Abstract
Solid tumors are well known for their genomic heterogeneity. While some aspects of this derive from so-called driver mutations, it is now clear that tumor cells possess a seemingly limitless capacity to evade cell death pathway activation, maintain essential survival programming, and initiate resistance networks that block efficacy of cytotoxic and targeted therapy. Given this amazing survival capability, how then to design approaches for effective eradication of malignant cells? Also present within all solid tumors is a diverse assemblage of genomically-stable immune cell types. While some of these possess documented activities that foster tumor progression, others possess inherent activities, that when favored, lead to rapid tumor cell elimination. This review focuses on aspects of dendritic cell (DC) biology in solid tumors, especially breast cancers, which point to DCs as a tractable tool to exploit for immune-based therapies.
Keywords: cancer, inflammation, immunogenic cell death, dendritic cells, Th2
INTRODUCTION
Novel therapeutic approaches are urgently needed for patients with breast cancer. Immunotherapies are amongst the most promising of these, including immune checkpoint-blockade where select T cell regulatory mechanisms are blocked, reported to alter the natural history of some refractory cancers [1, 2]. For example, improved survival has been documented for patients with metastatic melanoma treated with a blocking antibody targeting CTLA-4 [3], a T cell-intrinsic regulatory molecule [4]. Furthermore, objective responses in pretreated metastatic non-small cell lung cancer patients has been observed with PD-1-targeted therapy [5] (a T cell-extrinsic regulatory molecule that delivers an inhibitory signal via binding of ligand, PDL-1, expressed on some cancers [6]). In addition, delivery of immune activating signals can enhance anti-tumor responses to standard therapy, as has been recently illustrated with CD40-targeted therapy in pancreatic cancer [7]. These promising clinical findings indicate the power and therapeutic potential of leveraging aspects of immune-mediated mechanisms as anti-cancer therapy. Herein, we discuss recent insights and advances in the understanding aspects of tumor-promoting inflammation in breast cancer, focusing on the role of dendritic cells (DCs).
CHALLENGES IN BREAST CANCER THERAPY
Despite definitive reductions in breast cancer–related mortality, median survival of the ~ 25% of patients who develop metastatic disease remains poor at ~ 2–3 years [8]. While administration of preoperative therapies has not improved overall survival above that achieved with adjuvant therapy, the rate of pathologic complete response (pCR) after preoperative chemotherapy has been demonstrated to be a predictor of improved outcomes for estrogen receptor (ER)-negative breast cancer [9, 10]. Indeed, patients with triple-negative breast cancer (TNBC) that lacks ER, progesterone receptors (PR) and HER2, achieve higher pCR rates with preoperative chemotherapy as compared to ER-positive cancers, moreover, a pCR in TNBC predicts for highly favorable disease-free survival rates [11]. Conversely, patients with TNBC who do not achieve a pCR and have residual disease after preoperative chemotherapy, have a markedly increased risk of rapid recurrence, and death [11]. These patients represent a great unmet medical need as there is no known effective therapy that improves outcome. Many translational and clinical trials with new therapies for TNBC have been launched exploiting molecular insights to target tumor cell-intrinsic pathways regulating proliferation, survival, and chemoresistance (reviewed in [12]). With that TNNBC represents a heterogeneous assemblage of subtypes [13, 14], there is optimism for clinical trials evaluating sub-type-selective targeted-therapies for this patient group. That said, the inherent genomic instability and intratumoral heterogeneity of TNBC may instead limit efficacy, or enhance host toxicity that limits combinatorial strategies. Conversely, leveraging the diversity inherent to the immune response in these tumors for therapeutic gain has potential to overcome tumor cell genomic plasticity and clonal evolution.
