Abstract
In this paper, we developed a metal-enhanced fluorescence (MEF) substrate by modification of the commercially available surface enhanced Raman spectroscopy (SERS) substrate that may meet the reproducibility and sensitivity challenge of MEF. In spite of many studies and interest on MEF from a number of research groups, application to real-world situations and its commercial use remain challenging mainly due to the difficulties in fabricating reproducible MEF substrates. Specifically, one of the challenges is achieving a standardized MEF substrate for reproducible fluorescence intensity enhancement and/or changes in lifetime. The gold standard klarite substrates for SERS were coated with a thin layer of silver nanoparticles for MEF studies. To test the newly developed MEF substrates, a monolayer of streptavidin conjugated Alexa-647 was assembled on biotinylated-glass or MEF substrates. We observed over 50-fold increase in the fluorescence intensity from a monolayer of streptavidin conjugated Alexa-647 on the biotinylated MEF substrate compared to the same on glass substrate. A significant reduction in the lifetime and increased photostability of Alexa-647 on MEF substrate was observed. Fluorescence lifetime imaging was performed on the monolayer of dye assembled on the modified SERS substrates. We expect this study will serve as a platform to encourage the future use of a standardized MEF substrate for a plethora of sensing applications.
Keywords: metal-enhanced fluorescence, fluorescence, SERS substrates, fluorescence lifetime imaging
1.0. Introduction
Fluorescence technology has played a major role in many advances in biological and medical research. This technology has been applied to wide range of topics such as cell imaging, diagnostics, biomolecule interactions and even in-vivo testing. Fluorescence based assays are of particular interest because they are predominant analytical technology in which the specific interaction of an antibody with its antigen is exploited for molecular recognition. In spite of excellent properties of fluorescent probes, their applications to ultrahigh sensitive bioassays are limited by their insufficient brightness and photostability. We believe the interactions of fluorophores with metallic-surfaces and particles provide a means to bypass these limitations. As a result, we have studied the interactions of fluorophores with metallic particles and surfaces for last few years. This interest has been driven because of strong interaction of light with metallic nanostructures that leads to generating surface plasmons and amplified near-fields. The excited fluorophores (as dipoles) strongly interact with surface plasmons that result in significant improvements in the spectral properties of fluorescent probes. These improvements include increases in intensity, increases in photostability and decreases in lifetime. We referred these phenomena as metal-enhanced fluorescence (MEF).1–12 The potential use of MEF is far greater than we imagined several years ago and fluorophore-plasmon interactions are now being studied in many laboratories.13–21 We believe that metal-fluorophore interactions will result in a new generation of fluorometric assay formats for clinical testing because of near-field interaction that suits the dimension of typical bioassays. For example, the effects of metals on fluorophores are due to through-space interactions occurring over distances ranging from about 5 to 50 nm from the metal surface, which is ideal for most surface-based assays. Sub-wavelength size metal particles display strong interactions with incident light where the electric fields are concentrated around the particles, which provide selective excitation of surface-bound proteins. Metal particles also increase the radiative decay rates of the fluorophores. The radiative decay rate is determined by the transition probability, which is given by the extinction coefficient.22 The MEF phenomenon has been observed for many fluorophores in the UV, visible and near-infrared wavelength ranges.7–12
In spite of many studies and interest in metal-enhanced fluorescence from a number of research groups, application to real-world situations and its commercial use remain challenging mainly due to the difficulties in fabricating reproducible MEF substrates. Specifically MEF challenges are found in achieving a standardized substrate from which reproducible fluorescence intensity and/or lifetime measurements can be obtained. Several efforts have been focused toward increasing the enhancement ability, reproducibility, and mass production of MEF substrates. Towards this end, we believe a modification of the commercially available klarite substrate23,24 may meet the reproducibility and sensitivity challenge of MEF. Klarites are commercially available gold standard substrates often employed in surface enhanced Raman spectroscopy (SERS) and made in high volume and highly reproducible platform. These substrates were developed using Si-based semiconductor fabrication techniques. As a result, arrays of highly reproducible inverted pyramid structures are produced. The active surface of the klarite substrates are gold coated nanostructured silicon. Each pyramidal structure considered to have 'hot spots' or 'trapped plasmons' located inside the wells.
In this paper we modified the commercially available SERS substrates towards performing metal-enhanced fluorescence lifetime imaging and spectroscopy on a planar substrate. The klarite substrates were coated with a thermally deposited thin layer of silver for obtaining enhanced fluorescence in the visible spectral region. Based on our previous work, we observed that silver nanostructures are more suitable for performing MEF in the visible region compared to gold or aluminum. We have tested the MEF substrates by assembling a monolayer of streptavidin conjugated Alexa-647. We observed over 50-fold increase in the fluorescence intensity and several-fold decrease in lifetime from a monolayer of streptavidin (SA) conjugated Alexa-647 on the biotinylated metal nano-structured substrate compared to the same labeled SA on glass substrate. Although silver particles are present throughout the substrate only the patterned region showed the enhancement. We believe that the implementation of plasmonic nanostructures into a novel sensing devices will enable simple and inexpensive detection of multiple biomarkers of clinical samples.
2.0 Materials and Methods
Klarite substrates were purchased from Renishaw Diagnostics (UK). Silver wires, and silicon monoxide were purchased from Sigma-Aldrich and used as received. Distilled water (with a resistivity of 18.2 MΩ-cm) purified using Millipore Milli-Q gradient system was used for sample preparation. The active surface of the commercial klarite substrates is gold coated nanostructured silicon. Klarite substrates are fabricated using a well defined Silicon fabrication technique and then KOH surface etched. The process results in an array of highly reproducible inverted pyramid structures. The active surface area on these slides was a small 4 mm × 4 mm wafer with a gold surface. Klarite substrate is a commercially product optimized for SERS. The klarite slides were only used once and opened just prior to the deposition of silver to reduce any possible surface contamination. For our MEF experiments, 10 nm thick silver was deposited on klarite substrates using an Edwards Auto 306 Vacuum Evaporation chamber under high vacuum (<5×10−7Torr). In each case, the metal deposition step was followed by the deposition of 5 nm of silica via evaporation without breaking vacuum. This step served to protect the metal surface as well as it adds a spacer layer between the metal surface and fluorophore. The deposition rate was adjusted by the filament current and the thickness of film was measured with a quartz crystal microbalance. The surface morphology and topography of the modified klarite substrates were characterized by Witec α-300S AFM and Hitachi SU-70 scanning electron microscope (SEM) under ambient conditions. For SEM measurements, the substrates were mounted on an aluminum stub with conductive tape, and then observed by the SEM directly, i.e., without any further treatment of the sample. Samples were surveyed at low magnifications to see the general features and the homogeneity. Representative areas were selected for higher magnification investigation. From an application point of MEF based assays, large surface-area plasmonic structures with reproducible enhancement factors are needed. We believe the modified klarite substrates can be useful in this aspect.
