Abstract
Frozen tissue, a gold standard biospecimen, can yield well preserved nucleic acids and proteins after over a decade but is vulnerable to thawing and has substantial fiscal, spatial, and environmental costs. A long-term room temperature biospecimen storage alternative that preserves broad analytical utility can potentially empower tissue-based research. As there is scant data on the analytical utility of lyophilized brain tumor biospecimens, we evaluated lyophilized (freeze-dried) samples stored for 1 year at room temperature. Lyophilized tumor tissue processed into paraffin sections produced good histology. Yields of extracted DNA, RNA, and protein approximated those of frozen tissue. After 1 year, lyophilized samples yielded high molecular weight DNA that permitted copy number variation analysis, IDH 1 mutation detection, and MGMT promoter methylation PCR. A 27 % decrease in RIN scores over the 1 year suggests that RNA degradation was inhibited though incompletely. Nevertheless, RT-PCR studies on lyophilized tissue performed similarly to frozen tissue. In contrast to FFPE tissues where protein bands were absent or shifted to a lower molecular weight, lyophilized samples showed similar protein bands as frozen tissue on SDS-PAGE analysis. Lyophilized tissue performed similarly to frozen tissue for Western blots and enzyme activity assays. Immunohistochemistry of lyophilized tissue that were processed into FFPE blocks often required longer incubation times for staining than standard FFPE samples but generally provided robust antigen detection. This preliminary study suggests that lyophilization has promise for long-term room temperature storage while permitting varied tests; however, further work is required to better stabilize nucleic acids particularly RNA.
Keywords: Freeze-dry, Lyophilization, Brain tumor, Tissue, Nucleic acid, Protein, Biobank, Biorepository, Room temperature, Frozen tissue
Introduction
High quality biospecimens with broad analytical utility are critical to modern biomedical research. Frozen tissues stored for years can yield useful nucleic acids and protein [1, 2]. A major drawback is vulnerability to thawing but there are also financial, environmental, labor, and space issues. Freezers costing $6,000–$20,000 use 20–70 kilowatt-hours per day (kWh) and can generate 10,000–35,000 lbs CO2 annually [3]. Special handling and space demands can be problematic. Liquid nitrogen (LN2) tanks add safety concerns for technicians. In this era of limited resources, there is an imperative for developing room temperature storage approaches that could provide safer, lower cost, and lower maintenance alternatives. The ubiquitous formalin-fixed paraffin embedded (FFPE) specimens have cross-linked nucleic acids and proteins as well as fragmentation of nucleic acids [4] and their quality decreases with time [5]. While newer fixatives may better preserve nucleic acids [4, 6, 7], they have not gained wide acceptance. Dessicated tissues of Egyptian mummies can retain histologic details and yield protein and DNA after thousands of years [8, 9]. Therefore, dehydration of tissue is one mechanism by which to accomplish room temperature storage. Lyophilization, also known as a freeze-drying, is a two-step dehydration process achieved by manipulating temperatures and pressure. Primary drying involves lowering the pressure and raising the temperature sufficiently to cause sublimation where solid water (ice) converts directly to a gaseous state without transitioning through a liquid phase. In the secondary drying phase, conditions include elevated temperatures to break physicochemical bonds that bind residual water molecules to tissue leaving final tissue water content in the 1–4 % range [10, 11]. Lyophilization is used extensively for preservation of drugs, antibodies, and food. Freeze-dried blood products are being evaluated for transfusion in humans [12–14]. Lyophilized bacteria, fungi, virus, and hematopoietic stem cells can remain viable [14–16]. Previous investigations of lyophilized systemic tumors have showed variable RNA preservation along with well-preserved DNA and protein after 1 year [17, 18]. However, we were unable to identify similar studies of brain tumor specimens. In this study, we evaluate whether lyophilized brain tumor specimens stored at room temperature for 1 year can yield analyzable histology, DNA, RNA, and protein.
Materials and methods
Brain tumor biospecimens
Fresh frozen human brain tumor biospecimens were obtained under a protocol approved by the University of California, Los Angeles institutional review board. Fourteen brain tumor specimens including: One meningioma WHO Grade I, two atypical meningiomas WHO Grade II, one oligodendroglioma WHO Grade II, two oligoastrocytomas WHO grade II, one anaplastic oligoastrocytoma WHO Grade III, and seven glioblastomas WHO Grade IV, were used for this study. Samples from subsets of these tumors were used for the individual experiments in this study.
Lyophilization conditions
A Free Zone Triad Freeze Dry System (Labconco, Kansas City, MO, USA) was used to lyophilize brain tumor tissue samples. For primary drying, we subject tissues to a shelf temperature of –50 °C for 52 h and a collector temperature of –85 °C under vacuum of 0.01–0.02 mBar. For secondary drying, the tissues in this study were subjected to a shelf temperature of +20 °C for 20 h and a collector temperature of –85 °C under vacuum of 0.01–0.02 mBar. Under these conditions, tumor samples were dehydrated to a water content of 1–2 % as determined by Karl Fischer titration, a standard method for measuring water content [19].
