Abstract
Synesthetic color induced by graphemes is well understood to be an automatic perceptual phenomenon paralleling print color in some ways but also differing in others. We address this juxtaposition by asking how synesthetes are affected by synesthetic and print colors that are the same. We tested two groups of grapheme-color synesthetes using a basic color priming method in which a grapheme prime was presented followed by a color patch (probe), the color of which was to be named as quickly and accurately as possible. Primes induced either no color, print color only, synesthetic color only, or both forms of color (e.g., a letter “A” printed in red that also triggers synesthetic red). As expected, responses to name the probe color were faster if it was congruent with the prime color than if it was incongruent. The new finding (Expt 1) was that a prime that induced the same print and synesthetic color led to substantially larger priming effects than either one individually, an effect that could not be attributed to semantic priming (Expt 2). In addition, the synesthesia effects correlated with a standard measure of visual imagery. These findings are discussed as consistent with the hypothesis that print and synesthestic color converge on similar color mechanisms.
Keywords: synesthesia, grapheme, color, priming, visual imagery, VVIQ
1. Introduction
For a small percentage of the population, two colors may be triggered by one physical stimulus and reported as appearing simultaneously without mixing or canceling. This unique experience is hard to imagine for typical observers, but described vividly by grapheme-color synesthetes. These synesthetes experience automatic, conscious, and consistent colors triggered by a grapheme (e.g., a letter or number) independent of the color in which the grapheme is printed. Understanding the interaction of grapheme-induced synesthetic color and wavelength-induced color may help to explain the mechanisms underlying color vision more generally. Grapheme-induced colors in synesthetes are thought to arise through mechanisms similar to those in normal visual perception (Kim, Blake, & Palmeri, 2006) and be perceptually bound to shape by the same brain mechanisms as typical features of vision (Robertson, 2003; Sagiv, Heer, & Robertson, 2005; Ward, Li, Salih, & Sagiv, 2007). However, in typical observers two hues cannot be experienced simultaneously in the same spatial location. This seems to question both the anecdotal self-reports by some grapheme-color synesthetes who say that different synesthetic and printed hues appear in the exact same location simultaneously, and the hypotheses that synesthesia can function through normal mechanisms of perception and binding. On the contrary, this study will suggest a model based strongly on previous research that will help to explain how such a self-report by synesthetes is not only possible, but not unexpected given what is known about the representation of synesthetic color.
Maljkovic & Nakayama (1994) first showed that a color presented on one trial can influence response times (RT) on subsequent trials in a non-synesthetic group of participants, speeding the response to a specific color when it had appeared previously and slowing the response when it had not. Perceptual color priming effects were first extended to the study of synesthetic color by Mattingley, Rich, Yelland, & Bradshaw (2001). This group showed that synesthetic color, induced by a black printed grapheme, created a positive priming effect; synesthetes were faster to name the print color of a color patch probe when the probe was congruent to the synesthetic color induced by an achromatic grapheme prime. Mattingley, et al., (2001) name this effect a ‘congruency effect’ and we will use this terminology for the remainder of this manuscript. This finding suggests that synesthetic and print color pathways overlap enough that one form of color representation can trigger the other. Similar studies have validated such an interaction between synesthetic and print color behaviorally (Kim, Blake, & Palmeri, 2006; Kim & Blake, 2005) and neurophysiologically (Hubbard, Arman, Ramachandran, & Boynton, 2005; Brang, Hubbard, Coulson, Huang, & Ramachandran, 2010).
However, several studies have also shown that synesthetic and print colors do not operate in the same way. Synesthetic color requires attention and awareness of the grapheme in order to be induced (Mattingley, et al., 2001; Rich & Mattingley, 2003; Laeng, Svartdal, & Oelmann, 2004; Sagiv, Heer, & Robertson, 2005; Mattingley, Payne, & Rich, 2006; Rich & Mattingley, 2010), unlike the preattentive ‘pop out’ effects attainable with print color (Maljkovic & Nakayama, 1994; Treisman 1982). Likewise, many research groups have created synesthetic Stroop-like effects by presenting graphemes that are printed in hues incongruent to their synesthetic colors (e.g., letter “A” printed in blue while inducing synesthetic red, Odgaard, Flowers, & Bradman, 1999; Mills, Boteler, & Oliver, 1999; Dixon, Smilek, & Merikle, 2004; Ward, et al., 2007).
The synesthetic Stroop effect relies on the fact that synesthetic and print colors do not blend. Synesthetic Stroop, combined with the dual-color self-reports by synesthetes and recent brain-imaging studies (van Leeuwen, Petersson, & Hagoort, 2010; Hupé, Bordier, & Dojat, 2012), suggests that synesthetic and print colors are neurophysiologically independent and operate through different and possibly rivalrous networks. Synesthetic color has been hypothesized to result from either direct connections between hue-selective (V4) and shape-selective cortical maps (Brang, et al., 2010; Hubbard, 2007), via reentrant feedback between higher semantic and lower visual cortical regions (Smilek, Dixon, Cudahy, & Merikle, 2001), or from feed forward and feedback interactions of early visual and higher order cortical binding mechanisms through color processing pathways (Hubbard, 2007; Robertson, 2003). In the present studies we used a behavioral measure to determine how print and synesthetic color might interact in perception and discuss the results in relation to neurobiological evidence of print and synesthetic color processing.
We adopted a color priming method similar to Mattingley, et al., (2001). However, we created four conditions; a prime appeared that triggered either synesthetic color (s), print color (p), the combination (c) of synesthetic and print colors, or no color (b, baseline) on randomly interleaved trials. In the case where primes triggered the combination of synesthetic and print colors, we were careful to make sure that primes were printed in the same synesthetic color that they induced, triggering the perception of the same hue through print and synesthetic mechanisms. The prime was followed immediately by a print colored probe that was congruent or incongruent to the color of the prime (see Figure 1). In the first experiment, the prime appeared for 750 msec and was followed immediately by a colored probe. In the second experiment, all conditions were the same except that the prime appeared for only 200 msec. The shortened prime duration was created to address the possibility that congruency effects were the result of semantic priming (e.g., thinking “red” in conditions where print, synesthetic or both colors were primed) rather than perceptual priming. The shorter prime duration also allowed us to examine the interaction of print and synesthetic color priming at shorter and longer processing times.
Figure 1.

Participants were primed and asked to name the color of a subsequent probe aloud into a headset. Primes induced either no color (baseline, b), print color only (p), synesthetic color only (s), or the combination (c) of print and synesthetic color. Probes were congruent (CC) or incongruent (IC) to prime color. In Expt. 1, 13 synesthetes and 13 controls were presented with a 750 msec Prime. In Expt. 2, the Prime duration was reduced to 200 msec and presented to nine synesthetes.