While many immune effectors pathways are co-opted by tumors to foster neoplastic progression [15] other immune effector pathways can be harnessed to eliminate (breast) cancer cells. Perhaps the most compelling of these in humans is that observed by paraneoplastic diseases, some of which are neurological disorders that are a consequence of anti-tumor immune responses [16, 17]. Onconeural antigens (like cdr2), normally expressed on neurons, can also be expressed on breast cancer cells [16, 17]; some patients develop a strong antigen-specific CD8+ T cell-mediated response against their breast cancer resulting in autoimmune cerebellar degeneration and severe neurological dysfunction [16, 17]. The presence of naturally occurring immunity against a broad range of tumor-associated antigens including HER-2/neu, MUC1, cyclin B1 and survivin has now been documented in patients with breast cancer [18]. Indeed, some early clinical studies are attempting to augment this intrinsic immunity in patients at high risk for disease recurrence [19–21]. However, the native immune response to the cancer co-exists with the cancer, and is therefore not protective, either because of tumor escape, for example, through clonal evolution, or because it might have been generated in an inappropriate immunosuppressive microenvironment.
There is accumulating evidence that chronic inflammatory pathways play a key role in the initiation and progression of cancer [15, 22]. There are (at least) two types of chronic inflammation having opposing effects on tumors: (a) chronic inflammation that promotes cancer cell survival and metastasis [23–25], and (b) acute inflammation that can trigger cancer cell destruction as illustrated by regression of bladder cancer after treatment with microbial preparations [26]. Although chronic inflammation is often linked with the presence of type 2 polarized responses involving alternatively-activated macrophages (variably referred to as type 2, M2, Th2-type), acute inflammation associated with cancer destruction is instead linked with classically-activated macrophages (variably referred to as type 1, M1, Th1-type) [27]. Type 1 macrophages are induced by type 1 cytokine like interferon (IFN)-γ, whereas type 2 macrophages are induced by type 2 cytokines including interleukins (IL)-4 and IL-13 [27]. Clinically, there is evidence that chronic inflammation may increase the risk of breast cancer recurrence [28]; in a multi-center study of 734 women treated for early stage breast cancer, high levels of circulating acute phase proteins (APPs) approximately 3 years after treatment were associated with a two-fold elevated risk of disease recurrence and mortality [28]. Herein, we will discuss the mechanisms by which cancers can hijack dendritic cells (DCs) to promote chronic inflammation and accelerate tumor development, and how understanding this circuitry can offer new targets for cancer therapy.
DENDRITIC CELLS
Immunity results from a complex interplay between the innate arm of the immune system (which is antigen-nonspecific), and the adaptive arm of the immune system (which is antigen-specific). Cells of the innate arm utilize non-clonal recognition receptors, including lectins, Toll-like receptors (TLRs), NOD-like receptors (NLRs) and helicases for activation. B and T lymphocytes of the adaptive arm instead utilize clonal receptors that recognize antigens, or their derived peptides, in a highly specific manner. The nature of the immune response is regulated by DCs, a rare cell type in most tissues where under homeostatic conditions are key cellular sensors of microbes. DCs are linked to their environment through a wealth of molecular sensors enabling them to sense danger, and to transmit resulting information to lymphocytes. Thus, DCs provide an essential link between innate and adaptive immunity and thus represent an attractive vector for immunotherapy [29, 30].
DCs, discovered by Ralph Steinman in 1973, are bone marrow-derived cells that seed all tissues (reviewed in [31]), where they sample their environment and transmit information to adaptive immune cells [30, 31]. In peripheral tissues, DCs capture antigens (Ags) through several complementary mechanisms. DCs launch immune responses by presenting captured Ag in the form of peptide- major histocompatibility complex (MHC) complexes to naïve, i.e., antigen-inexperienced, T cells in lymphoid tissues. Upon interaction with DCs, naïve CD4+ and CD8+T cells differentiate into antigen-specific memory T cells with distinct functions. CD4+ T cells for example, can become T helper (Th)-1, Th2, or Th17 cells, T follicular helper cells (Tfh) that help B cells differentiate into antibody secreting cells, or regulatory T cells (Tregs) that modulate functions of other lymphocytes. Naïve CD8+ T cells can give rise to cytotoxic effector lymphocytes (CTLs).