Preparation of formalin fixed paraffin embedded (FFPE) blocks
Fresh tumor tissues were fixed in 10 % buffered formalin for 18 h and then subjected to tissue processing in a Sakura Tissue-Tek VIP (Sakura Finetek, Torrance, CA, USA) as follows: (1) 80 % ethanol for 105 min, (2) three treatments of 95 % ethanol for 45, 105, and 45 min, (3) three treatments of 100 % ethanol for 105, 45, and 45 min, (4) three treatments of xylene for 105, 45, and 45 min, (5) four treatments of paraffin for 105, 45, 45, and 45 min at 60 °C, and (6) a cleaning cycle consisting of a xylene treatment followed by an alcohol treatment at 40 °C. Tissues were then embedded in paraffin blocks.
Storage conditions
Lyophilized tissues were vacuum-sealed in 2 ml amber glass vials and stored in a dark chamber containing 2.5 kg of desiccant (calcium sulfate) while FFPE blocks were stored in cardboard boxes, all at room temperature (21–22 °C). Frozen tissue was stored in a –80 °C freezer.
DNA, RNA and protein extraction and quality analysis
Lyophilized tissue was rehydrated in phosphate buffer solution pH 7.2 at room temperature for 15 min and then homogenized with a handheld grinder (Fisher Scientific, Pittsburgh, PA, USA) for 1 min on ice. Genomic DNA, total RNA and denatured protein from lyophilized or frozen tissue were extracted using the Illustrate TriplePrep Kit (GE Healthcare, Little Chalfont, UK). DNA and total protein from FFPE samples were isolated by using a QIAamp DNA FFPE Tissue Kit and Qproteome FFPE Tissue Extraction Buffer respectively (Qiagen, Hilden, Germany). Total RNA from FFPE samples was extracted with the RNA High Pure FFPE RNA Micro Kit (Roche Diagnostics, Mannheim, Germany). DNA was electrophoresed on 1 % agarose gels (BioRad, Hercules, CA, USA). Total RNA quality was assessed by RNA Integrity Number (RIN) obtained with the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The quality of total protein was evaluated by SDS-PAGE on a 4–20 % Tris– glycine gradient gel (Invitrogen, Carlsbad, CA, USA).
Single nucleotide polymorphism analysis
200 ng of DNA from relevant tissue samples were analyzed using the HumanCytoSNP-12 BeadChip (300, 000 SNPs) on an iScan array scanner according to the manufacturer’s protocol (Illumina, San Diego, CA, USA) at the Microarray Laboratory, Southern California Genotyping Consortium, UCLA. Copy number variation was determined using the B Allele Freq algorithm of Genome Studio v2010.3.
Sequencing of isocitrate dehydrogenase 1 (IDH1)
Sanger sequencing primers were designed to include the R132 codon within exon 4 of the IDH1 gene. Samples were analyzed using forward 5′-GCG TCA AAT GTG CCA CTA TC-3′ and reverse 5′-CA AAA TCA CAT TAT TGC CAA C-3′ primers to generate a 236 base pair fragment. Purified polymerase chain reaction (PCR) products were sequenced using the BigDye Terminator v1.1 and analyzed on a 3730 sequencer (Applied Biosystems, Foster City, CA, USA).
O-6-Methylguanine-DNA methyltransferase (MGMT) methylation
MGMT methylation analysis was performed by methylation-specific PCR (MSP) according to a previously published protocol with slight modifications [20]. To generate bisulfite modified DNA, genomic DNA was modified with the EZ DNA Methylation-Gold Kit (ZymoResearch, Orange, CA, USA). Samples were subjected to a two-stage nested PCR strategy using: first-stage primers (5′GGATAT GTTGGGATAGTT-3′ and 5′-CCAAAAACCCCAA ACCC-3′) and second-stage primers (unmethylated reaction: 5′-TTTGTGTTTTGATGTTTGTAGGTTTTTGT-3′ and 5′-AACTCCACACTCTTCCAAAAACAAAACA-3′; methylated reaction: 5′-TTTCGACGTTCGTAGGTTT TCGC-3′ and 5′-CACTCTTCCGAAAACGAAACG-3′).Positive and negative control samples for the MSP reaction were U87MG DNA treated with SssI methyltransferase (New England Biolabs, Ipswich, MA, USA) and whole-genome amplification of U87MG DNA using the GenomiPhi V2 Amplification kit (Amersham Biosciences, Piscat-away, NJ, USA).
Reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR was performed using an Illustra Ready-to-Go RT-PCR kit (GE Healthcare, Little Chalfont, UK). All reactions were done in a Mastercycler gradient (Eppendorf, Hauppauge, NY, USA) under an initial denaturing step at 94 °C for 5 min, followed by 35 cycles (40 cycles for EGFRvIII mRNA detection [21]) of 94 °C denaturation for 30 s, 55 °C annealing for 30 s, and 72 °C extension for 1 min. Primers were as follows: GAPDH- (5′-GAG TCA ACG GAT TTG GTC GT-3′ and 5′-TTG ATT TTG GAG GGA TCT CG-3′), GFAP—(5′-GCT TCC TGG AAC AGC AAA AC-3′ and 5′-CAG CCT CAG GTT GGT TTC AT-3′), EGFR wt—(5′-CCA AGG GAG TTT GTG GAG AA-3′ and 5′-CTT CCA GAC CAG GGT GTT GT-3′), EGFRvIII—(5′-CCC CTG ACT CCG TCC AGT ATT G-3′ and 5′-CGA GTA TGA TAG GAG GCA CCA GTA C-3′).