We propose two likely models based on the available research and test them with our behavioral priming paradigm. In one model, synesthetic and print colors operate through discrete neurophysiological pathways that do not converge before hue has been processed. In this model, a letter that induces the same hue through synesthetic and print pathways (e.g., print-colored red “A” that also triggers the experience of synesthetic red) will only create as much of a priming effect as either a print colored, or synesthetically colored letter on its own; since the two color pathways remain separate, the effects of the combination condition cannot exceed either individual effect. In the second model, synesthetic and print colors begin via separate pathways for shape and wavelength respectively, and then converge on overlapping cortical regions that process hue, such as hue-selective visual area V4 (Hubbard, et al., 2005; Brang, et al., 2010). This model predicts that when a letter is printed in the same color it triggers synesthetically, the two color signals will converge and the resulting priming effect will be larger than either effect triggered from only synesthetic color or print color primes alone. Neurophysiological and psychological studies have confirmed the presence of hue-selective regions in several early visual areas (Xiao, Casti, Xiao, & Kaplan, 2007; Xiao, Wang, & Felleman, 2003; Conway, 2001) including area V4 (Conway & Tsao 2009; Tanigawa, Lu, & Roe, 2010) and studies have validated the role of the inferotemporal regions of the cortex (Rouw and Scholte, 2007) and specifically area V4 (Hubbard et al., 2005; Brang et al., 2010) in synesthetic color experience for grapheme-color synesthetes.
As a final point, grapheme-color synesthesia can be experienced simply by imagining a grapheme without the presence of a physical stimulus (Elias, Saucier, Hardie, & Sarty, 2003; Jansari, Spiller, & Redfern, 2006; Spiller and Jansari, 2008). The relationship of synesthetic experience to visual imagery was proposed by Ramachandran and Hubbard (2001) and has since been validated in synesthetes (Barnett and Newell, 2008; Price 2009). Barnett and Newell (2008) used a qualitative self-report survey called the Vividness of Visual Imagery Questionnaire (Marks, 1973) to show that a population of synesthetes had more vivid imagery than non-synesthetic controls. We were curious whether this difference in visual imagery was simply a passive phenomenon or whether it might correlate with a behavioral metric of visual perception. For this reason, we also used the same measure of the vividness of visual imagery (VVIQ) to test for a correlation between synesthetic congruency effects and visual imagery in our experiment. We hypothesized that more vivid imagery would correlate with larger congruency effects for synesthetes, perhaps due to a general increase in hue sensitivity (Ward, et al., 2007) in perceptual networks that overlap with those responsible for generating internal visual representations (Mechelli, Price, Friston, & Ishai, 2004).
2. Methods
2.1. Participants
All participants had normal or corrected to normal vision, no reported history of neurological or psychiatric disorder, and gave signed informed consent before entering the study (as approved by the UCB Committee for Protection of Human Subjects). All participants participated for cash compensation.
Thirteen grapheme-color synesthetes between the ages of 19 and 40 (M = 27.7, SD = 7.23, 9 female) participated in Experiment 1, and nine synesthetes between the ages of 19 and 32 (M = 24.0, SD = 5.5, 8 female) participated in Experiment 2. Five synesthetes participated in both experiments. In these experiments, a prime was followed immediately by a colored patch probe on each trial. Each subject was given instructions to name the color of the probe as rapidly as possible. 13 non-synesthete controls matched for age and sex (M = 27.2, SD = 5.32, 9 female) also participated in Experiment 1. Synesthetes and controls in Experiment 1 were yoked: each control participant named the same specific probe colors as the synesthete that he or she was matched to.
Data from two additional synesthetic participants were excluded from Experiment 1, one because of a post-participation report of being in a coma for several days during the weeks just prior to participation, and the other because of too many errors during the task (26 percent of all trials were removed for this subject, which was far beyond the 10 percent maximum criterion).
2.2. Materials and Procedure
Synesthesia was assessed and color matches were gathered using the online Synesthesia Battery (Eagleman, Kagan, Nelson, Sagaram, & Sarma, 2007). Upon arriving at the lab, each synesthete used the testing computer to re-match colors for 3 letters chosen as primes that induced the most consistent color representations for that synesthete. We only used colors that could be named using easily spoken basic color terms (red, yellow, blue, or green).
Synesthetes and yoked controls performed a color-naming task (Figure 1) in which a prime was followed by a print colored probe. Primes consisted of one of four randomized stimulus types: achromatic non-inducing symbols (baseline, b), print-colored symbols (print, p), achromatic letters that induce synesthetic color (synesthesia, s), or letters that induced synesthetic color and were colored in their congruent synesthetic color (combo, c). Probes appeared in either the congruent or incongruent color relative to the prime. Note that in the case of the baseline condition the congruent/incongruent distinction is irrelevant since baseline primes do not induce any form of color. On each trial, one of three possible achromatic grapheme primes (‘A’ in Figure 1) appeared in the center of the display for either 750 msec (Experiment 1) or 200 msec (Experiment 2). A colored probe then replaced the prime, appearing in the same foveal location and remaining until response. Participants sat 57 cm from the monitor and named the color of the target patch as quickly and accurately as possible. Voice onset time of the verbal response was recorded using a head-mounted microphone and data were filtered for outliers and hardware errors after the experiment was complete.
Stimuli were presented with a Dell Dimension DM051 2.80 GHz Intel Pentium processor on a Viewsonic G225f CRT computer monitor, with a refresh rate of 120 Hz (8.3 msec per frame). Graphics were presented using a NVIDIA Quadro fx 1300 graphics driver and response time to name the target patch color was recorded with millisecond accuracy using a Plantronics DSP 400 digitally enhanced headset.
A frame remained on the screen for the duration of each block and acted as a fixation guide during the inter-trial interval. This frame was made of thin white lines outlining the four corners of a box (4.3 degrees wide) with 2 degrees of separation between each of the four corner sections (see Figure 1). Letter primes were presented in Arial font and subtended an average visual angle of 1.65 × 2.25 degrees. Non-inducing grapheme shapes (e.g., ❄, ⌘, ♎) were created using Wingdings fonts, Greek alphabet, or Pesenti symbols (Pesenti, Thioux, Seron, & Volder, 2000) and subtended the same average visual angle as letters. Probes were circular colored patches (2.04 deg). A complete trial began with a prime (750 msec Exp. 1, 200 msec Exp. 2), followed immediately by a probe which remained on the screen until voice onset. The response was followed, by an inter-trial interval (750 msec, both experiments) where only the reference frame remained on the monitor.
In Experiment 1, a total of 240 trials were run, divided into 4 blocks of 60 trials, and in Experiment 2 the number was reduced to 192 trials, divided into 4 blocks of 48 trials. We reduced the number of trials to shorten the overall presentation. All blocks in both experiments contained equal numbers of all possible combinations of the conditions (4 primes × 2 color congruency). Trials were pseudo-randomized so that primes appeared randomly but no more than two trials of the same color probe could appear consecutively. Incongruent colors were also pseudo-randomly matched to primes so that a patch congruent to the color of one prime was assigned as the incongruent color to another prime. This assured that there were equal numbers of congruent and incongruent trials. This counterbalanced, pseudo-randomized design controlled for semantic priming effects that could occur if one color was named several times in a row as well as variance in luminance between colored probes. Luminance was not equated because it was essential that the probe colors chosen matched synesthetic colors.