Streptavidin conjugated Alexa-647 dye was purchased from Invitrogen. Glass cover slide or metal substrates were covered with 250 µL of 10 µM biotinylated bovine serum albumin (BSA-biotin, Sigma) aqueous solution and placed in a humid chamber for 20 hr (5°C). Following this step, the slides were then washed 3 times with PBS buffer, and were placed again in the humid chamber. After that, 250 µL sample of 100 nM streptavidin conjugated Alexa-647 in 100mM PBS buffer was then added to each BSA-biotin-coated surface for 2 hrs at 5°C. The slides were then washed multiple times with PBS buffer. The resulting streptavidin-Alexa-647 dye monolayer on glass or metal nano-structured surfaces was used for fluorescence measurement. The immobilized protein was always kept in the wet condition while performing fluorescence measurements to prevent protein unfolding or de-naturation through drying. Observations of fluorescence were made with a scanning confocal Picoquant MicroTime 200 microscope with time-correlated single-photon counting capabilities. The excitation laser was reflected by a dichroic mirror to a high numerical aperture (NA) air objective (100×, NA 0.95) and focused to a diffraction limited spot (~300 nm) on the sample surface. The fluorescence from the samples was collected by a single-photon counting avalanche photodiode (SPAD) (SPCM-AQR-14, Perkin Elmer Inc) through the dichroic beam splitter and long-pass (Chroma) filters. Fluorescence or reflectance images were recorded by raster scanning the sample through the excitation light focus by means of a linearized piezo scanner. The reproducibility of the enhancement on the modified klarite substrates are verified from the intensity and lifetime images of each pattern. For Alexa-647, we used an excitation wavelength of 638 nm (20-MHz repetition rate, 80-ps FWHM). Intensity vs time traces and intensity-time decays were obtained by positioning the excitation beam at different positions on the samples. The arrival time of each photon (100-ns resolution) as well as the fluorescence delay time relative to the laser pulse (37-ps resolution) were recorded for each detection channel and stored for later analysis. For spectroscopy of fluorophores on metal or glass substrates, a 150 mm spectrograph (Princeton Instruments Acton) has been employed. The spectrograph consists of high-reflectance mirrors (used for collimation and focusing), and a 150 G/mm dispersion grating with 500 nm blaze wavelength; this grating provides efficient imaging from 450–750 nm. The detector for the spectrograph is a high quantum efficiency (>90% visible range) electron-multiplied CCD (Princeton Instruments Photon Max 512). These EM-CCDs are commonly used for high sensitivity spectral imaging.
The fluorescence intensity decays were analyzed in terms of the multi-exponential model as the sum of individual single exponential decays:25
| (1) |
In the above expression, τj are the decay times and αi are the amplitudes. The fractional contribution of each component to the steady-state intensity is described by:
| (2) |
the mean (intensity weighted) lifetime is represented by:
| (3) |
and the amplitude weighted lifetime is given by:
| (4) |
The values of αi and τj were determined using the PicoQuant Symphotime software with the deconvolution of instrument response function and nonlinear least squares fitting. The goodness-of-fit criterion was determined by the χ2 value.
3.0 Results and Discussion