Histology and immunohistochemistry
FFPE blocks are created from fresh tissue then stored. Lyophilized tissues are stored for 1 year then rehydrated with phosphate buffered saline supplemented with Halt Protease Inhibitor Cocktail (Thermo Scientific, Rockford, IL, USA) for 1 h (37 °C) prior to making a FFPE block. Frozen tissues are stored for 1 year then processed into a FFPE block. Antigen retrieval was achieved with a pH 6 Target retrieval solution (DAKO, Carpinteria, CA, USA) in a Decloaking Chamber at 95 °C for 20 min. Tissue sections were then treated with 3 % H2O2 and with Background Sniper (Biocare Medical, Concord, CA, USA) to reduce nonspecific background staining. Primary antibodies for Ki67 (DAKO, Carpinteria, CA, USA), GFAP (Millipore, Temecula, MA, USA), EMA (AbD Serotec, Raleigh, NC, USA) were applied in a 1:100 dilution for 1 h followed by detection with the MACH4 Universal HRP Polymer detection system (Biocare Medical, Concord, CA, USA).
Western blot
Protein aliquots were mixed with 2× Laemmli buffer, boiled at 95 °C for 5 min, loaded onto 4–20 % Tris–glycine gradient gel (Invitrogen, Carlsbad, CA, USA) and electrophoresed at 120 V for 1 h 30 min. After separation, proteins were transferred to a nitrocellulose membrane at 100 V for 1 h. Blots were blocked with 5 % milk in TBST for 30 min and probed with mouse monoclonal primary antibodies for GFAP (1:3,000, Millipore, Temecula, CA, USA), and GAPDH (1:5,000, Sigma-Aldrich, St. Louis, MO, USA), at room temperature for 1 h. Membranes were washed with TBST and probed with anti-mouse HRP-conjugated secondary antibody (1:10,000, Sigma-Aldrich, St. Louis, MO, USA), at room temperature for 1 h. An ECL kit (Thermo Scientific, Rockford, IL, USA) was used for chemoluminescent signal development.
Enzyme activity measurements
Lactate dehydrogenase (LDH, EC 1.1.1.27) and Pyruvate kinase (PK, EC 2.7.1.40) activity was measured in the tissue homogenates with LDH and PK Assay Kits (BioVision, Mountain View, CA, USA). In this colorimetric LDH quantification assay, LDH reduces NAD+ to NADH, which then interacts with a chromogen to produce a color. LDH activity was expressed in mU/mg protein, where one unit LDH is the amount of enzyme that catalyzes the conversion of lactate to pyruvate to generate 1.0 μmol to NADH per min at 37 °C. In the PK assay, phosphoenolpyruvate and adenosine diphosphate are catalyzed by PK to generate pyruvate and adenosine triphosphate. The pyruvate generated is oxidized by pyruvate oxidase to produce a color. Total protein concentration was estimated with a Micro BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA).
Statistical analysis
Statistical analyses were conducted by a statistician (D.T.). We compared mean yields of lyophilized against fresh frozen tissue for DNA, RNA and protein levels at Time zero using paired two sample t tests. Our comparison of DNA, RNA and Protein yields over time (1 year vs. Time zero) is based on paired t tests. Our comparison of mean PK and LDH activity of lyophilized against frozen tissue is based on independent t tests for differences in mean. Confidence Intervals and P-values for differences in mean yield are computed under the assumption of Gaussian sampling. As we consider a paired design and balanced sample sizes, in the case of independent tests, these quantities are known to be robust to potential violations of sampling model assumptions. In order to compare missing data rates between technologies, we considered independent McNeimar tests of homogeneity. P values for the McNeimar test are based on exact calculations.
Results
DNA, RNA, and protein yields
Wet tumor tissue weighing 30–50 mg was either lyophilized or put in frozen storage at –80 °C. To ascertain whether the process of lyophilization affected yield as opposed to loss during long-term storage, tissue samples were analyzed for yield immediately after lyophilization (time zero) and compared to frozen samples (see Online Resource 1). Yields per mg of tissue were calculated using the original weight of wet tissue prior to lyophilization or freezing. DNA, RNA, and protein yields were not statistically significantly different for lyophilized compared to frozen samples (p = 0.09, p = 0.21, p = 0.98 respectively). The yield of DNA, RNA, and protein also did not statistically significantly differ between lyophilized and frozen samples stored for 1 year (p = 0.32, p = 0.91, p = 0.87 respectively).