In the steady state, non-activated (immature) DCs present self-antigens to T cells, thereby inducing tolerance either through T cell deletion or differentiation of regulatory/suppressor T cells [32]. These immature DCs have special characteristics including: 1) ability to efficiently capture Ags, 2) accumulation of MHC class II molecules in the late endosome-lysosomal compartment, 3) low level expression of costimulatory molecules, 4) a unique set of chemokine receptors allowing their migration to lymphoid tissues (e.g. CCR7), and 5) limited capacity to secrete cytokines [33]. In contrast, mature Ag-loaded DCs can launch differentiation of Ag-specific T cells into effector cells with unique functions and cytokine profiles. DC maturation is associated with: 1) down-regulation of Ag-capture activity, 2) increased expression of surface MHC class II molecules and costimulatory molecules, 3) ability to secrete cytokines [33], and 4) acquisition of CCR7 expression thus enabling migration of DCs into draining lymph nodes [33]. However, DC maturation does not result in a unique phenotype. Rather, in response to various signals provided by different microbes, either directly or through the surrounding cells, DCs acquire distinct phenotypes that eventually contribute to diverse immune responses. In addition to cytokines or direct microbial signals, ligation of CD40 represents an essential signal for differentiation of fully mature DCs able to launch adaptive T cell immunity [34]. The plasticity of DCs in response to extrinsic signals, and the existence of distinct DC subsets with specific functions, contributes to the mounting of highly diverse immune responses. DCs that sit in tissues under steady state are dependent upon FLT3 (fms-related tyrosine kinase receptor 3) and macrophage-colony stimulating factor receptor (MCSF-R) [35]. However, inflammatory processes such as those initiated by microbial invasion, or developing cancers, substantially alter DC compartments. While the origin of DCs recruited to sites of inflammation is still unclear, it is clear that monocytes give rise to inflammatory DCs in vivo [36].
Human blood DC subsets can be distinguished by differential expression of three surface molecules: CD303 (BDCA-2), CD1c (BDCA-1), and CD141 (BDCA3) [37]. CD303+ plasmacytoid DCs (pDCs) represent a front-line of anti-viral immunity through their ability to secrete large quantities of type I IFN in response to viral encounter [38]. Their pre-synthesized stores of MHC class I permit a rapid initial CD8+ T cell response to viral infection [39]. pDC-derived type I IFN may promote the immunogenic maturation of other DC populations [40] therefore helping to activate novel T cell clones. In their resting state, pDCs are considered to play an important role in tolerance, including oral tolerance [40]. This functional plasticity could be exploited in cancer as we will discuss below.
Human CD141+CD1c− DCs uniquely express Toll-like Receptor 3 (TLR3), produce IL-12, and efficiently cross-prime CD8+ T cells when activated by the TLR3 ligand, poly I:C [41–47]; however, other human DC subsets such as Langerhans cells (LCs) [48, 49] and CD1c+ DCs also cross-present antigens to CD8+ T cells [43, 45, 46]. Recent data from humanized mice, human blood, and lung tissue reveal that both CD1c+ and CD141+ DC subsets can acquire viral antigens and thereby drive antiviral effector CD8+ T cell responses [50]. In contrast, CD1c+ DCs are uniquely able to drive differentiation of CD103+CD8+ mucosal T cells [50]. This is important because CD103 expression by CTLs mediates adherence to E-cadherin resulting in tumor cell rejection [51]. Indeed, mucosal homing and retention of CD8+ T cells is important for mucosal cancer vaccine efficacy [52]. These results highlight the critical role the route of immunization plays in trafficking of effector T cells [53, 54], and the critical role tissue DCs play in imprinting the trafficking patterns of elicited T cells [55].