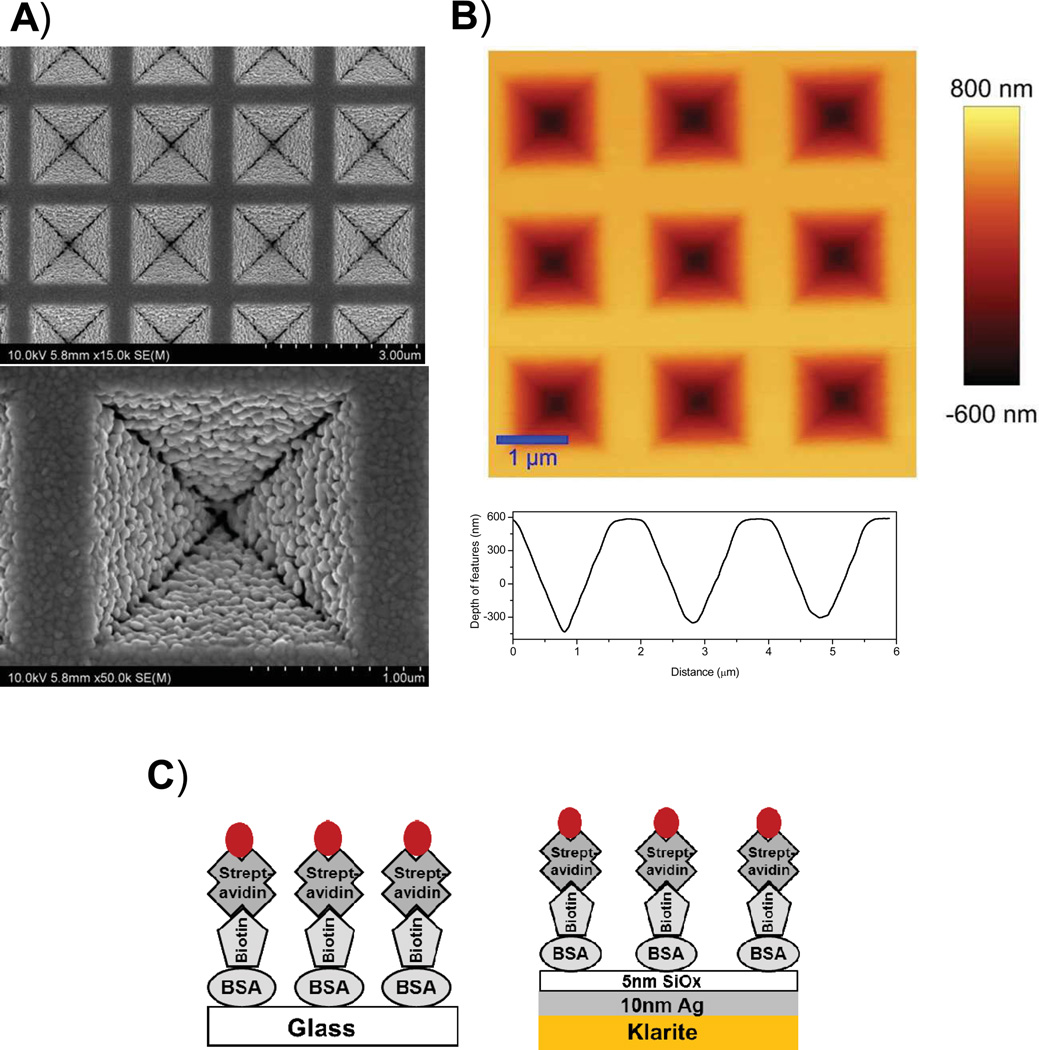
The commercially available klarite substrates have a well-defined pattern of inverted square-pyramid micro-wells coated with 100nm of gold. Figure 1A shows the high resolution FE-SEM image of 10 nm thick silver and 5nm thick silica deposited on commercial klarite substrate. The FE-SEM image reveals the uniform distribution of metal nanoparticles with an average particle size of approximately 80 nm. Figure 1B shows the topography of the modified klarite substrate obtained by an AFM where the tip is in contact with the surface of the substrate. Images presented in Figure 1 clearly show the well-defined inverted pyramidal features of the silver coated klarite substrate where the dimensions of each individual feature is 1.5 µn in width, and 1 µn in depth. Surface characterizations of these substrates suggest that the deposition of thin silver film did not actually cause any change to the overall nanostructures. A line scan to reveal the cross section of the features is shown in the bottom panel of Figure 1B. It is well-known that surface roughness promotes coupling of incident light (plane waves) to surface plasmons to create localized regions of enhanced field especially in the near-fields around the particles.25 Fluorophores located in these enhanced field areas experience a much higher excitation field than if it were isolated in a dielectric and directly excited only by the incident light. This leads to higher excitation rates of the fluorophore, which leads to greater excitation-emission cycles in a given time period.
Figure 1.
A) FESEM images B) AFM image of 10nm thick silver and 5nm thick silica coated klarite substrates. A cross-sectional line scan is shown in panel (B). Panel (C): Schematic representation of the BSA-bt-SA-Alexa647 assembly on glass or modified SERS substrate.
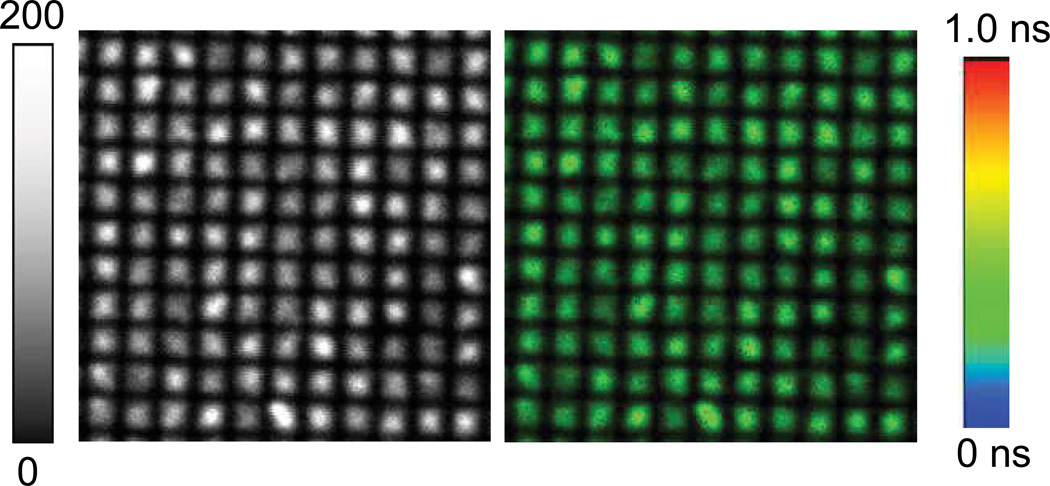
In our experiments, the active assay surface was composed of a klarite substrate coated with 10nm thick metallic silver layer covered with a thin silica layer onto which BSA-bt was immobilized. This is schematically represented in Figure 1. The silica layer protects the silver against the chemicals and provides the way for well-established immobilization of biomolecules on the glass surfaces. Alexa-647-labeled streptavidin were used as the analyte which binds to immobilized BSA-bt near the silver surface. Figure 2 shows the fluorescence intensity image (20 µm × 20 µm) of streptavidin conjugated Alexa-647 bound to the BSA-biotinylated metal substrate. It is important to note that the entire surface is covered with a monolayer of fluorophores. The fluorescence is intense only in the structured portion of the substrate. The highest intensity was found at the center of the individual structure. There is very weak fluorescence from the Alexa-647 molecules embedded between the structural features. This is especially remarkable considering that both the excitation and the emission are performed for the monolayer of SA-Alexa-647 immobilized on the whole substrate with or without the inverted pyramidal features. These results are promising to guide the design of improved substrates for MEF.
Figure 2.
(left) Fluorescence intensity and (right) fluorescence lifetime images of streptavidin conjugated Alexa-647 bound to the BSA-biotinylated nano-structured substrate. Image size is 20 µm × 20 µm. Excitation laser (638 nm) power was 19 w/cm2.