To begin the experiment participants completed four practice trials presented at the beginning of the first block that contained a letter prime not used in the test trials. These practice trials allowed participants to practice naming colors into the head-mounted microphone.
2.3. Data Analysis
To assess whether participants responded correctly to probe color patches, the researcher sat next to the participant and checked whether the spoken response matched the target color that appeared. If the participant did not clearly produce the correct color name on the first try, the response was discarded. Response times three standard deviations or more above the mean were excluded as were those occurring earlier than 150 msec after the offset of the prime. Less than 1% of responses fell within this early period, and those that did were due to accidental noises made by the participant (e.g., mouth smacking, coughing, etc). Mechanical and response errors resulted in the removal of 4.11 percent of data in Experiment 1 (synesthetes 4.01%, controls 4.39%) and 4.63 percent of data in Experiment 2 (synesthetes only).
In Experiment 1, a mixed design (group as a between-subjects factor) was first used to compare the priming effects of synesthetes to yoked controls after outliers and errors were removed. Next, data were analyzed separately for synesthetes and controls in Experiment 1, and synesthetes in Experiment 2, using a 2 by 4 (color congruency, prime type) within-subjects analysis of variance. Afterwards, a congruency effect metric was calculated for each participant by subtracting congruent color (CC) means from incongruent color (IC) means separately for each type of prime (baseline (no color) (b), print color only (p), synesthetic color only (s), combination of print and synesthetic color (c)) to compare the size of the congruency effect (CC – IC) across prime types (b, p, s, c). A one way analysis of variance was run on these 4 difference means and a Sidak-Bonferroni post-hoc analysis was used to correct for pair-wise comparisons on conditions for which there were no a priori hypotheses (as discussed in more detail below).
Data from a slightly modified (instructions were shortened, questions remained identical) version of the Vividness of Visual Imagery Questionnaire (VVIQ, Marks, 1973) were also collected from all synesthetes in both experiments and from yoked controls, and separate averages were made for the eyes open and eyes closed ratings on that scale for each participant (16 identical questions each). A paired-samples t-test indicated that scores were not significantly different for synesthetes in the eyes open condition (M = 2.31, SD = 0.86) and the eyes closed condition (M = 2.19, SD = 0.85), for Experiment 1, t(12) = 1.27, p = .23, or for synesthetes in Experiment 2 (eyes open M = 2.15, SD = 0.82 eyes closed M = 2.15, SD = 0.86, t(8) = 0.00, p = 1). Scores were also not significantly different for controls in the eyes open condition (M = 2.34, SD = 0.98) and the eyes closed condition in Experiment 1 (M = 2.55, SD = 1.04), t(12) = -0.76, p = .46. Thus, an average VVIQ score was created by combining the eyes open and eyes closed conditions and this average score was used.
3. Results
3.1. Experiment 1: Synesthetes and yoked controls, 750 msec prime duration
Group differences were analyzed with a mixed design ANOVA. Our interest at the group level was to identify whether synesthetes and yoked controls showed overall differences in the pattern of congruency effects for different priming conditions (Figure 2). The congruency effect is measured by subtracting congruent from incongruent RTs for each of the four priming conditions (Prime Type: b, p, s, c) resulting in a difference mean for each condition. Group was a between subjects factor while Color Congruency (congruent (CC) and incongruent (IC) probe colors relative to the prime color), Prime Type (b, p, s, or c primes), and Congruency Effect (the difference mean between congruent and incongruent RTs for b, p, s, and c conditions for each subject) were used as within-subjects factors. The critical component of this analysis was the interaction of Group by Congruency Effect, which if different would support the hypothesis that different experiences of the primes (i.e., the presence or absence of synesthetic color) would create different patterns of priming effects for synesthetes than non-synesthetes. As expected, this interaction was significant F(3,22) = 3.35, p = .037, η2 = .31. The main effect of Congruency Effect was also significant when collapsed across groups F(3,22) = 9.15, p = .0004, η2 = .56. Table 1 shows mean RTs for synesthetes and controls, and also the difference means (Congruency Effect) for each condition of each group.
Figure 2.
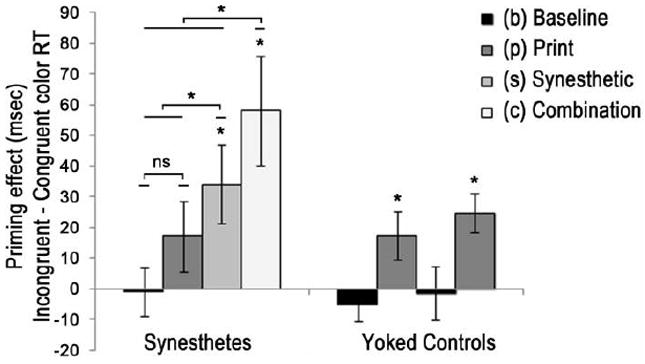
Congruency effects for all conditions and groups in Expt 1. A congruency effect is the difference between the mean RT of an incongruent and congruent color condition. This is arbitrary for black bars since these conditions induced no color for participants. For synesthetes, congruency effects were only significant for conditions that induced synesthetic color (s) and (c). The combo priming effect (c) was significantly larger than all other conditions for synesthetes and the synesthetic color condition (s) was larger than print (p) or baseline (b). Controls do not experience synesthetic color and so their data is categorized as either containing acrhomatic stimuli (black bars) or print colored stimuli (dark gray bars). Only print colored stimuli congruency effects for controls. Asterisks indicate significant effects (p < .05). Error bars indicate SEM. See Table 1 for mean RTs, priming effects, and p values.
TABLE 1.
Experiment 1, 750 msec prime duration
| Group | Synesthetes | Yoked Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| Prime Type | b | P | s | c | b | P | s | c |
|
|
||||||||
| IC (msec) | 495.6 | 508.6 | 509.3 | 515.7 | 474.0 | 476.6 | 471.5 | 478.0 |
| CC (msec) | 496.5 | 491.4 | 475.2 | 457.7 | 479.4 | 459.0 | 472.9 | 455.3 |
| Congruency Effect (msec) | -0.9 | 17.2 | 34.1* | 58.1* | -5.4 | 17.5* | -1.4 | 24.7* |
| p-value | .900 | .176 | .020 | .008 | .326 | .045 | .883 | .002 |
Mean response times for synesthetes and yoked controls across four different prime types. For synesthetes, p = print color only, s = synesthetic color only, c = combination of synesthetic and print color). For controls, b and s represent achromatic conditions, and p and c represent color conditions. Asterisks mark a difference score (the Congruency Effect) that is significant between the incongruent (IC) and congruent (CC) color conditions. Note that for controls, color congruency only applies to the p and c conditions, where primes contained print color. Minor inaccuracies in difference scores are the result of rounding to one decimal place in this table. See Fig 2 for a visualization of congruency effects.