The human skin hosts epidermal LCs and dermal interstitial DCs (dermal DCs). The dermal DCs can be further subdivided into CD1a+ DCs and CD14+ DCs. Earlier studies of human cutaneous DCs demonstrated their phenotypic and functional heterogeneity with regards to cellular immunity and priming of highly efficient CTLs [56]. Our studies concluded that human CD14+ DCs can directly help activated B cells, as well as induce naïve T cells, differentiate into cells with properties of T follicular helper cells (Tfh) [48], thus, they may be specialized for development of humoral responses [48]. On the contrary, LCs are more efficient in cross-presenting peptides from protein Ags to CD8+ T cells, and in priming CD8+ T cells in becoming potent CTLs [48]. With this evolving understanding of the biologic function of DCs, the challenge becomes deciphering which DCs control T cell differentiation and trafficking in vivo in human breast cancer.
CD4+ T CELLS IN BREAST CANCER-ASSOCIATED INFLAMMATION
An expanding list of Th subsets, specialized for promoting particular types of inflammation, function through secretion of a restricted set of cytokines leading to unique classes of immune response (reviewed in [57]). Thus, in response to intracellular microbes, such as viruses and certain bacteria, CD4+ T cells differentiate into Th1 cells, secrete IFN-γ, and possess a specific range of functions. In contrast, extracellular pathogens such as helminths induce development of Th2 cells, whose cytokines [IL-4, IL-5, IL-10 and IL-13] direct immunoglobulin E- and eosinophil-mediated destruction of pathogens [57]. Since discovery of Th1 and Th2, a large spectrum of CD4+ T cell phenotypes have been described based on their cytokine secretion profiles and function (reviewed in [57]). The main subsets of CD4+ T cells also express unique transcription factors: Th1 cells can be identified by expression of T-bet, Th2 cells express GATA-3, Th17 cells express RORγT, whereas Tregs express Fox-P3 [57]. All of these CD4+ T cell types contribute to tumorigenesis in various ways. For example, Tregs can inhibit effector functions of CD8+ T cells thereby preventing tumor rejection [58]. Although in general favoring tumor rejection, Th1 cells can contribute to tumor escape via secretion of IFN-γ which in turn triggers expression of programmed cell death ligand (PDL)-1 in tissues providing an off-signal to effector T cells [6]. Furthermore, selective evolutionary pressure exerted by IFN-γ can lead to tumor editing and selection of resistant clones, thereby also facilitating tumor development [59].
Such plasticity of cells and outcomes is even further exemplified by the more recently identified Th17 cells [60] that exert pro- and anti-tumor activity depending on the tissue environment in which they find themselves. Th17 cells are detected at strikingly high frequency in tumors, but not blood, of patients with diverse cancer types, including ovarian and pancreatic cancer (reviewed in [61]). The major pro-tumor effects of Th17 cells are manifest by their capacity to promote angiogenesis, and recruit other immune cells, in particular neutrophils, which in turn can secrete elastase, another pro-tumor factor [61]. Interestingly, IL-17, derived from Th17 cells, can synergize with IFN-γ to induce secretion of Th1 type chemokines, CXCL9 and CXCL10, by tumor cells, which in turn attract effector T cells to tumor sites [61]. IFN-γ+IL17+T cells have been reported in human tumors and in patients with autoimmune disease [61]. Whereas they are pathogenic in autoimmune disease, the synergistic effects of IL-17 and IFN-γ could be exploited for cancer therapy. Another recently characterized pathway for pro-tumor inflammation that can be a target for therapy is Th2 inflammation, discussed in greater detail hereunder.