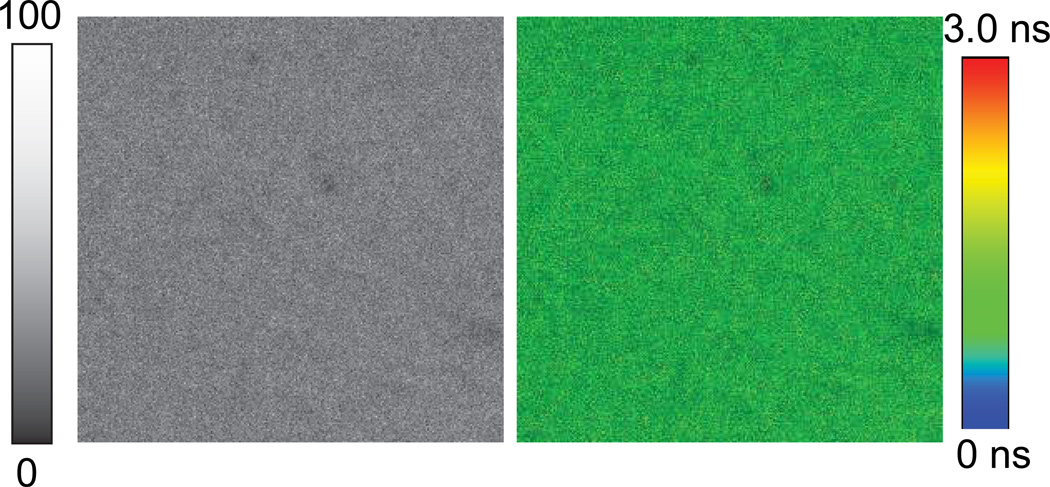
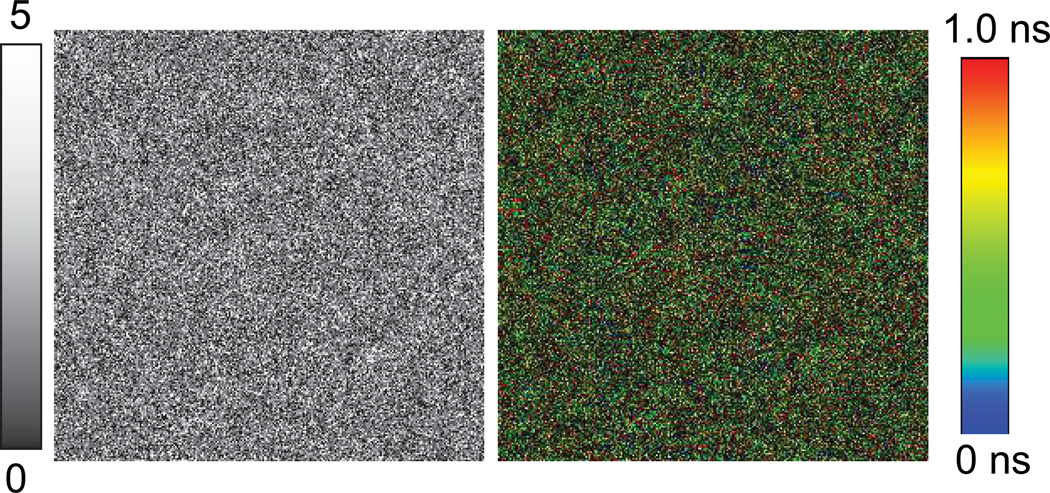
To compare the intensity and lifetime of fluorophore attached to the MEF substrate, fluorescence intensity and lifetime images of Alexa-647-SA bound to the BSA-biotinylated on glass substrate are recorded as shown in Figure 3. The excitation power used for glass substrate is 32-fold more than that used for imaging with metal substrates (600w/cm2for glass vs. 19 w/cm2for metal nano-structured substrate). The image size is (20 µm × 20 µm). We note that the intensity and lifetime remains constant all through the images as one may expect. However, the image brightness on glass is significantly (50-fold) less than the BSA-bt-SA-Alexa-647 assay observed with the modified SERS substrate. Fluorescence intensity and lifetime images of Alexa-647-SA bound to the BSA-biotinylated on smooth portion (non-featured) of the modified klarite substrate are shown in Figure 4. It is important to note that the fluorescence intensity is decreased substantially on the non-featured portion of the 10nm thick silver and 5nm thick silica coated klarite substrate than the glass substrate.
Figure 3.
(left) Fluorescence intensity and (right) fluorescence lifetime images of streptavidin conjugated Alexa-647 bound to the BSA-biotinylated on glass substrate. Image size is 20 µm × 20 µm. Excitation laser (638 nm) power was 600 w/cm2.
Figure 4.
(left) Fluorescence intensity and (right) fluorescence lifetime images of streptavidin conjugated Alexa-647 bound to the BSA-biotinylated on smooth area of the metal nano-structured substrate. Image size is 10 µm × 10 µm. Excitation laser (638 nm) power was 600 w/cm2.