Synesthetic Group Only
Data from synesthetes alone were analyzed using a 2 by 4 ANOVA with the within-subjects factors of Color Congruency (prime Congruent or Incongruent relative to probe) and Prime Type (b, p, s, c). There was a trend towards a main effect of Prime Type F(3,36) = 2.57, p = .07, η2 = .18 and a significant main effect of Color Congruency F(1,12) = 5.61, p = .035, η2 = .32. Mauchly’s test indicated that the assumption of sphericity had been violated for the Color Congruency by Prime Type interaction (Χ2(5) = 14.2, p = .015), and degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε = .55). The original effect of Color Congruency and Prime Type was verified by a significant interaction between the two factors F(1.6, 19.6) = 12.66, p = .0005, η2 = .51 that was due to a decrease in RT from print to synesthetic to combination prime types in the CC condition F(3,36) = 9.26, p = .0001, η2 = .436. A one-way ANOVA also confirmed a significant difference among the four incongruent RT values F(3,36) = 4.29, p = .011, η2 = .263 (see Table 1). However, this was due to significant differences between each color-based prime type (p, s, c) and the baseline condition (b). The size of the Congruency Effect for incongruent trials only was not significantly different for color-based prime types. These differences for congruent and incongruent trials show that Congruency Effects in this study are due to positive priming resulting in faster RTs over prime types rather than increased interference.
We hypothesized that all priming conditions that involved color (print, synesthetic, and combination) would produce congruency effects for synesthetes, measured by subtracting the congruent from the incongruent RTs within each condition. Paired-samples t-tests confirmed these a priori hypotheses of a significant congruency effect for the combination prime condition t(12) = 3.26, p = .007, for the synesthetic color condition t(12) = 2.72, p = .019, and an insignificant difference for the print color condition t(12) = 1.48, p = .166. RTs from the baseline condition were also compared even though these primes did not induce any form of color. This was important because a prior study had reported that synesthetes can learn novel grapheme-color associations in one testing day (Mroczko, Metzinger, Singer, & Nikolić, 2009) and it was possible that participants learned these color pairings to symbols from their print-colored counter parts. However, RTs from the baseline condition showed no significant congruency effects t(12) =.113, p = .912, confirming that these novel color pairings were not associated.
Further analyses of the difference scores showed that the size of the Congruency Effect was larger for colored prime conditions (p, s, c) than baseline (F(3,10) = 5.11, p = .021, η2 = .605). Paired-samples t-tests confirmed that the size of the Congruency Effect for the baseline condition was significantly smaller than the combination color condition, t(12) = 3.97, p = .002, the synesthetic color condition, t(12) = 3.37, p = .006, and at trend compared to the print color condition, t(12) = 2.10, p = .057 that was clearly in the hypothesized direction.
We did not predict a priori whether print color, synesthetic color, or the combination of the two colors would create the greatest congruency effects. Thus, post-hoc analyses were conducted on these three comparisons. A Šidàk-Bonferonni post-hoc correction (cutoff for three comparisons is p < .0170) indicated that the combination (c) Congrueny Effect was significantly greater than synesthetic (s) color priming, t(12) = 3.04, p = .010, and greater than print (p) color priming, t(12) = 4.09, p = .001. Synesthetic color Congruency Effects were also found to be greater than print color Congruency Effects, t(12) = 2.97, p = .012.
Yoked Control Group Only
Data from non-synesthetic controls were also analyzed using a two by four ANOVA with the within-subjects factors of Color Congruency (prime Congruent or Incongruent relative to probe) and Prime Type (colored letters, colored symbols, black letters, black symbols). Note that controls viewed the exact same prime stimuli as synesthetes, but did not experience synesthetic color for letter shapes. Thus, print colored letter primes and print colored symbol primes were expected to produce congruency effects, and black letter and symbol primes were not expected to produce congruency effects. There was no main effect of Prime Type, F(3,36) = 1.28, p = .295, η2 = .096, and a trend towards a significant main effect of Color, F(1,12) = 4.41, p = .058, η2 = .269. However, the interaction of Prime Type by Color was highly significant, F(1,12) = 4.78, p = .007, η2 = .285.
Paired-samples t-tests confirmed the a priori hypotheses that primes with print color would create congruency effects in non-synesthetes and achromatic primes would not. Controls showed significant congruency effects when primed with print-colored letters, t(12) = 3.88, p =.002 (the “combination” condition for synesthetes), and print-colored symbols, t(12) = 2.23, p = .046 (the “print color only” condition for synesthetes). Controls did not show significant congruency effects when primed with achromatic letters, t(12) = 0.160, p = .875 (the “synesthetic color only” condition for synesthetes), or achromatic symbols, t(12) = 1.01, p = .331 (the “baseline” condition for synesthetes). In summary, controls showed color priming when primed with print-colored primes, and did not show color priming when primed with achromatic primes (no synesthesia). These results were as expected.
3.2. Experiment 2: Synesthetes, 200 msec prime duration
We collected data in Experiment 2 to check whether semantic priming of the response color (e.g., priming of “red”) influenced our results1. This type of semantic priming has traditionally been thought to occur after about 250ms after stimulus onset (Neely, 1977) and if present in this study, would be expected to decrease RTs for congruent conditions and increase RTs for incongruent conditions in a similar way to that in Experiment 1. Thus, in Experiment 2, we shortened the prime duration to 200ms to reduce the chance of semantic priming while preserving synesthetic color priming; synesthetic color priming has been demonstrated at this prime duration previously (Spruyt, Koch, Vandromme, Hermans, & Eelen 2009).
We used the same within subject statistical comparisons in Experiment 2 as were used to analyze the synesthetic group in the first experiment2. A two by four ANOVA with the within-subjects factors of Color Congruency and Prime Type was run first. The main effect of Prime Type was not significant F(3,24) = 0.543, p = .657, η2 = .06. The main effect of color Congruency was significant F(1,8) = 44.1, p = .0002, η2 = .85 and there was a Congruency by Prime Type interaction F(3, 24) = 12.93, p = .00003, η2 = .62. All three comparisons passed Mauchly’s Test of Sphericity so a normal distribution was assumed.
We again hypothesized a priori that all priming conditions (p, s, c) with colored primes would show significant color congruency effects, measured by comparing RTs to congruent versus incongruent probe colors relative to the color induced in each prime condition. As expected, the Print, t(8) = 6.23, p = .0003, Synesthetic, t(8) = 3.17, p = .013, and Combo, t(8) = 5.51, p = .0006 conditions produced significant congruency effects, while the Baseline condition did not, t(12) = .787, p = .454.
A one-way ANOVA revealed significant differences between individual Congruency Effects (b, p, s, c), F(3,6) = 6.30, p = .028, η2 = .759 so we compared individual difference means of the Congruency Effect sizes for each colored-prime condition (p, s, c) to baseline (b) and to each other using paired-samples t-tests as in Experiment 1. We again assumed a priori that all Congruency Effect sizes would be larger than the Baseline condition and found significant differences in each case: Print > Baseline t(8) = 4.21, p = .003, Synesthetic > Baseline t(8) = 2.45, p = .039, Combo > Baseline t(12) = 4.43, p = .002 (Table 2). This would be expected whether perceptual or semantic priming produced the effects. However, if only semantic priming were involved then print color, synesthetic color, and the combination of the two should not differ from each other.