Th2 INFLAMMATION IN PATHOGENESIS OF EPITHELIAL TUMORS
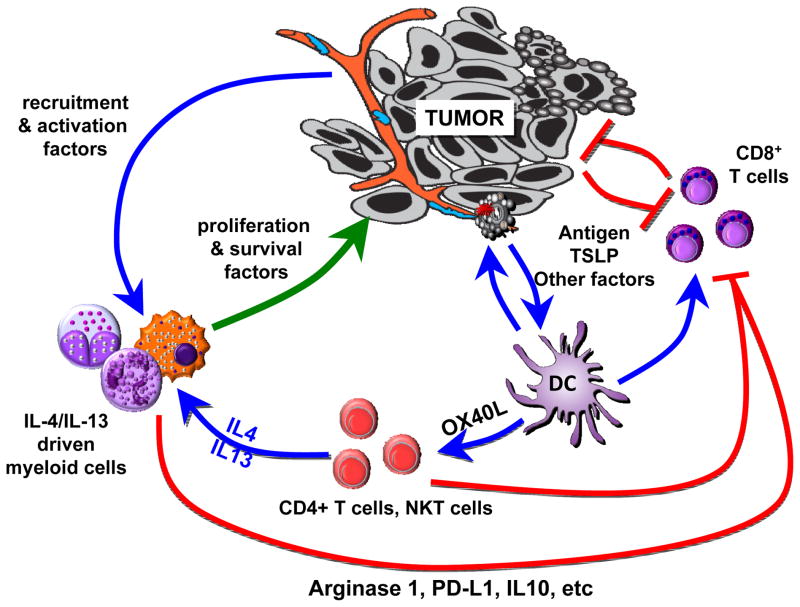
Breast and pancreatic cancers contain significant presence of inflammatory Th2 (iTh2) cells [62] (Figure 1). These iTh2 cells are differentiated from the classical Th2 cells by their co-expression of tumor necrosis factor (TNF)-α and lack of IL-10 secretion [63]. They are driven by OX40L (CD134)-expressing DCs in response to cancer-derived thymic stromal lymphopoietin (TSLP) [64]. These iTh2 cells accelerate breast tumor development in humanized mouse models through production of IL-13 [62]. Blocking of OX40L and/or TSLP in vivo results in inhibition of IL-13 secretion, and consequently leads to inhibition of breast cancer development [64]. In genetically-engineered mouse models of mammary cancer, Th2 cells accelerate development of pulmonary metastasis via IL-4R signaling [65]. IL-4 and IL-13 can contribute to tumorigenesis in several ways. For example, IL-13 produced by NKT cells induces myeloid cells to secrete transforming growth factor (TGF)-β;, which ultimately inhibits CTL function (reviewed in [66]). Spontaneous autochthonous breast carcinomas arising in Her-2/neu transgenic mice arise with shorter latency when mice are depleted of T cells, thus providing evidence for T cell–mediated immunosurveillance slowing tumor growth [66]. This immunosurveillance can be further enhanced by blockade of IL-13, which slows appearance of autologous tumors as compared to controls [66]. IL-4 and IL-13 can also generate type 2 macrophages [67] that promote tumor development via several mechanisms [68] including secretion of growth factors such as epidermal growth factor (EGF), enzymes involved in tissue remodeling such as cathepsins, as well as through direct inhibitory effects on CD8+ T cell function (reviewed in [69]).
Figure 1. Pathogenic type 2 cytokine loop in breast cancer.
DCs in breast cancer are exposed to cancer-derived factors—for example, TSLP—that skew their maturation toward expression of OX40L and capacity to activate CD4+ T cells to secrete IL-13 and IL-4, type 2 cytokines. In this environment, responding lymphocytes secreting IL-4 and IL-13 promote tumor development either directly or indirectly via myeloid cells including macrophages. Direct effects include triggering anti-apoptotic pathways and steroid metabolism in epithelial cancer cells, as well as promoting stromal fibroblast proliferation and differentiation. Indirect effects include triggering secretion of growth (EGF) and pro-angiogenic (VEGF) factors by tumor-infiltrating macrophages as well as PDL-1 expression and IL-10 secretion that blunt CD8+ T cell effector function. Cancers cells are likely to also directly activate innate lymphocytes secreting IL-13. The molecular and cellular factors contributing to the global IL-13 production in epithelial cancers likely extend beyond TSLP, and are topics of intense study. Another active area is the question of TSLP regulation, whether all breast cancer express it, and at which stages, as well as its role in metastatic niche formation.