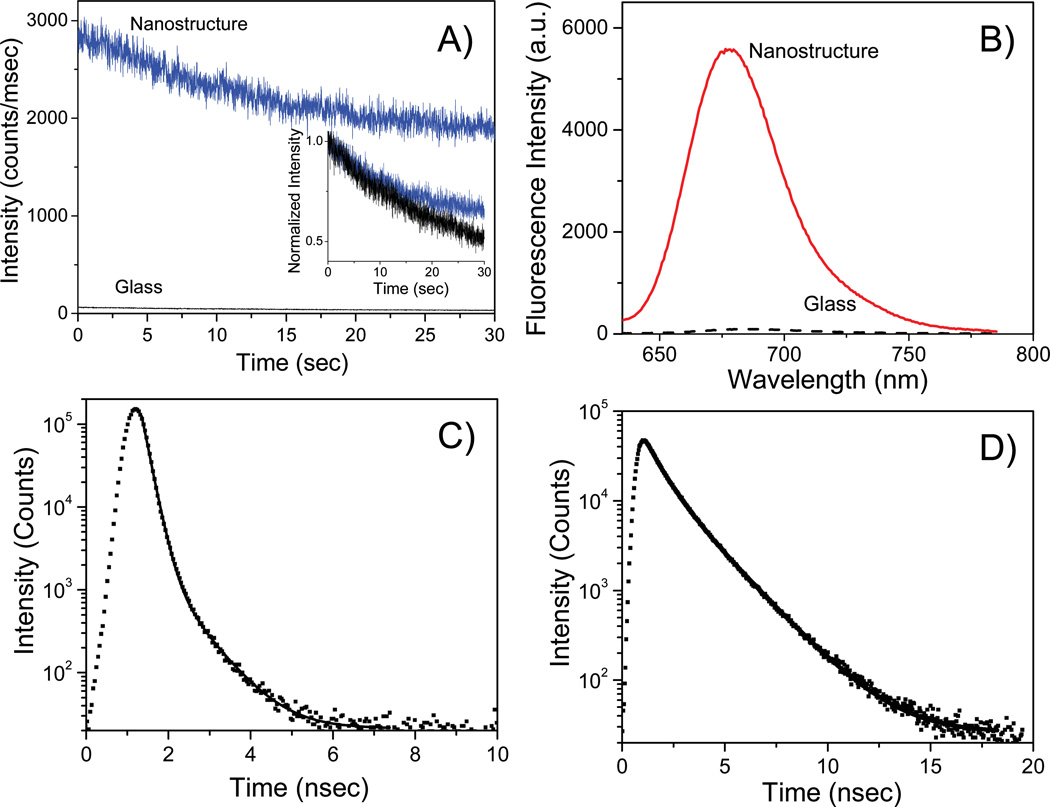
In Figure 5A we present the photostability of streptavidin conjugated Alexa-647 bound to the BSA-biotinylated layer on the MEF and glass substrates. Using the same incident excitation power, we observed significantly more fluorescence from the individual structure of the metal substrates when compared to the glass sample. We observed that Alexa-647 molecules are more photostable on the MEF substrate when compared to the glass substrate when the incident excitation yields similar initial emission intensities. Our results confirm that an increase in emission intensity coupled with a decrease in lifetime of the Alexa-647 emission on MEF substrates causes a significant increase in its photostability, which in turn is expected to increase its detectability as the excited state molecule is able to go through a larger number of excitation-emission cycles in a given period of time without photobleaching.
Figure 5.
(A) Fluorescence intensity vs time (B) emission spectra (C) & (D) intensity-decays of streptavidin conjugated Alexa-647 bound to the BSA-biotinylated on glass or nano-structured substrates. The excitation beam for panels (A), (B) and (C) for metal samples were positioned on the tip of the pyramidal structure.
To more quantitatively compare the emission and explore the changes in underlying photophysics of fluorophores on glass and nano-structured substrates, we recorded the fluorescence spectra of Alexa-647-SA molecules by positioning the excitation beam on specific positions on the substrates in our stage-scanning confocal set-up and collected the spectra with a spectrometer coupled with an EMCCD camera, while under continuous excitation. Figure 5B shows the fluorescence emission spectra of Alexa-647 on a glass substrate as well as on the inverted-pyramidal structure of the modified klarite substrate. We observe over 50-fold improvement in fluorescence intensity relative to glass microscope slide. It can be seen that the emission peak of both the spectra are located at approximately 670 nm thus indicating minimal change in spectral properties of Alexa-647 by the interaction with the nanostructures. There is a significant spectral overlap between the emission spectra of Alexa-647 molecules from glass and nanostructures thus further confirming that the emission spectra collected were indeed from the Alexa-647 molecules and not corrupted by any noise in the system (such as metal nanoparticle luminescence).
There is often a misunderstanding of the reasons behind the observation of enhanced emission of fluorophores on metallic substrates. At times, it has been attributed simply to conventional far-field reflection from the metallic film surface. Hence it is essential to clarify this point. It is important to note that reflection from a metallic surface normally occur when the 'metal' is extended over several wavelengths in size. However, in this particular case, the deposited silver particles over the gold coated klarite substrate for silver film thickness of 10 nm are smaller than the wavelength of light, so these silver coated substrate essentially behave like particulate surfaces as confirmed by FESEM images (Figure 1). Hence we believe that excited fluorophore couples with the silver nanoparticle and the induced dipole radiates coherently with the fluorophore's dipole. In this sample setup the fluorophores are located approximately within 5 to 15 nm away from the metal surface, i.e. essentially in the near-field of the metal where the excited state molecule couples to the plasmons on the surface of the silver particles and the combined excited fluorophore-metal complex acts as a unified radiating system (plasmophore). We would like to point out that the typical extinction spectra of 80 nm silver nanoparticles show distinct plasmon resonance bands or peaks in the visible region around 450 nm. The plasmon resonance spectra or extinction spectra represent far-field properties of the silver. However, the fluorescence measurements show consistent enhancements in the inverted pyramidal structured portion of the substrate. This is another indication that the near-field interactions between the excited state molecules with the surface plasmons of the metal nanoparticles and/or nanostructures causes the observed enhancement in the emission intensity.
The fluorophore-metal coupling depends on fluorophore distances and orientations relative to the metal surfaces. We have reported a few systematic steady-state and time-resolved fluorescence studies with metal nanostructures which provided important information on the effect of fluorophore distance on the metal-enhanced fluorescence phenomenon.7,9,26 These studies indicated how the fluorophore-metal interactions changes with the distances between metal and probe. These studies suggested a distance of 5–20 nm at which the fluorophores are located from the metal surface to render maximum fluorescence intensity. In some cases, a dielectric (such as silica) coating of required thickness can be evaporated over the metal surfaces, or a molecular spacer that binds directly to metal surfaces (e.g. thiols with silver/gold) of the desired length can be used. We have used inert fatty acid (Langmuir-Blodgett) layers26 or polyelectrolyte layers (by layer-by-layer assembly) to control the distance.9 Another way to obtain the required distance from the metal surface is to use the protein-layers such as BSA-biotin-Streptavidin or IgG-anti-IgG assemblies. One layer of BSA-biotin-streptavidin adds a distance of ~ 9 nm which is the ideal distance of putting the probe from the metal surface for MEF. In our present study, we have used the BSA-bt-SA-Alexa-647 layer which positioned Alexa-647 probes approximately 14 nm from the particulate silver nanostructures, including 5 nm thick silica layer on modified klarite substrates. In earlier studies, we have also reported the effect of the fluorophore-metal distance on the extent of the radiation power enhancement for the metal nanoparticle-fluorophore system.7 As the efficiency of metal-fluorophore coupling is distance dependent, this property could be exploited for background suppression for biological samples that would be useful in a variety of analytical and medical sensing applications.