TABLE 2.
Experiment 2, synesthetic priming at a 200 msec prime duration
| Group | Expt 1 (750 msec) | Expt 2 (200 msec) | ||||||
|---|---|---|---|---|---|---|---|---|
| Prime Type | b | p | s | C | B | p | S | c |
|
|
||||||||
| IC (msec) | 495.6 | 508.6 | 509.3 | 515.7 | 495.8 | 536.4 | 511.7 | 538.1 |
| CC (msec) | 496.5 | 491.4 | 475.2 | 457.7 | 502.4 | 475.4 | 481.9 | 476.4 |
| Prime Effect (msec) | -0.9 | 17.2 | 34.1* | 58.1* | -6.6 | 61.0* | 29.7* | 70.7** |
| p-value | .900 | .176 | .020 | .008 | .45 | .0003 | .013 | .0006 |
Mean response times for the 13 synesthetes who participated in Experiment 1 and the 9 synesthetes who participated in Experiment 2. b = baseline condition with achromatic primes, p = print color only, s = synesthetic color only, c = combination of synesthetic and print color for Experiment 1 (750 ms) and Experiment 2 (200 ms). Asterisks mark a difference score (the Congruency Effect) that is significant between the incongruent (IC) and congruent (CC) color conditions. Minor inaccuracies in scores are the result of rounding to one decimal place in this table.
The results indicated that the combination (c) Congruency Effect was significantly greater than the synesthetic (s) effect, t(8) = 3.91, p = .005, and greater than print (p) color congruency, t(8) = 2.73, p = .026. There was no significant difference between Synesthetic and Print color Congruency Effects with a 200 msec prime duration t(8) = 0.925, p = .382.
Five of the synesthetes in Experiment 1 participated in Experiment 2, and it could be argued that the different pattern of results between the 2 experiments was the result of increased variability introduced by four new synesthetic participants. For this reason, Table 3 presents the mean RTs for the 5 participants who were in both Experiment 1 and Experiment 2 compared to the full set of 9 subjects who participated in Experiment 2. As can be seen, this subgroup showed a pattern of RTs that mirrored the respective means of the larger group.
TABLE 3.
5 synesthetes participating in both experiments compared to all Expt 2 participants
| Group | Synesthetes (n=9) | Synesthetes (n=5) | ||||||
|---|---|---|---|---|---|---|---|---|
| Prime Type | B | p | S | C | B | p | S | c |
|
|
||||||||
| IC (msec) | 495.8 | 536.4 | 511.7 | 538.1 | 515.8 | 563.2 | 471.5 | 478.0 |
| CC (msec) | 502.4 | 475.4 | 481.9 | 467.4 | 531.8 | 500.4 | 472.9 | 455.3 |
| Prime Effect (msec) | -6.6 | 61.0* | 29.7* | 70.7* | -16.0 | 62.7* | 36.0* | 82.0* |
| p-value | .454 | .0002 | .013 | .0006 | .246 | .016 | .044 | .013 |
Mean response times for all nine synesthetes in Experiment 2 (left) and the five synesthetes who participated in both Experiments (right), across four different prime types. b = achromatic baseline, p = print color only, s = synesthetic color only, c = combination of synesthetic and print color. Asterisks mark a difference score (the Congruency Effect) that is significant between the incongruent (IC) and congruent (CC) color conditions. Minor inaccuracies in difference scores are the result of rounding to one decimal place in this table.
3.3. Correlations with self-reported vividness of visual imagery
Scores from the vividness of visual imagery questionnaire (VVIQ) were regressed with the size of the congruency effects for each group in each Experiment. It was hypothesized that more vivid imagery would correlate with a stronger synesthetic representation and thus lead to larger priming effects in conditions where synesthetic color acted as a color prime.
The congruency effect for each of the four priming conditions (b, p, s, c) were correlated with the average VVIQ scores for synesthetes and for controls (Figure 3). We were careful not to assume a Gaussian distribution for the correlational comparisons, since RTs are often skewed and our survey was categorical. For this reason we analyzed the data using a non-parametric Spearman’s Rho correlation, which also corrects for outliers.
Figure 3.

Correlations between synesthetic color congruency effects and the vividness of visual imagery. Note that smaller imagery values indicate more vivid imagery. The plot in the top middle is a reminder that the congruency effect is the difference between congruent (e.g., red) and incongruent (e.g., green) mean RTs. As this effect increases (ordinate axis), vividness of visual imagery also increases (abscissa). 3.a. Correlation between the vividness of visual imagery and color priming with a prime duration of 750 msec. Imagery correlates significantly with both the synesthetic color congruency effect (black line) and the combination of synesthetic and print color (red line). 3.b. When the prime duration is reduced to 200 msec only the correlation between visual imagery and synesthetic color congruency is significant. Individual data for each synesthete are shown in the background as faint black triangles (s condition) and red squares (c condition).
In Experiment 1, synesthetes showed a positive correlation between the vividness of visual imagery and Congruency Effect magnitude for the condition in which primes induced only synesthetic color (s), Spearman’s r(13) = -.657, p = .015, and the combination condition (c) in which primes induced synesthetic and print color, Spearman’s r(13) = -.765, p = .002.
Correlations between VVIQ and print or baseline congruency conditions were not significant (Spearman’s r(13) = -.311, p = .301, Spearman’s r(13) = -.322, p = .284 respectively) nor was the correlation between VVIQ and grand mean of reaction time for all conditions, Spearman’s r(13) = -.003, p = .993. For non-synesthetic controls, there were no significant correlations between congruency effect magnitude and the vividness of visual imagery (all p > .354). However, controls did show a significant correlation between VVIQ and the grand mean of the reaction time of all conditions, Spearman’s r(13) = -.560, p = .046. Note that a negative r-value represents a positive correlation because lower values on the VVIQ represent more vivid imagery. Thus, for the significant correlations found for synesthetes, the congruency effect size increases as imagery becomes more vivid (smaller VVIQ scores). For controls, the significant correlation is positive, meaning that smaller (faster) reaction times are associated with smaller values (more vivid imagery) on the VVIQ.
In Experiment 2, synesthetes showed a positive correlation between the vividness of visual imagery and Congruency Effect magnitude only for the synesthesia condition (s) Spearman’s r(9) = .667, p< .05. It is worth pointing out again that a negative r-value indicates a positive correlation (Congruency Effects increase as imagery becomes more vivid) since the VVIQ rates vivid imagery as lower values. Correlations between the vividness of visual imagery and all other Congruency Effect conditions (b, p, c) did not reach significant levels (all p > .765).