DNA quality and DNA tests
DNA from all lyophilized samples produced a high molecular weight band on 1 % agarose gels (Fig. 1). Some lyophilized samples can show mild smearing suggesting DNA fragmentation. In contrast, all tested FFPE samples consistently yielded only smeared DNA without a high molecular weight band. DNA sequencing and Methylation-specific PCR for determination of IDH1 mutations and MGMT methylation status produced accurate results from lyophilized samples (see Online Resource 2). SNP analysis was used to evaluate chromosomes 7, 10, 19, and 22 q in three brain tumors as they commonly demonstrate copy number variation (CNV) in gliomas or meningiomas [22–25]. The CNV and loss of heterozygosity (LOH) profiles were highly similar for paired frozen and lyophilized tumors (Fig. 2). Chromosome 22 q LOH, a well described finding in meningiomas, was identified in the meningioma but not in the gliomas. Chromosome 10 LOH was detected in the glioblastoma as is common. No differences between lyophilized and frozen samples were identified in the SNP profiles for the low grade glioma. In the glioblastoma, CNVs largely matched with only a slight variation for chromosome 7 perhaps due to tumor heterogeneity.
Fig. 1.

Genomic DNA extracted from frozen, lyophilized, and FFPE brain tumor tissue M–DNA marker, MEN(F) frozen meningioma, MEN(L) lyophilized meningioma MEN(P) FFPE meningioma, LGG(F) frozen low grade glioma, LGG(L) lyophilized low grade glioma, LGG(P) FFPE low grade glioma, GBM(F) frozen glioblastoma, GBM(L) lyophilized glioblastoma, GBM(P) FFPE glioblastoma
Fig. 2.
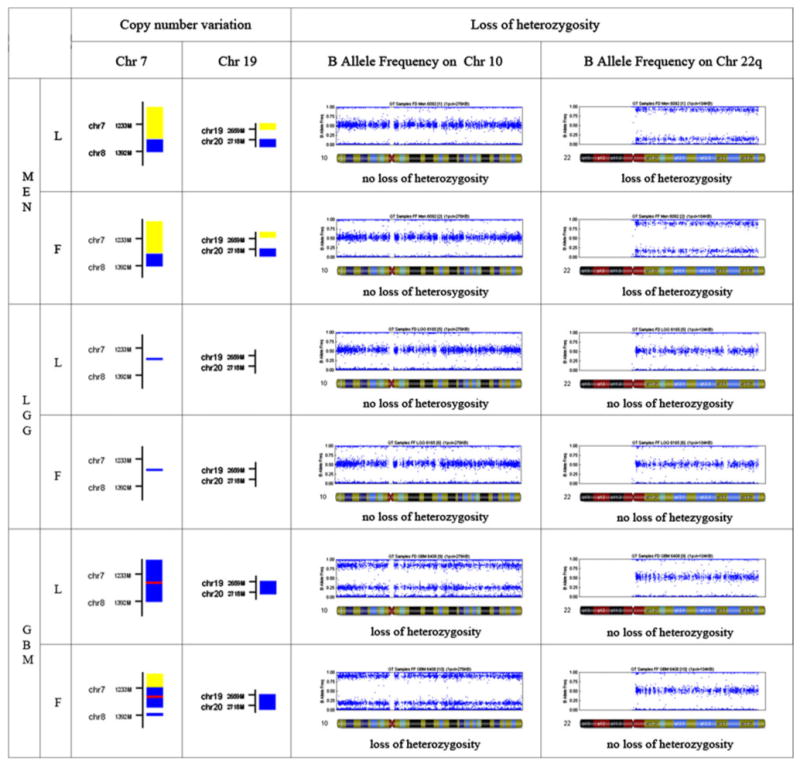
SNP analysis of genomic DNA, lyophilized (L) vs. frozen (F) tissue of three types of brain tumor: meningioma (MEN), low grade glioma (LGG), glioblastoma (GBM). On the left half of the figure, copy number variation is shown for chromosome 7 and 19, where the colored squares represent gain at specific chromosome loci (yellow–1 copy, blue–3 copies, red–4 copies). On the right half of the figure, loss of heterozygosity (LOH) on chromosome 10 and 22 q are shown in a B Allele frequency plot
RNA quality and RT-PCR
Thirteen brain tumor samples including meningioma, glioblastoma, and other glioma were assessed for RNA degradation. After 1 year, RIN scores were lower in lyophilized tissues than their frozen tissue counterparts by 30.8 % (meningioma), 31.0 % (Grade II, III gliomas), and 21.4 % (glioblastoma) respectively (Fig. 3a). The average difference in RIN scores between lyophilized samples and frozen tissue samples was 26.7 % and was statistically different (n = 13; p = 0.015). Nevertheless, RT-PCR for EGFRvIII, EGFR, GFAP, GAPDH mRNA resulted in similar and distinct bands for paired lyophilized and frozen samples (Fig. 3b). Mutant EGFRvIII transcripts were detected only in glioblastoma samples as previously reported [26, 27]. Glioblastoma and low grade glioma contained detectable GFAP mRNA by RT-PCR while, as anticipated, meningioma did not.
Fig. 3.