The metal-enhanced fluorescence phenomenon is generally accompanied by a reduction in the lifetime of the emission that occurs simultaneously with an increase in the emission intensity. The shorter lifetimes for emission from excited-state fluorophores in close proximity to metallic nanostructures, coupled with the enhanced emission intensities are indicative of the radiative decay rate modification, and have been reported in numerous publications. The intensity decays of the streptavidin conjugated Alexa-647 bound to the BSA-biotinylated layer on glass and structured portion of the modified klarite substrates are shown in Figure 5 (c) and (d). The lifetimes of the Alexa-647 probes were measured using the time-correlated single-photon counting (TCSPC) method and were recovered by non-linear least-squares (NLLS). The solid lines indicate the best fit to the experimental decay curves. It can be clearly seen from the figure that the intensity decay of Alexa-647 on the MEF substrate surface is faster than on the glass substrate. The intensity-decay of the Alexa-647-SA on the glass substrate was fitted with a double exponential with two lifetime components of 3 ns (31%) and 1 ns (69%) with the total amplitude-weighted lifetime being 1.5 ns. The intensity decay of Alexa-647-SA on the MEF substrate was also fitted with a double-exponential with two lifetimes of 1 ns (19%) and 0.3 ns (81%). Hence the amplitude-weighted lifetime of Alexa-647 on nanostructures was calculated to be 0.35 ns. Therefore the intensity decays in Figure 5 show that the lifetime of Alexa-647 was decreased by over 5-fold on the featured metal substrate when compared to the glass control substrate. This shortening of the Alexa-647 lifetime on the nano-structured substrate suggests that the enhanced emission intensity observed on inverted pyramidal structure of metal substrate cannot be from the Alexa-647 molecules alone but rather due to the radiation from the plasmon-fluorophore complex7 that results when excited fluorophores interact with the silver nanoparticles in their immediate proximity (near-field). This supports our radiating plasmon model16 where we believe the enhanced fluorescence emission observed in MEF experiments is due to radiation from the entire excited state fluorophore-metal nanoparticle complex acting as a singular radiating entity.7,11 We have coined the term called "plasmophore" to describe this entity. Additionally, we would like to point out that precise agreement between the increases in emission intensity (over 50-fold) and decrease in lifetime (5-fold) cannot be expected. This is because time-domain measurements often result in over-weighting of the lifetime by the longer lifetime components in cases involving a heterogeneous decay, especially when the decay of the short components overlaps IRF. An advantage of a shorter fluorescence lifetime is that it would allow a higher photon emission rate at optical saturation.
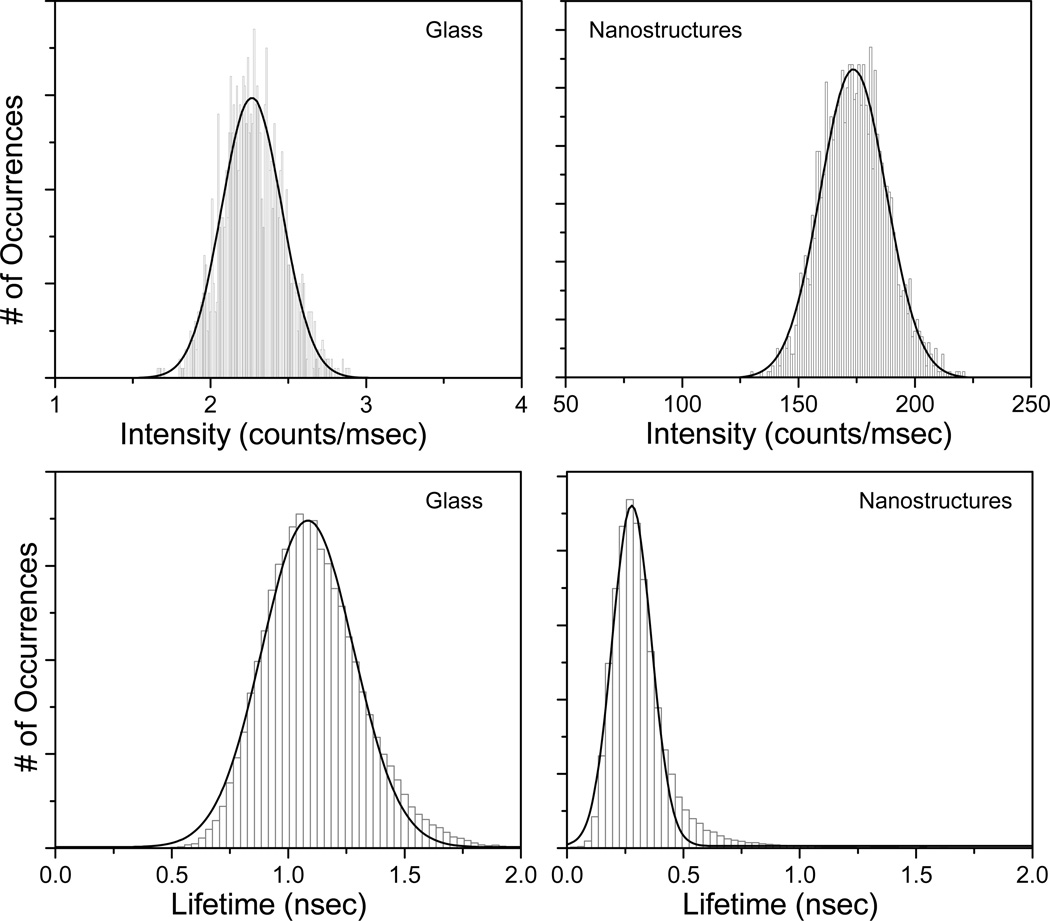
To compare the brightness and fluorescence lifetimes of Alexa647-SA molecules on the metal nanostructures and glass substrates, intensity and average fluorescence lifetime histograms were constructed. We measured the intensities and lifetimes at different positions on glass and inverted pyramidal tip of the metal substrates. The intensity histograms of Alexa-647-SA molecules on glass or metal surfaces are shown in figure 6. With the same illumination power, examination of the intensity histograms shows that the Alexa-647 molecules have an average intensity of about 170 counts/msec on the inverted-pyramidal position of the modified klarite substrate compared to an average intensity of ~2.3 kHz on glass (Figure 6, top panels). This suggests over 50-fold brighter intensity on the MEF substrates than that from the glass surface. It is probable that the molecules reside in the "hotspots" on the klarite substrate,23 where the fluorophore-plasmon coupling is very efficient. Such plasmonic hotspots, often the nanoscale clefts, gaps, or fissures in nano-structured metals, are known to amplify the electromagnetic fields to a very large extent.23,27,28 From the lifetime distributions, the average