4. Discussion
This study used a perceptual priming task to explore how print and synesthetic color might be related in grapheme-color synesthesia. We presented synesthetes with primes of two different durations (750 msec and 200 msec) and found that synthetic color priming was robust and comparable in size at both durations (34.1 msec in Experiment 1 and 36 msec in Experiment 2). Conversely, print color was much larger (62.7 msec) at the 200 msec prime duration than 750 msec duration (17.2 msec). The 200 msec prime duration was also used as a means to rule out semantic priming as creating the effects. Additionally, congruency effects for synesthetes increased in size significantly from print color priming, to synesthetic color priming, to the prime that induced the combo of both color simultaneously at 750 msec. This change in priming mostly reflected improved performance when the colors of the prime and probe were congruent.
The results of Experiment 1 support the conclusion that synesthetic color and print color initially rely on different (form-based versus wavelength-based) pathways but converge at some point to form a stronger color signal than either color type alone. The presence of color congruency at a shorter duration in Experiment 2 confirms these results are not due to semantic priming and also shows that synesthetic color does in fact prime at 200 msec (Spruyt, et al., 2009).
Whereas previous studies have validated the independence of synesthetic and print color (van Leuween, et al., 2010; Odgaard, et al., 1999; Mills, et al., 1999; Dixon, et al., 2004; Ward, et al., 2007), we demonstrate an exception where synesthetic and print color can combine into a single amplified signal when the hues of both colors match. The current results are consistent with a model of processing whereby synesthetic and print colors are represented through distinct but overlapping pathways that converge on a map of perceived hue (see Figure 4 for a schematic). When a prime triggers the same print and synesthetic color, both forms of color converge on the same hue-selective map (e.g., possibly a red-selective cortical hyper column) that results in a larger color signal (e.g., more “red” signal) and a larger perceptual color congruency effect (faster RTs when naming a red probe). When a prime triggers different print and synesthetic colors (e.g., print green and synesthetic red), the two hues are still processed in the same general color region, but rely on independent hue-selective maps (e.g., print-induced green and synesthetically induced red cortical hyper columns). Such a model can account for how a prime can lead to faster priming RTs when it’s print and synesthetic color match, relative to a prime that triggers only one form of color. The same model can also explain how synesthetic color can be perceptually bound to the same location of an incongruent print color without blending since different hue-selective channels do not likely interact directly within early visual color-selective cortical areas and thus cannot blend.
Figure 4.

a) A printed letter “A” (bottom left) induces the color green through normal wavelength-dependent opponency processing (green pathway). First color is processed by hue-selective cortical columns (green circle) within the schematic color region (marked “COLOR”), and then bound to a spatial location with attention (yellow circle at top). The shape of “A” is processed within a shape-selective cortical region (marked “SHAPE”) and triggers a second, synesthetic color (red arrows). Synesthetic and print colors are different in hue but triggered from the same spatial location and so both hues are bound to the same location simultaneously, creating a dual color percept (red and green “A” at top). b) The synesthetic color “red” is triggered from the shape of “A”, while print color “red” is triggered from the printed text. In this example, the print-induced and shape-induced (synesthetic) red hue signals converge on the same hue-selective cortical column (red circle) within the color region (COLOR) and a single, more robust color signal (thick red arrow) emerges.
Although synesthetic and print colors clearly arise through independent sources (shape-induced color versus wavelength-induced color) the present results suggest that they may converge on the same perceptual, hue-selective cortical region. There is evidence for hue-selective cortical columns in the blobs of V1 (Xiao, et al., 2007), the thin stripes of V2 (Xiao, et al., 2003; Conway, 2001), and within globs in V4 (Conway & Tsao 2009; Tanigawa, et al., 2010). These hue-selective cortical columns are thought not to connect directly within a cortical region, allowing different hues to be processed independently within a column without blending with other columns. Accordingly, in Figure 4, synesthetic and print colors both converge on the same hue-selective region and do not blend when they are different hues (e.g., letter “A” printed in green that induces synesthetic red). However, synesthetic and print color would converge on a single hue-selective cortical column when they both represent the same hue (e.g., letter “A” printed in red that also induces the same synesthetic red). Thus, when synesthetic and print colors are the same, congruency effects will increase because the perceptual signal (e.g., the color red) is amplified within a hue-selective channel. This combination of synesthetic and print color will create larger congruency effects than is possible when the same hue-selective channel is stimulated by either print or synesthetic color alone. The color priming data in Experiment 1 match this hypothesis; priming from a combination of synesthetic and print color exceeds the effects from either form of color individually.
The schematic in Figure 4 also offers an explanation to how it is possible for a synesthete to experience two colors in the same spatial location without blending. When synesthetic and print colors are different, they are represented by different hue-selective cortical columns simultaneously, but can still be bound to a single location through mechanisms thought to play an important role in synesthetic and non-synesthetic binding (Robertson 2003). For synesthetes, color arises through both wavelength and shape-based pathways, which may share the same spatial coordinates but do not converge at any stage early enough to blend into one single hue.
It is, of course, possible that a distinct synesthetic hue system exists that co-activates with normal hue selective channels to produce the redundancy gain reported here. However, this would seem a less parsimonious account as it would require a completely separate hue processing system.
Data from Experiment 2 confirm that our initial results (Experiment 1) cannot be accounted for by semantic priming effects alone (the letter or print color priming the response code “red”). Synesthetes still show congruency effects for all three prime conditions (p, s, c) at 200 msec)3. At this prime duration, print color congruency effects are not significantly different in size to combo congruency effects and it is possible to interpret this to mean that print and synesthetic color pathways have not fully converged after a prime duration of 200 msec.
A particularly unique finding of the present study is that synesthetic congruency effects correlate significantly with the self-reported vividness of visual imagery on the standardized vividness of visual imagery questionnaire (VVIQ scores). Synesthetes with more vivid visual imagery produced larger congruency effects in Experiment 1 for both the conditions where synesthesia was involved (s and c). In Experiment 2, the vividness of visual imagery correlated significantly only with the synesthetic (s) priming effect RTs. As can be seen in Tables 1 and 2, congruency effect sizes remained constant for synesthetic and combo conditions, while the print color congruency effect size decreased significantly in the 200 msec experiment compared to priming at 750 msec.
Print color priming has been shown to occur pre-attentively at very short durations (i.e., 14.3 msec prime, Brietmeyer, Ro, & Singhal, 2004) whereas synesthetic color priming is attention-dependent (Mattingley, 2009) and requires the processing of a grapheme shape first (Brang, et al., 2010). Thus, it is possible that print color contributed more than synesthetic color in the combo condition at 200 msec leading to a lack of correlation between this condition (c) and imagery. Our findings show that at 750 msec print color effects faded while synesthetic color effects remained, contributing more to the combo condition and likely leading to a positive correlation between combo and imagery.
Although researchers have previously suggested that visual imagery may be associated with synesthesia (Barnett & Newell, 2008; Ramachandran & Hubbard, 2001; Spiller & Jansari, 2008), the present results are the first to demonstrate a clear behavioral link. We cannot claim that synesthetic color is a form of mental imagery, or even that it acts through the same mechanisms of visual imagery, but the present results demonstrate a strong connection between visual imagery in general and the strength of the synesthetic color experience. Crude assessments taken from measures of the VVIQ can be combined with quantitative studies (e.g., Cui, et al., 2007) to correlate imagery and early visual activity. It is also possible that visual imagery measures correlate with top-down activity. Some theories of synesthesia suggest that synesthetic colors arise from re-entrant feedback, disinhibition, or hyper-binding from higher cortical regions (Smilek, et al., 2001; Grossenbacher & Lovelace, 2001; Robertson 2003). The VVIQ may correlate with these top-down mechanisms as well.