Assessment of RNA quality. A–RNA integrity number (RIN) of total RNA extracted from lyophilized (open bars) and frozen (grey bars) brain tumor specimens B–RT-PCR products amplified from mRNA of following specimens: MEN(F) frozen meningioma, MEN(L) lyophilized meningioma, LGG(F) frozen low grade glioma, LGG(L) lyophilized low grade glioma, GBM(F) frozen glioblastoma, GBM(L) lyophilized glioblastoma
Protein analysis
SDS-PAGE electrophoresis followed by Coomassie blue staining show similar protein band profiles in lyophilized and frozen samples (Fig. 4a). FFPE protein samples in contrast demonstrate absence of multiple bands and a generalized shift to lower molecular weights. Western blot detection of GFAP and the housekeeping protein GAPDH shows comparable bands for lyophilized and frozen samples (Fig. 4b). GFAP and GAPDH were relatively weakly detected in FFPE samples. Meningioma which is a non-glial tumor did not show evidence of GFAP expression regardless of mode of storage.
Fig. 4.

Analysis of protein samples. A Coomassie staining of total proteins on 4–20 % gradient tris–glycine gel, where M protein marker, MEN(F) frozen meningioma, MEN(L) lyophilized meningioma, MEN(P) FFPE meningioma, LGG(F) frozen low grade glioma, LGG(L) lyophilized low grade glioma, LGG(P) FFPE low grade glioma, GBM(F) frozen glioblastoma, GBM(L) lyophilized glioblastoma, GBM(P) FFPE glioblastoma. B–Western blot for GFAP and GAPDH
Enzymatic activity assessment
The enzymatic activity of lactate dehydrogenase (LDH) of lyophilized and frozen tissue demonstrated no statistically significant difference for meningioma, low grade glioma, and glioblastoma; p = 0.61, p = 0.81, p= 0.76 respectively (see Online Resource 3A). Similarly, the activity of pyruvate kinase (PK) was not detectably different in frozen or lyophilized meningioma, low grade glioma, and glioblastoma; p = 0.73, p = 0.80, p= 0.79 respectively (see Online Resource 3B). The brain tumor specimens showed a greater degree of LDH activity than for brain tissue as previously reported [28].
Histology and immunohistochemistry
Microscopic examination of H&E stained-lyophilized tissue after additional FFPE processing showed histology similar to FFPE except that there can be vacuolization that are presumably ice crystallization artifacts (see Online Resource 4). In lyophilized and other tissue samples, GFAP immunopositivity was present in the low grade glioma and glioblastoma (Fig. 5d–f, j–l). In lyophilized and other tissue samples, the meningioma stained for EMA (Fig. 5p–r). Immunostaining for Ki-67 was qualitatively similar for lyophilized, frozen, and FFPE tissue. Glioblastoma tissue displays a high Ki-67 labeling index (Fig. 5a–c) whereas the low grade glioma and meningioma have relatively low labeling indices (Fig. 5g–i, m–o).
Fig. 5.
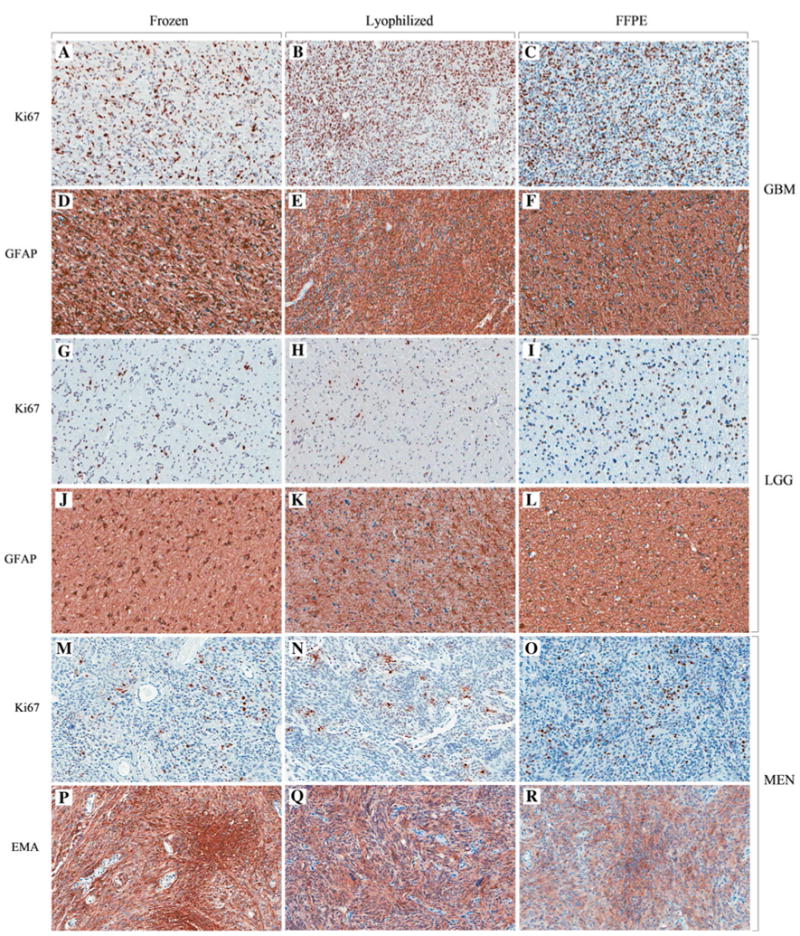
Immunohistochemical staining of frozen–A, D, G, J, M, P,lyophilized–B, E, H, K, N, Q and FFPE–C, F, I, L, O, R brain tumor tissue. (Aperio digital image 200× magnification)
Discussion
The number of archived biospecimens in the Unites States has steadily increased to over 600 million [29]. It is not feasible to obtain or store all biospecimens as frozen tissue. In additional to logistical barriers, there are cost constraints. An European study has estimated that 1 year storage of a lyophilized sample costs 3 euros compared to 24 euros at –80 °C and 31 euros in LN2 [30]. Development of room temperature tissue storage that preserves broad analytical utility can increase access of patients to trials and lower the costs for biomedical research. We have demonstrated that lyophilized brain tumor specimens stored at ambient temperature for 1 year can yield DNA, RNA, and protein of good quantity and sufficient quality for a number of current analytical assays. However, our study suggests that, for optimal long term storage, elucidating the basis for low-level nucleic acid degradation will be essential. Causes of the degradation may include humidity, oxygen, lipid peroxidation, light, and elevated temperature [30, 31]. Reactive oxygen species (ROS) are detectable in lyophilized tissue samples and may cause nucleic acid fragmentation [32]. Brain tumor cells may be especially susceptible to lipid peroxidation that generates reactive oxygen species [33–37]. Antioxidants, dessicants, oxygen absorbers, and other cell stabilizer additives are potential avenues of study. While protein preservation including enzymatic activity appears promising, testing of more immunostains and detailed quantitative comparisons of markers like Ki-67 are also necessary to verify the utility of lyophilized tissue.