fluorescence lifetime of Alexa-647-SA molecules on the MEF substrates is ~ 0.3 nsec, and that on glass is 1.4 nsec. The lifetime histograms indicate that on average the lifetimes are about 5-fold shorter on the nanostructures than on glass. The relative width of the distribution of the histograms could be a result of a number of mechanisms such as different local nano-environments, variation in the nanostructure of the klarite substrates. An important observation is that the fluorophores with the shorter lifetimes had higher intensities (over 50-fold), which suggests that the radiative decay rate of the Alexa-647 molecules are much larger on the metal nano-structured substrate than on glass. The results indicate that plasmonic nanostructures can increase the brightness and reduce the lifetime of Alexa molecules. This also suggests that Alexa-647 molecules can potentially have higher photostability on plasmonic substrate when compared to glass. We believe this is the first observation of the lifetime imaging and spectroscopy on a commercially available plasmonic substrate.
Figure 6.
Intensity (top panels) and lifetime (bottom panels) histograms of streptavidin conjugated Alexa-647 bound to the BSA-biotinylated on glass or nano-structured substrates.
The effect of metals can be described from the perspective of the fluorophore. We know the quantum yield and lifetime of a fluorophore are interrelated as defined below29:
| (5) |
| (6) |
where Γ and knr are the radiative and nonradiative decay rates respectively. In the presence of metal, the quantum yield and lifetime are given by:
| (7) |
| (8) |
where Γm is the radiative rates in the presence of metal particles. In this discussion we do not consider the quenching effect of the metal (kmnr). Increases in radiative rates near metal nanostructures result in increased quantum yields and decreased lifetimes. The above equations result in one unique property of metal-fluorophore interactions, i.e. the lifetime decreases as the intensity increases. A decrease in lifetime has several favorable consequences: (a) It allows for increased fluorophore photostability, as there is less time for excited state photo-destructive processes to occur; (b) Additionally, fluorophores can become less prone to optical saturation and have higher maximum emission rates. Thus increase in emission intensity of over 50-fold on a MEF substrate for a common dye in the red-region of the spectra accompanied by approximately 5-fold decrease in lifetime result in increased detectability that can be utilized for any surface based fluorescence assay.
4.0 Conclusions
The focus of this study was to determine whether a commercially available plasmonic SERS substrate can be modified to observe substantial metal-enhanced fluorescence. Deposition of a 10nm film of silver (Ag) and a 5nm film of silica on the commercial klarite substrates generated significant improvement in fluorescence intensity relative to glass microscope slides. We observed over 50-fold increase in the fluorescence intensity from a monolayer of streptavidin conjugated Alexa-647 on the biotinylated plasmonic substrate compared to the same on glass substrate. A reduction in the lifetime and increased photostability of Alexa-647 on plasmonic substrate was observed. The implementation of plasmonic nanostructures into a novel sensing devices will enable simple and inexpensive detection of multiple biomarkers of clinical samples. Fluorescence based assays are of particular interest because they are predominant analytical technology in which the specific interaction of an antibody with its antigen is exploited for molecular recognition. In spite of excellent properties of fluorescent probes their applications to ultrahigh sensitive bioassays are limited by their insufficient brightness and photostability. We believe this study will facilitate the wide use of fluorescence based bioassays utilizing reproducible and standardized MEF substrates for a plethora of sensing applications.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) - Grant Nos. AI087968 (KR) and HG002655, EB006521 (JRL). The authors would like to thank Dr. S. Dutta Chowdhury for helpful comments.