5. Conclusion
The findings in these studies are consistent with the model shown schematically in Figure 4. This model may explain the unusual phenomenon in which a grapheme-color synesthete reports experiencing two colors bound to the same spatial location simultaneously without blending. Our hypothesis is that the same cortical region selective to hue is able to support print-based and synesthetic color representations simultaneously, allowing for both color percepts to emerge even when they are incongruent. The exception to this is when the synesthetic and print colors trigger the same color experience (e.g., letter “A” printed in red that triggers the same red synesthetically), which then results in an amplified color signal and faster response times to a probe that matches such a doubly congruent prime. Future studies are clearly needed to assess the cortical networks involved in the competition and combination of synesthetic and print color. The present results further suggest that the synesthetic pathways are strongly linked to visual imagery. Additional studies may also address whether specific elements of the VVIQ (e.g., color imagery) are especially strong predictors of synesthetic effects and whether VVIQ correlates with the perceived vividness of synesthetic colors in grapheme-color and other types of synesthesia.
Acknowledgments
Funding for this project was provided by NIH#EY16975 (L.C.R.) and NIH #EY021446 (B.D.A.). We would like to thank the Robertson Lab for their invaluable input and Miren Edelstein for assisting in experimental design and data collection.
Footnotes
We thank an anonymous reviewer for suggesting the possibility of semantic priming that inspired experiment 2.
Note that we did not use a non-synesthete control population in Experiment 2 since this follow up experiment was performed primarily to confirm that semantic priming was not accounting for synesthetic congruency effects.
The mean RT magnitudes also do not vary statistically between Experiment 1 and Experiment 2. This point is important because if semantic priming was present, it would facilitate responses to 750 msec prime durations (Experiment 1) and not affect 200 msec prime durations (Experiment 2), resulting in faster responses overall for a 750 msec prime than a 200 msec prime. We compared RT magnitudes for all 4 conditions separately (b, p, s, c) between experiments 1 and 2, and found no significant differences for congruent prime RTs (all p > .597) or incongruent prime RTs (all p > .366). We also compared the size of congruency effects for each condition separately between experiments and found no significant differences in RT magnitude for baseline, synesthetic, or combo priming effects (baseline congruency effects, t(20) = .48, p = .636; synesthetic t(20) = .26, p = .799; combo t(20) = .53, p = .604). Finally, we found a significant difference in print color priming effects t(20) = 2.7, p = .014 that went in the opposite direction expected if semantic priming was present. Synesthetes showed greater print color congruency effect sizes when presented with 200 msec primes. As a final check, we compared data from the five synesthetes who were present in both experiments. Again, all comparisons mentioned above came out insignificant for individual Prime Type (all p > .43), and Congruency Effect conditions (all p > .19).
References
- Barnett KJ, Newell FN. Synaesthesia is associated with enhanced, self-rated visual imagery. Consciousness and Cognition. 2008;17(3):1032–1039. doi: 10.1016/j.concog.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Brang D, Hubbard EM, Coulson S, Huang M, Ramachandran VS. Magnetoencephalography reveals early activation of V4 in grapheme-color synesthesia. Neuroimage. 2010;53(1):268–274. doi: 10.1016/j.neuroimage.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Breitmeyer Bruno G, Ro Tony, Singhal Neel S. Unconscious Color Priming Occurs at Stimulus- Not Percept-dependent Levels of Processing. Psychological Science. 2004 Mar;15(3):198–202. doi: 10.1111/j.0956-7976.2004.01503009.x. [DOI] [PubMed] [Google Scholar]
- Conway BR, Tsao DY. Color-tuned neurons are spatially clustered according to color preference within alert macaque posterior inferior temporal cortex. Proceedings of the National Academy of Sciences. 2009;106(42):18034. doi: 10.1073/pnas.0810943106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway Bevil R. Spatial Structure of Cone Inputs to Color Cells in Alert Macaque Primary Visual Cortex (V-1) The Journal of Neuroscience. 2001 Apr 15;21(8):2768–2783. doi: 10.1523/JNEUROSCI.21-08-02768.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Jeter CB, Yang D, Montague PR, Eagleman DM. Vividness of mental imagery: Individual variability can be measured objectively. Vision research. 2007;47(4):474–478. doi: 10.1016/j.visres.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MJ, Smilek D, Merikle PM. Not all synaesthetes are created equal: Projector versus associator synaesthetes. Cognitive, Affective, & Behavioral Neuroscience. 2004;4(3):335–343. doi: 10.3758/cabn.4.3.335. [DOI] [PubMed] [Google Scholar]
- Eagleman DM, Kagan AD, Nelson SS, Sagaram D, Sarma AK. A standardized test battery for the study of synesthesia. Journal of neuroscience methods. 2007;159(1):139–145. doi: 10.1016/j.jneumeth.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias LJ, Saucier DM, Hardie C, Sarty GE. Dissociating Semantic and Perceptual Components of Synaesthesia: Behavioural and Functional Neuroanatomical Investigations. Cognitive Brain Research. 2003;16(2):232–237. doi: 10.1016/s0926-6410(02)00278-1. [DOI] [PubMed] [Google Scholar]
- Grossenbacher PG, Lovelace CT. Mechanisms of synesthesia: cognitive and physiological constraints. Trends in Cognitive Sciences. 2001;5(1):36–41. doi: 10.1016/s1364-6613(00)01571-0. [DOI] [PubMed] [Google Scholar]
- Hubbard EM. Neurophysiology of synesthesia. Current psychiatry reports. 2007;9(3):193–199. doi: 10.1007/s11920-007-0018-6. [DOI] [PubMed] [Google Scholar]
- Hubbard EM, Arman AC, Ramachandran VS, Boynton GM. Individual differences among grapheme-color synesthetes: brain-behavior correlations. Neuron. 2005;45(6):975–985. doi: 10.1016/j.neuron.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Hupé Jean-Michel, Bordier Cécile, Dojat Michel. The Neural Bases of Grapheme–Color Synesthesia Are Not Localized in Real Color-Sensitive Areas. Cerebral Cortex. 2012;22(7):1622–1633. doi: 10.1093/cercor/bhr236. [DOI] [PubMed] [Google Scholar]