Supplementary Material
Acknowledgments
This work is supported in part by the Art of the Brain Fund, Harry E. Singleton Cancer Fund, NIH P50 NS044378, and U01 MH083500. We thank the Translational Pathology Core Laboratory at UCLA for resources provided. We thank Joe DeYoung and the Southern California Genotyping Consortium for assistance in SNP genotyping.
Footnotes
Conflict of interestWe have no conflicts of interest to report.
Electronic supplementary material The online version of this article (doi:10.1007/s11060-013-1135-1) contains supplementary material, which is available to authorized users.
Contributor Information
Sergey Mareninov, Department of Pathology and Laboratory Medicine (Neuropathology), UCLA School of Medicine, Los Angeles, CA 90095, USA.
Jason De Jesus, Department of Pathology and Laboratory Medicine (Neuropathology), UCLA School of Medicine, Los Angeles, CA 90095, USA.
Desiree E. Sanchez, Department of Pathology and Laboratory Medicine (Neuropathology), UCLA School of Medicine, Los Angeles, CA 90095, USA
Andrew B. Kay, Department of Pathology and Laboratory Medicine (Neuropathology), UCLA School of Medicine, Los Angeles, CA 90095, USA
Ryan W. Wilson, Department of Pathology and Laboratory Medicine (Neuropathology), UCLA School of Medicine, Los Angeles, CA 90095, USA
Ivan Babic, Department of Pathology and Laboratory Medicine (Neuropathology), UCLA School of Medicine, Los Angeles, CA 90095, USA.
Weidong Chen, Department of Neurology (Neuro-oncology), David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA.
Donatello Telesca, Department of Biostatistics, UCLA School of Public Health, Los Angeles, CA 90095, USA.
Jerry J. Lou, Department of Neurology (Neuro-oncology), David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA
Leili Mirsadraei, Department of Pathology and Laboratory Medicine (Neuropathology), UCLA School of Medicine, Los Angeles, CA 90095, USA.
Tracie P. Gardner, Department of Pathology and Laboratory Medicine (Neuropathology), UCLA School of Medicine, Los Angeles, CA 90095, USA
Negar Khanlou, Department of Pathology and Laboratory Medicine (Neuropathology), UCLA School of Medicine, Los Angeles, CA 90095, USA.
Harry V. Vinters, Department of Pathology and Laboratory Medicine (Neuropathology), UCLA School of Medicine, Los Angeles, CA 90095, USA, Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, CA 90095, USA, Brain Research Institute, UCLA, Los Angeles, CA 90095, USA
Bob B. Shafa, Department of Neurosurgery, David Geffen School of Medicine at UCLA, UCLA School of Medicine, Los Angeles, CA 90095, USA
Albert Lai, Department of Neurology (Neuro-oncology), David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA, Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, CA 90095, USA, Brain Research Institute, UCLA, Los Angeles, CA 90095, USA.