References
- 1.Drexhage KH. Influence of a Dielectric Interface on Fluorescence Decay Time. J. Luminescence. 1970;2:693–701. [Google Scholar]
- 2.Ford GW, Weber WH. Electromagnetic Effects on a Molecule at a Metal Surface. Surface Sci. 1981;109:451–481. [Google Scholar]
- 3.Chance RR, Prock A, Silbey R. Molecular Fluorescence and Energy Transfer near Interfaces. Adv. Chem. Phys. 1973;37:1–65. [Google Scholar]
- 4.Ford GW, Weber WH. Electromagnetic Interactions of Molecules with Metal Surfaces. Phys. Reports. 1984;113:195–287. [Google Scholar]
- 5.Novotny K, Hecht B. Principles of Nano-Optics. Cambridge University Press; 2006. p. 539. [Google Scholar]
- 6.Gersten JI. Theory of fluorophore-metallic surface interactions. In: Geddes CD, Lakowicz JR, editors. Topics in Fluorescence Spectroscopy, Vol. 8: Radiative Decay Engineering. New York: Springer Science Business Media, Inc; 2005. pp. 197–221. [Google Scholar]
- 7.Lakowicz JR, Ray K, Chowdhury M, Szmacinski H, Fu Y, Zhang J, Nowaczyk K. Plasmon-Controlled Fluorescence: A New Paradigm in Fluorescence Spectroscopy. The Analyst. 2008;133:1308–1346. doi: 10.1039/b802918k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray K, Badugu R, Lakowicz JR. Metal-Enhanced Fluorescence from CdTe Nanocrystals: A Single Molecule Fluorescence Study. J. Am. Chem. Soc. 2006;128:8998–8999. doi: 10.1021/ja061762i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray K, Badugu R, Lakowicz JR. Polyelectrolyte Layer-by-Layer Assembly To Control the Distance between Fluorophores and Plasmonic Nanostructures. Chem. Mat. 2007;19:5902–5909. doi: 10.1021/cm071510w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury MH, Lakowicz JR, Ray K. Ensemble and Single Molecule Studies on the Use of Metallic Nanostructures to Enhance the Intrinsic Emission of Enzyme Cofactors. J Phys Chem C. 2011;115:7298–7308. doi: 10.1021/jp112255j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray K, Szmacinski H, Lakowicz JR. Enhanced Fluorescence of Proteins and Label-Free Bioassays using Aluminum Nanostructures. Anal. Chem. 2009;81:6049–6054. doi: 10.1021/ac900263k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakowicz JR. Radiative Decay Engineering 5: Biophysical and Biomedical Applications. Anal. Biochem. 2005;337:171–194. doi: 10.1016/j.ab.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pompa PP, Martiradonna L, Delia Torre A, Delia Sala F, Manna L, De Vittorio M, Calabi F, Cingolani R, Rinaldi R. Metal-Enhanced Fluorescence of Colloidal Nanocrystals with Nanoscale Control. Nature Nanotechnology. 2006;1:126–130. doi: 10.1038/nnano.2006.93. [DOI] [PubMed] [Google Scholar]
- 14.Nooney R, Clifford A, LeGuevel X, Stranik O, McDonagh C, MacCraith B. Enhancing the Analytical Performance of Immunoassays that Employ Metal-Enhanced Fluorescence. Anal. Bioanal. Chem. 2010;396:1127–1134. doi: 10.1007/s00216-009-3357-9. [DOI] [PubMed] [Google Scholar]
- 15.Osorio-Román IO, Ortega-Vásquez V, Vargas CV, Aroca RF. Surface-Enhanced Spectra on D-Gluconic Acid Coated Silver Nanoparticles. Appl. Spectrosc. 2011;65:838–843. doi: 10.1366/11-06279. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Chen CY, Wei X, Qiang W, Li Z, Cheng Q, Xu D. Highly Sensitive Detection of Proteins Based on Metal-Enhanced Fluorescence with Novel Silver Nanostructures. Anal. Chem. 2012;84:8656–8662. doi: 10.1021/ac301787x. [DOI] [PubMed] [Google Scholar]
- 17.Liang J, Li K, Gurzadyan GG, Lu X, Liu B. Silver Nanocube-Enhanced Far-red/Near-infrared Fluorescence of Conjugated Polyelectrolyte for Cellular Imaging. Langmuir. 2012;28:11302–11309. doi: 10.1021/la302511e. [DOI] [PubMed] [Google Scholar]
- 18.Gryczynski I, Luchowski R, Matveeva EG, Shtoyko T, Sarkar P, Borejdo J, Akopova I, Gryczynski Z. Metal-Enhanced Immunoassays. Methods Mol. Biol. 2012;875:217–229. doi: 10.1007/978-1-61779-806-1_10. [DOI] [PubMed] [Google Scholar]
- 19.Deng W, Goldys EM. Plasmonic Approach to Enhanced Fluorescence for Applications in Biotechnology and the Life Sciences. Langmuir. 2012;28:10152–10163. doi: 10.1021/la300332x. [DOI] [PubMed] [Google Scholar]
- 20.Aouani H, Wenger J, Gérard D, Rigneault H, Devaux E, Ebbesen TW, Mahdavi F, Xu T, Blair S. Crucial Role of the Adhesion Layer on the Plasmonic Fluorescence Enhancement. ACS Nano. 2009;3:2043–2048. doi: 10.1021/nn900460t. [DOI] [PubMed] [Google Scholar]
- 21.Karolin J, Geddes CD. Reduced Lifetimes are Directly Correlated with Excitation Irradiance in Metal-Enhanced Fluorescence. J. Fluorescence. 2012;22:1659–1662. doi: 10.1007/s10895-012-1132-3. [DOI] [PubMed] [Google Scholar]
- 22.Strickler SJ, Berg RA. Relationship Between Absorption Intensity and Fluorescence Lifetimes of Molecules. J. Chem. Phys. 1962;37:814–822. [Google Scholar]
- 23.Hankus M, Stratis-Cullum D, Pellegrino P. Surface Enhanced Raman Scattering (SERS)-Based Next Generation Commercially Available Substrate: Physical Characterization and Biological Application, SPIE-Optics and Photonics West. 2011;8099:7. [Google Scholar]
- 24.Jiao Y, Ryckman JD, Ciesielski P, Escobar C, Jennings G, Weiss S. Patterned Nanoporous Gold as an Effective SERS Template. Nanotechnology. 2011;22:295–302. doi: 10.1088/0957-4484/22/29/295302. [DOI] [PubMed] [Google Scholar]
- 25.Lakowicz JR. Principles of Fluorescence Spectroscopy. 3rd Ed. Springer: New York; 2006. [Google Scholar]
- 26.Ray K, Badugu R, Lakowicz JR. Sulforhodamine Adsorbed Langmuir-Blodgett Layers on Silver Island Films: Effect of Probe Distance on the Metal-Enhanced Fluorescence. J. Phys. Chem C. 2007;111:7091–7097. doi: 10.1021/jp067635q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moskovits M. Imaging: Spot the Hotspot. Nature. 2011;469:307–308. doi: 10.1038/469307a. [DOI] [PubMed] [Google Scholar]
- 28.Bek A, Jansen R, Ringler M, Mayilo S, Klar TA, Feldmann J. Fluorescence Enhancement in Hot Spots of AFM-Designed Gold Nanoparticle Sandwiches. Nano Lett. 2008;8:485. doi: 10.1021/nl072602n. [DOI] [PubMed] [Google Scholar]
- 29.Lakowicz JR. Radiative Decay Engineering: Biophysical and Biomedical Applications. Anal. Biochem. 2001;298:1–24. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]