Autocrine IL-13 is important in the pathophysiology of Hodgkin’s disease (reviewed in [70]). IL-13 and IL-13R are frequently expressed by Hodgkin’s and Reed-Sternberg cells, where IL-13 stimulates growth. Similar to Hodgkin’s cells, breast cancer cells express pSTAT6 [62], indicating that IL-13 may regulate cancer cell physiology. Phosphorylation of STAT6 can lead to up-regulation of anti-apoptotic pathways in cancer cells [71] leading to chemotherapy resistance, or to immune-mediated cytotoxicity driven by granzymes and resulting in tumor growth rather than rejection. Clinically, the Th2 signature in breast cancer [14, 72, 73] and the expression of the Th2 master regulator GATA-3 in pancreatic cancer [74] are associated with poor outcomes. Furthermore, the pathogenic TSLP/IL-13 pathway has also been detected in the context of Helicobacter pylori infection which leads to chronic gastritis, the causative factor in gastric cancer [75]. There, H. pylori triggers human gastric epithelial cells to produce TSLP [75]. DCs exposed to supernatants of H. pylori-infected epithelial cells trigger naïve CD4+ T cells to produce high levels of the Th2 cytokines IL-4 and IL-13, and of inflammatory cytokines TNF-α and IFN-γ [75]. Thus, disrupting this inflammatory, pro-tumor TSLP-OX40L-IL13/4 axis could be considered as a novel investigational therapeutic approach for several cancers. The molecular and cellular factors contributing to global IL-4/IL-13 production in epithelial cancers likely extend beyond TSLP, and are topics of intense study.
MODULATING DCs IN THE TUMOR ENVIRONMENT
DCs are found in most tumors in humans and mice. Tumors can prevent Ag presentation and establishment of tumor-specific immunity through a variety of mechanisms. Tumor-derived factors can alter DC maturation so as to yield cells that indirectly help tumor growth (“pro-tumor” inflammation) as discussed above. Furthermore, by converting immature DCs into macrophages, i.e., through IL-6 and M-CSF, breast cancers can prevent priming of tumor-specific T cells [76, 77]. Alternatively, the tumor glycoproteins carcinoembryonic antigen (CEA) and MUC-1 (mucin-1) that are endocytosed by DCs may stay confined in early endosomes, therefore preventing efficient processing and presentation to T cells [78].
pDCs that infiltrate breast carcinomas produce little type I interferon upon TLR ligation [79]. These pDCs induce naïve CD4+T cells to differentiate into IL-10-producing T cells having suppressive functions. Such inhibition of type I interferon secretion might also impact generation of effector T cells as DCs require type I interferon signals to cross-present tumor Ags [80, 81]. Whether this mechanism explains why pDC are associated with poor prognosis in early breast cancer [82] remains to be determined. Consistently however, pDC depletion delayed tumor growth in vivo, and intratumoral administration of TLR7L led to pDC activation, and displayed potent curative effects [83].
Recent studies point to an unexpected role for DCs in response to cancer therapy via so-called “immunogenic cancer cell death” [84]. Certain cytotoxic agents such as anthracyclines or oxaliplatin can induce immunogenic cancer cell death, characterized by secretion of HMGB1 (high mobility group protein B1) from dying cells that engages TLR4 on DCs [84]. This signal facilitates cancer Ag processing and presentation by DCs to T cells [84] that in turn plays an important role in boosting anti-cancer immunity via endogenous vaccination. Indeed, absence of HMGB1 expression by dying tumor cells compromises DC-dependent T cell priming by tumor-associated Ags [85]. Furthermore, early stage breast cancer patients who carry a TLR4 loss-of-function allele have a higher risk of recurrence following radiotherapy and chemotherapy than those who carry the wild type TLR4 allele [86]. Exploiting this unique molecular mechanism of Ag delivery and DC activation could be another way to harness DCs for breast cancer immunotherapy.