Linda M. Liau, Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, CA 90095, USA, Brain Research Institute, UCLA, Los Angeles, CA 90095, USA, Department of Neurosurgery, David Geffen School of Medicine at UCLA, UCLA School of Medicine, Los Angeles, CA 90095, USA
Paul S. Mischel, Department of Pathology and Laboratory Medicine (Neuropathology), UCLA School of Medicine, Los Angeles, CA 90095, USA, Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, CA 90095, USA, Brain Research Institute, UCLA, Los Angeles, CA 90095, USA
Timothy F. Cloughesy, Department of Neurology (Neuro-oncology), David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA, Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, CA 90095, USA, Brain Research Institute, UCLA, Los Angeles, CA 90095, USA
William H. Yong, Email: wyong@mednet.ucla.edu, Department of Pathology and Laboratory Medicine (Neuropathology), UCLA School of Medicine, Los Angeles, CA 90095, USA, Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, CA 90095, USA, Brain Research Institute, UCLA, Los Angeles, CA 90095, USA
References
- 1.Chu TY, Hwang KS, Yu MH, Lee HS, Lai HC, Liu JY. A research-based tumor tissue bank of gynecologic oncology: characteristics of nucleic acids extracted from normal and tumor tissues from different sites. Int J Gynecol Cancer. 2002;12(2):171–176. doi: 10.1046/j.1525-1438.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 2.Leonard S, Logel J, Luthman D, Casanova M, Kirch D, Freedman R. Biological stability of mRNA isolated from human postmortem brain collections. Biol Psychiatry. 1993;33(6):456–466. doi: 10.1016/0006-3223(93)90174-c. [DOI] [PubMed] [Google Scholar]
- 3.U.S. EPA . Personal Emissions Calculator Assumptions and References–What you can do—Climate Change—US EPA. 2011 Available via DIALOG. http://www.epa.gov/climatechange/wycd/calculator/ind_assumptions.html. Accessed 28 Dec 2011.
- 4.Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002;161(6):1961–1971. doi: 10.1016/S0002-9440(10)64472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizuno TNH, Iwamoto KS, Ito T, Fukuhara T, Tokunaga M, Tokuoka S, Mabuchi K, Seyama T. RNA from decades-old archival tissue blocks for retrospective studies. Diagn Mol Pathol. 1998;7(4):202–208. doi: 10.1097/00019606-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Preusser M, Plumer S, Dirnberger E, Hainfellner JA, Mannhalter C. Fixation of brain tumor biopsy specimens with rcl2 results in well-preserved histomorphology. Immunohistochem Nucleic Acids Brain Pathol. 2010;20(6):1010–1020. doi: 10.1111/j.1750-3639.2010.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincek V, Nassiri M, Nadji M, Morales AR. A tissue fixative that protects macromolecules (DNA, RNA, and protein) and histomorphology in clinical samples. Lab Invest. 2003;83(10):1427–1435. doi: 10.1097/01.lab.0000090154.55436.d1. [DOI] [PubMed] [Google Scholar]
- 8.Hawass Z, Gad YZ, Ismail S, Khairat R, Fathalla D, Hasan N, Ahmed A, Elleithy H, Ball M, Gaballah F, Wasef S, Fateen M, Amer H, Gostner P, Selim A, Zink A, Pusch CM. Ancestry and pathology in king Tutankhamun’s family. JAMA. 2010;303(7):638–647. doi: 10.1001/jama.2010.121. [DOI] [PubMed] [Google Scholar]
- 9.Mekota AM, Vermehren M. Determination of optimal rehydration, fixation and staining methods for histological and immunohistochemical analysis of mummified soft tissues. Bio-tech Histochem. 2005;80(1):7–13. doi: 10.1080/10520290500051146. [DOI] [PubMed] [Google Scholar]
- 10.Jennings TA. Lyophilization: introduction and basic principles. CRC Press LLC; Boca Raton: 2008. [Google Scholar]
- 11.Mellor JD. Fundamentals of freeze drying. Elsevier/Academic Press; Maryland Heights: 1978. [Google Scholar]
- 12.Bode AP, Fischer TH. Lyophilized Platelets: fifty Years in the making. Artif Cells Blood Substit Immobil Bio-technol. 2007;35(1):125–133. doi: 10.1080/10731190600974962. [DOI] [PubMed] [Google Scholar]
- 13.Hellstern P. Fresh-frozen plasma, pathogen-reduced single-donor plasma or bio-pharmaceutical plasma? Transfus Apher Sci. 2008;39(1):69–74. doi: 10.1016/j.transci.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Wolkers WF, Tablin F, Crowe JH. From anhydrobiosis to freeze-drying of eukaryotic cells. Comp Biochem Physiol A. 2002;131(3):535–543. doi: 10.1016/s1095-6433(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 15.Bolla PA, Serradell Mde L, de Urraza PJ, De Antoni GL. Effect of freeze-drying on viability and in vitro probiotic properties of a mixture of lactic acid bacteria and yeasts isolated from kefir. J Dairy Res. 2011;78(01):15–22. doi: 10.1017/S0022029910000610. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan SS, Pyatt DW, Carpenter JF. Preservation of differentiation and clonogenic potential of human hematopoietic stem and progenitor cells during lyophilization and ambient storage. PLoS One. 2010;5(9):12518. doi: 10.1371/journal.pone.0012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu WW, Wang Z, Hollister SJ, Krebsbach PH. Localized viral vector delivery to enhance in situ regenerative gene therapy. Gene Ther. 2007;14(11):891–901. doi: 10.1038/sj.gt.3302940. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo S, Sugiyama T, Okuyama T, Yoshikawa K, Honda K, Takahashi R, Maeda S. Preservation of pathological tissue specimens by freeze-drying for immunohistochemical staining and various molecular biological analyses. Pathol Int. 1999;49(5):383–390. doi: 10.1046/j.1440-1827.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi R, Matsuo S, Okuyama T, Sugiyama T. Degradation of Macromolecules during Preservation of Lyophilized Pathological Tissues. Pathol Res Pract. 1995;191(5):420–426. doi: 10.1016/S0344-0338(11)80729-6. [DOI] [PubMed] [Google Scholar]