- Jansari AS, Spiller MJ, Redfern S. Number Synaesthesia: When Hearing ‘four Plus Five’ Looks Like Gold. Cortex. 2006;42(no 2):253–258. doi: 10.1016/s0010-9452(08)70350-2. [DOI] [PubMed] [Google Scholar]
- Kim CY, Blake R, Palmeri TJ. Perceptual interaction between real and synesthetic colors. Cortex. 2006;42(2):195–203. doi: 10.1016/s0010-9452(08)70344-7. [DOI] [PubMed] [Google Scholar]
- Kim CY, Blake R. Watercolor illusion induced by synesthetic colors. Perception. 2005;34(12):1501. doi: 10.1068/p5422. [DOI] [PubMed] [Google Scholar]
- Laeng B, Svartdal F, Oelmann H. Does color synesthesia pose a paradox for early-selection theories of attention? Psychological Science. 2004;15(4):277–281. doi: 10.1111/j.0956-7976.2004.00666.x. [DOI] [PubMed] [Google Scholar]
- van Leeuwen Tessa M, Petersson Karl Magnus, Hagoort Peter. Synaesthetic Colour in the Brain: Beyond Colour Areas. A Functional Magnetic Resonance Imaging Study of Synaesthetes and Matched Controls. PLoS ONE. 2010;5(8):e12074. doi: 10.1371/journal.pone.0012074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maljkovic V, Nakayama K. Priming of pop-out: I. Role of features. Memory and Cognition. 1994;22:657–657. doi: 10.3758/bf03209251. [DOI] [PubMed] [Google Scholar]
- Marks DF. Visual imagery differences in the recall of pictures. British journal of psychology (London, England: 1953) 1973;64(1):17. doi: 10.1111/j.2044-8295.1973.tb01322.x. [DOI] [PubMed] [Google Scholar]
- Mattingley JB. Attention, Automaticity, and Awareness in Synesthesia. Annals of the New York Academy of Sciences. 2009;1156(1):141–167. doi: 10.1111/j.1749-6632.2009.04422.x. [DOI] [PubMed] [Google Scholar]
- Mattingley JB, Payne JM, Rich AN. Attentional load attenuates synaesthetic priming effects in grapheme-colour synaesthesia. Cortex. 2006;42(2):213–221. doi: 10.1016/s0010-9452(08)70346-0. [DOI] [PubMed] [Google Scholar]
- Mattingley JB, Rich AN, Yelland G, Bradshaw JL. Unconscious priming eliminates automatic binding of colour and alphanumeric form in synaesthesia. Nature. 2001;410:580–582. doi: 10.1038/35069062. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ishai A. Where bottom-up meets top-down: neuronal interactions during perception and imagery. Cerebral Cortex. 2004;14(11):1256–1265. doi: 10.1093/cercor/bhh087. [DOI] [PubMed] [Google Scholar]
- Mills CB, Boteler EH, Oliver GK. Digit synaesthesia: A case study using a Stroop-type test. Cognitive Neuropsychology. 1999;16(2):181–191. [Google Scholar]
- Mroczko A, Metzinger T, Singer W, Nikolić D. Immediate Transfer of Synesthesia to a Novel Inducer. Journal of Vision. 2009;9(12):1–8. doi: 10.1167/9.12.25. http://www.journalofvision.org/content/9/12/25. [DOI] [PubMed] [Google Scholar]
- Neely JH. Semantic Priming and Retrieval from Lexical Memory: Roles of Inhibitionless Spreading Activation and Limited-capacity Attention. Journal of Experimental Psychology: General. 1977;106(3):226. [Google Scholar]
- Odgaard EC, Flowers JH, Bradman HL. An Investigation of the Cognitive and Perceptual Dynamics of a Color–Digit Synesthete. Perception. 1999;28:651–664. doi: 10.1068/p2910. [DOI] [PubMed] [Google Scholar]
- Pesenti M, Thioux M, Seron X, Volder AD. Neuroanatomical substrates of Arabic number processing, numerical comparison, and simple addition: A PET study. Journal of Cognitive Neuroscience. 2000;12(3):461–479. doi: 10.1162/089892900562273. [DOI] [PubMed] [Google Scholar]
- Price MC. Spatial Forms and Mental Imagery. Cortex. 2009;45(10):1229–1245. doi: 10.1016/j.cortex.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Hubbard EM. Synaesthesia–a window into perception, thought and language. Journal of Consciousness Studies. 2001;8(12):3–34. [Google Scholar]
- Rich AN, Mattingley JB. The effects of stimulus competition and voluntary attention on colour-graphemic synaesthesia. Neuroreport. 2003;14(14):1793. doi: 10.1097/00001756-200310060-00007. [DOI] [PubMed] [Google Scholar]
- Rich Anina N, Mattingley Jason B. Out of sight, out of mind: The attentional blink can eliminate synaesthetic colours. Cognition. 2010 Mar;114(3):320–328. doi: 10.1016/j.cognition.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Robertson LC. Binding, spatial attention and perceptual awareness. Nature Reviews Neuroscience. 2003;4(2):93–102. doi: 10.1038/nrn1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouw R, Scholte HS. Increased structural connectivity in grapheme-color synesthesia. Nature Neuroscience. 2007;10(6):792–797. doi: 10.1038/nn1906. [DOI] [PubMed] [Google Scholar]
- Sagiv N, Heer J, Robertson L. Does binding of synesthetic color to the evoking grapheme require attention? Cortex. 2006;42(2):232–242. doi: 10.1016/s0010-9452(08)70348-4. [DOI] [PubMed] [Google Scholar]
- Smilek D, Dixon MJ, Cudahy C, Merikle PM. Synaesthetic photisms influence visual perception. Journal of Cognitive Neuroscience. 2001;13(7):930–936. doi: 10.1162/089892901753165845. [DOI] [PubMed] [Google Scholar]
- Spiller MJ, Jansari AS. Mental imagery and synaesthesia: Is synaesthesia from internally-generated stimuli possible? Cognition. 2008;109(1):143–151. doi: 10.1016/j.cognition.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Spruyt A, Koch J, Vandromme H, Hermans D, Eelen P. A Time Course Analysis of the Synesthetic Colour Priming Effect. Canadian Journal of Experimental Psychology. 2009;63(3):211. doi: 10.1037/a0015299. [DOI] [PubMed] [Google Scholar]
- Tanigawa H, Lu HD, Roe AW. Functional organization for color and orientation in macaque V4. Nature Neuroscience. 2010;13(12):1542–1548. doi: 10.1038/nn.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A. Perceptual grouping and attention in visual search for features and for objects. Journal of Experimental Psychology: Human Perception and Performance. 1982;8(2):194–214. doi: 10.1037//0096-1523.8.2.194. [DOI] [PubMed] [Google Scholar]
- Ward Jamie, Li Ryan, Salih Shireen, Sagiv Noam. Varieties of grapheme-colour synaesthesia: A new theory of phenomenological and behavioural differences. Consciousness and Cognition. 2007 Dec;16(4):913–931. doi: 10.1016/j.concog.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Casti A, Xiao J, Kaplan E. Hue maps in primate striate cortex. Neuroimage. 2007;35(2):771–786. doi: 10.1016/j.neuroimage.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Wang Y, Felleman DJ. A spatially organized representation of colour in macaque cortical area V2. Nature. 2003;421(6922):535–539. doi: 10.1038/nature01372. [DOI] [PubMed] [Google Scholar]


