. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 20.Tsimberidou AM, Fu S, Ng C, Lim JA, Wen S, et al. A phase 1 study of hepatic arterial infusion of oxaliplatin in combination with systemic 5-fluorouracil, leucovorin, and bevacizumab in patients with advanced solid tumors metastatic to the liver. Cancer. 2010;116:4086–94. doi: 10.1002/cncr.25277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ducreux M, Ychou M, Laplanche A, Gamelin E, Lasser P, et al. Hepatic arterial oxaliplatin infusion plus intravenous chemotherapy in colorectal cancer with inoperable hepatic metastases: A trial of the gastrointestinal group of the federation nationale des centres de lutte contre le cancer. J Clin Oncol. 2005;23:4881–7. doi: 10.1200/JCO.2005.05.120. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Therapy Evaluation Program (CTEP) National cancer institute common toxicity criteria version 3.0 [Google Scholar]
- 23.Billewicz WZ, Kemsley WF, Thomson AM. Indices of adiposity. Br J Prev Soc Med. 1962;16:183–8. doi: 10.1136/jech.16.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 25.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, et al. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97:2333–8. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 26.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, et al. Visceral adipose tissue: Relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80:271–8. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–22. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 28.Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, et al. Appendicular skeletal muscle mass: Measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–8. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- 29.Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, et al. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998;351:871–5. doi: 10.1016/S0140-6736(97)11518-5. [DOI] [PubMed] [Google Scholar]
- 30.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 31.Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, et al. Phase ii placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–12. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 32.Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:6973–9. doi: 10.1158/1078-0432.CCR-09-1525. [DOI] [PubMed] [Google Scholar]
- 33.Parsons HA, Baracos V, Dhillon N, Kurzrock R. A preliminary investigation of body composition, symptom burden and survival in a phase i clinical trials service (abstr) Support Care Cancer. 2010;18:S140. [Google Scholar]
- 34.Reuters Thompson. Micromedex. 2010;1.0 [Google Scholar]
- 35.Dzodic R, Gomez-Abuin G, Rougier P, Bonnay M, Ardouin P, et al. Pharmacokinetic advantage of intra-arterial hepatic oxaliplatin administration: Comparative results with cisplatin using a rabbit vx2 tumor model. Anticancer Drugs. 2004;15:647–50. doi: 10.1097/01.cad.0000131684.06390.fe. [DOI] [PubMed] [Google Scholar]
- 36.Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13:3264–8. doi: 10.1158/1078-0432.CCR-06-3067. [DOI] [PubMed] [Google Scholar]