- 20.Sturgeon R, Lam J, Windust A, Grinberg P, Zeisler R, Oflaz R, Paul R, Lang B, Fagan J, Simard B, Kingston C. Determination of moisture content of single-wall carbon nanotubes. Anal Bioanal Chem. 2012;402(1):429–438. doi: 10.1007/s00216-011-5509-y. [DOI] [PubMed] [Google Scholar]
- 21.Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M, Filka E, Yong WH, Mischel PS, Liau LM, Phuphanich S, Black K, Peak S, Green RM, Spier CE, Kolevska T, Polikoff J, Fehrenbacher L, Elashoff R, Cloughesy T. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29(2):142–148. doi: 10.1200/jco.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimoto K, Dang J, Zhu S, Nathanson D, Huang T, Dumont R, Seligson DB, Yong WH, Xiong Z, Rao N, Winther H, Chakravarti A, Bigner DD, Mellinghoff IK, Horvath S, Cavenee WK, Cloughesy TF, Mischel PS. Development of a real-time RT-PCR assay for detecting EGFRvIII in glioblastoma samples. Clin Cancer Res. 2008;14(2):488–493. doi: 10.1158/1078-0432.ccr-07-1966. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho L, Smirnov I, Baia G, Modrusan Z, Smith J, Jun P, Costello J, McDermott M, VandenBerg S, Lal A. Molecular signatures define two main classes of meningiomas. Mol Cancer. 2007;6(1):64. doi: 10.1186/1476-4598-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Idbaih A, Carvalho Silva R, Crinière E, Marie Y, Carpentier C, Boisselier B, Taillibert S, Rousseau A, Mokhtari K, Ducray F, Thillet J, Sanson M, Hoang-Xuan K, Delattre JY. Genomic changes in progression of low-grade gliomas. J Neurooncol. 2008;90(2):133–140. doi: 10.1007/s11060-008-9644-z. [DOI] [PubMed] [Google Scholar]
- 25.Network TCGAR . Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wada K, Maruno M, Suzuki T, Kagawa N, Hashiba T, Fujimoto Y, Hashimoto N, Izumoto S, Yoshimine T. Chromosomal and genetic aberrations differ with meningioma subtype. Brain Tumor Pathol. 2004;21(3):127–133. doi: 10.1007/bf02482188. [DOI] [PubMed] [Google Scholar]
- 27.Ekstrand AJ, Sugawa N, James CD, Collins VP. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proc Natl Acad Sci USA. 1992;89(10):4309–4313. doi: 10.1073/pnas.89.10.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA. 1987;84(19):6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corridori F, Cremona T, Tagliabue G. Glutamic-oxalacetic transaminase and lactic dehydrogenase activities in brain tumour homogenates. J Neurochem. 1960;6(2) doi: 10.1111/j.1471-4159.1960.tb13459.x. [DOI] [PubMed] [Google Scholar]
- 30.Vaught J, Rogers J, Carolin T, Compton C. bio-bankonomics: developing a sustainable business model approach for the formation of a human tissue biobank. J Natl Cancer Inst Monogr. 2011;42:24–31. doi: 10.1093/jncimonographs/lgr009. [DOI] [PubMed] [Google Scholar]
- 31.Leboeuf C, Ratajczak P, Zhao WL, François Plassa L, Court M, Pisonero H, Murata H, Cayuela JM, Ameisen JC, Garin J, Janin A. Long-term preservation at room temperature of freeze-dried human tumor samples dedicated to nucleic acids analyses. Cell Preserv Technol. 2008;6(3):191–198. doi: 10.1089/cpt.2008.0003. [DOI] [Google Scholar]
- 32.Matsuo S, Toyokuni S, Osaka M, Hamazaki S, Sugiyama T. Degradation of DNA in dried tissues by atmospheric oxygen. Biochem Biophys Res Commun. 1995;208(3):1021–1027. doi: 10.1006/bbrc.1995.1436. [DOI] [PubMed] [Google Scholar]
- 33.Commoner B, Townsend J, Pake GE. Free radicals in biological materials. Nature. 1954;174(4432):689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- 34.Cirak B, Inci S, Palaoglu S, Bertan V. Lipid peroxidation in cerebral tumors. Clin Chim Acta. 2003;327(1–2):103–107. doi: 10.1016/s0009-8981(02)00334-0. [DOI] [PubMed] [Google Scholar]
- 35.Colquhoun A. Lipids, mitochondria and cell death: implications in neuro-oncology. Mol Neurobiol. 2010;42(1):76–88. doi: 10.1007/s12035-010-8134-4. [DOI] [PubMed] [Google Scholar]
- 36.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7(2):97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Zengin E, Atukeren P, Kokoglu E, Gumustas MK, Zengin U. Alterations in lipid peroxidation and antioxidant status in different types of intracranial tumors within their relative peritumoral tissues. Clin Neurol Neurosurg. 2009;111(4):345–351. doi: 10.1016/j.clineuro.2008.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