Conclusions
Interrogating the functions of DCs in tumor parenchyma is a fertile area for investigation. Ultimately, re-programming patients’ “pro-tumor” DCs into “anti-tumor” DCs may be part of effective cancer immunotherapy.
Acknowledgments
Thanks to all of our patients and healthy volunteers who agreed to participate in this research. Thanks to Dr. Jacques Banchereau for critical reading of the manuscript; to Drs Luz S. Muniz, and Joseph Fay, the former and current members of BIIR, the Clinical Core, the Apheresis Core, the Flow Cytometry Core, the Imaging Core and the Animal Facility team at BIIR for contributions. KP acknowledges support from the BIIR, Baylor University Medical Center Foundation, Baylor Sammons Cancer Center, Susan B. Komen Foundation, Cancer Prevention Research Institute of Texas, and NIH/NCI. LMC acknowledges support from the NIH/NCI, Susan B Komen Foundation, the Dept of Defense Breast Cancer Research Program, and the Breast Cancer Research Foundation.
References
- 1.Topalian SL, Weiner GJ, Pardoll DM. Cancer Immunotherapy Comes of Age. J Clin Oncol. 2011;29(36):4828–36. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lizee G, et al. Harnessing the power of the immune system to target cancer. Annu Rev Med. 2012;64:71–90. doi: 10.1146/annurev-med-112311-083918. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharpe AH, et al. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 7.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSantis C, et al. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61(6):409–18. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15(7):2483–93. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 10.Rastogi P, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 11.Liedtke C, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 12.Crown J, O’Shaughnessy J, Gullo G. Emerging targeted therapies in triple-negative breast cancer. Ann Oncol. 2012;23(Suppl 6):vi56–65. doi: 10.1093/annonc/mds196. [DOI] [PubMed] [Google Scholar]
- 13.Schneider BP, et al. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res. 2008;14(24):8010–8. doi: 10.1158/1078-0432.CCR-08-1208. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen VN, et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci U S A. 2012;109(8):2802–7. doi: 10.1073/pnas.1108781108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Albert ML, et al. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med. 1998;4(11):1321–4. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- 17.Darnell JC, Albert ML, Darnell RB. Cdr2, a target antigen of naturally occuring human tumor immunity, is widely expressed in gynecological tumors. Cancer Res. 2000;60(8):2136–9. [PubMed] [Google Scholar]
- 18.Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–15. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 19.Park KH, et al. Insulin-like growth factor-binding protein-2 is a target for the immunomodulation of breast cancer. Cancer Res. 2008;68(20):8400–9. doi: 10.1158/0008-5472.CAN-07-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107(4):477–84. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Disis ML, Schiffman K. Cancer vaccines targeting the HER2/neu oncogenic protein. Semin Oncol. 2001;28(6 Suppl 18):12–20. [PubMed] [Google Scholar]
- 22.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani A, et al. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371(9614):771–83. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 25.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–91. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9(1):57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 27.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Pierce BL, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27(21):3437–44. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 30.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 31.Steinman RM. Decisions About Dendritic Cells: Past, Present, and Future. Annu Rev Immunol. 2011;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 32.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 33.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 34.Caux C, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180(4):1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helft J, et al. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234(1):55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 36.Segura E, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;38(2):336–48. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Ueno H, et al. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234(1):199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284(5421):1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 39.Di Pucchio T, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9(5):551–7. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 41.Lauterbach H, et al. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med. 2010;207(12):2703–17. doi: 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crozat K, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207(6):1283–92. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jongbloed SL, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207(6):1247–60. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachem A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207(6):1273–81. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poulin LF, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207(6):1261–71. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mittag D, et al. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J Immunol. 2011;186(11):6207–17. doi: 10.4049/jimmunol.1002632. [DOI] [PubMed] [Google Scholar]
- 47.Haniffa M, et al. Human Tissues Contain CD141(hi) Cross-Presenting Dendritic Cells with Functional Homology to Mouse CD103(+) Nonlymphoid Dendritic Cells. Immunity. 2012;37(1):60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29(3):497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klechevsky E, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116(10):1685–97. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu CI, et al. Human CD1c(+) Dendritic Cells Drive the Differentiation of CD103(+) CD8(+) Mucosal Effector T Cells via the Cytokine TGF-beta. Immunity. 2013;38(4):818–30. doi: 10.1016/j.immuni.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Floc’h A, et al. Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med. 2007;204(3):559–70. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandoval F, et al. Mucosal Imprinting of Vaccine-Induced CD8+ T Cells Is Crucial to Inhibit the Growth of Mucosal Tumors. Sci Transl Med. 2013;5(172):172ra20. doi: 10.1126/scitranslmed.3004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mullins DW, et al. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198(7):1023–34. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheasley-O’neill SL, et al. Dendritic cell immunization route determines integrin expression and lymphoid and nonlymphoid tissue distribution of CD8 T cells. J Immunol. 2007;178:1512–1522. doi: 10.4049/jimmunol.178.3.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424(6944):88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 56.Joffre OP, et al. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12(8):557–69. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 57.Bluestone JA, et al. The functional plasticity of T cell subsets. Nat Rev Immunol. 2009;9(11):811–6. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanchot C, et al. Tumor-Infiltrating Regulatory T Cells: Phenotype, Role, Mechanism of Expansion In Situ and Clinical Significance. Cancer Microenviron. 2012;6(2):147–57. doi: 10.1007/s12307-012-0122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsushita H, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 482(7385):400–4. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8(5):337–48. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 61.Wei S, et al. Th17 cells have stem cell-like features and promote long-term immunity. Oncoimmunology. 1(4):516–519. doi: 10.4161/onci.19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aspord C, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204(5):1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu YJ, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 64.Pedroza-Gonzalez A, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208(3):479–90. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeNardo DG, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terabe M, Park JM, Berzofsky JA. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunol Immunother. 2004;53(2):79–85. doi: 10.1007/s00262-003-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geissmann F, et al. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10(6):453–60. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35(5):585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 69.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29(2):309–16. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99(12):4283–97. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
- 71.Zhang WJ, et al. IL-4-induced Stat6 activities affect apoptosis and gene expression in breast cancer cells. Cytokine. 2008;42(1):39–47. doi: 10.1016/j.cyto.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 72.Teschendorff AE, et al. Improved prognostic classification of breast cancer defined by antagonistic activation patterns of immune response pathway modules. BMC Cancer. 2010;10:604. doi: 10.1186/1471-2407-10-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Denardo DG, et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Monte L, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208(3):469–78. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kido M, et al. Helicobacter pylori promotes the production of thymic stromal lymphopoietin by gastric epithelial cells and induces dendritic cell-mediated inflammatory Th2 responses. Infect Immun. 2010;78(1):108–14. doi: 10.1128/IAI.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chomarat P, et al. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1(6):510–4. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 77.Chomarat P, et al. TNF skews monocyte differentiation from macrophages to dendritic cells. J Immunol. 2003;171(5):2262–9. doi: 10.4049/jimmunol.171.5.2262. [DOI] [PubMed] [Google Scholar]
- 78.Hiltbold EM, et al. The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells. J Immunol. 2000;165(7):3730–41. doi: 10.4049/jimmunol.165.7.3730. [DOI] [PubMed] [Google Scholar]
- 79.Cao W, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206(7):1603–14. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diamond MS, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuertes MB, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Treilleux I, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10(22):7466–74. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 83.Le Mercier I, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. 73(15):4629–40. doi: 10.1158/0008-5472.CAN-12-3058. [DOI] [PubMed] [Google Scholar]
- 84.Kroemer G, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 85.Yamazaki T, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2013 doi: 10.1038/cdd.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]