Abstract
Objective
To determine whether treatment with the CXC chemokine receptor (CXCR) 4 agonist ubiquitin results in beneficial effects in a polytrauma model consisting of bilateral femur fractures plus blunt chest trauma (Injury Severity Score 18-25).
Design
Treatment study.
Setting
Research Laboratory.
Subjects
Seventeen Yorkshire pigs.
Interventions
Intravenous (i.v.) injection of 1.5 mg/kg ubiquitin or albumin (=control) at 60 min after polytrauma.
Measurements and Main Results
Anesthetized, mechanically ventilated pigs underwent polytrauma, followed by a simulated 60 min shock phase. At the end of the shock phase ubiquitin or albumin were administered and animals were resuscitated to a mean arterial blood pressure of 70 mmHg until t = 420 min. After i.v. ubiquitin, ubiquitin plasma concentrations increased sixteen-fold to 2870 ± 1015 ng/mL at t = 90 min and decreased with t1/2 = 60 min. Endogenous plasma ubiquitin increased two-fold in the albumin group with peak levels of 359 ± 210 ng/mL. Plasma levels of the cognate CXCR4 ligand stromal cell-derived factor (SDF)-1α were unchanged in both groups. Ubiquitin treatment reduced arterial lactate levels and prevented a continuous decrease in arterial oxygenation, which occurred in the albumin group during resuscitation. Wet weight to dry weight ratios of the lung contralateral from the injury, heart, spleen and jejunum were lower with ubiquitin. With ubiquitin treatment, tissue levels of IL-8, IL-10, TNFα and SDF-1α were reduced in the injured lung and of IL-8 in the contralateral lung, respectively.
Conclusions
Administration of exogenous ubiquitin modulates the local inflammatory response, improves resuscitation, reduces fluid shifts into tissues and preserves arterial oxygenation after blunt polytrauma with lung injury. This study further supports the notion that ubiquitin is a promising protein therapeutic and implies CXCR4 as a drug target after polytrauma.
Keywords: Exogenous ubiquitin, CXC chemokine receptor 4, stromal cell-derived factor-1α, resuscitation, blunt chest trauma, femur fractures, organ protection
Introduction
Ubiquitin, an intracellular post-translational protein modifier, is released into the systemic circulation during various disease processes, including trauma and sepsis (1, 2). We have previously shown that extracellular ubiquitin functions as an anti-inflammatory immune modulator in trauma patients and identified ubiquitin as a natural CXC chemokine receptor (CXCR) 4 agonist (3-6). Furthermore, we observed that low systemic ubiquitin concentrations are associated with a higher degree of organ dysfunction and increased morbidity and mortality in burn patients, which points towards a beneficial role of endogenous extracellular ubiquitin after injury (7). Accordingly, we have tested the therapeutic potential of exogenous ubiquitin and detected that ubiquitin treatment attenuated third spacing of fluids and reduced organ injury in various species and disease models (8-13). Importantly, the immune modulatory actions and therapeutic potential of ubiquitin have been confirmed independently (14-18). As these findings suggest ubiquitin as a promising protein therapeutic, rigorous preclinical testing of its therapeutic efficacy in conditions that closely resemble the clinical scenario is required to further define its therapeutic potential and understand its possible side effect profile.
Although we have previously demonstrated therapeutic potential of ubiquitin in trauma models, these models either consisted of a combination of isolated injuries and arterial hemorrhage to simulate the physiology of a severely injured patient, or resulted in a mild to moderate injury (9, 11, 12). However, the effects of ubiquitin treatment after severe blunt injuries without superimposed hemorrhage are unknown.
The majority of severely injured patients who survive the pre-hospital phase present with blunt polytrauma, with the combination of chest and extremity trauma being the most common injury pattern (19, 20). Thus, the assessment of the therapeutic efficacy of ubiquitin in a clinically relevant polytrauma model is essential to further advocate its development as a therapeutic agent for trauma patients. Based on the effects of ubiquitin in previous models, we hypothesized that the same dose of ubiquitin will also preserve lung function and reduce edema formation in a large animal model designed to mimic the typical polytrauma patient (8, 11, 13). Therefore, we evaluated the effects of exogenous ubiquitin in a polytrauma model, consisting of bilateral femur fractures plus blunt chest trauma. When translated to humans, this model corresponds to an injury severity score of 18-25 (abbreviated injury scale: Extremities: 3, Chest: 3-4) (21).
Materials and Methods
General Animal Protocol
All procedures were performed according to National Institutes of Health Guidelines for Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. Seventeen male and female Yorkshire pigs (30-40 kg body weight, Michael Fanning Farms, IN) were fasted overnight. Anesthesia was induced with 10 mg/kg ketamine, 1 mg/kg xylazine intramuscularly and maintained by continuous intravenous (i.v.) infusion of 10 mg/kg/h ketamine, 0.25 mg/kg/h xylazine and 50 μg/kg/h fentanyl. After orotracheal intubation animals were mechanically ventilated (Evita XL, Draeger Medical) with intermittent mandatory ventilation adjusted to tidal volumes of 12 mL/kg at 12-18 breaths/min to maintain a partial pressure of CO2 (pCO2) between 35-45 mmHg. A fraction of inspired oxygen (FiO2) of 0.4 with positive end expiratory pressure (PEEP) of 5 mmHg was used, except where otherwise noted. Core body temperature was maintained using warming blankets (Gaymar T/Pump 500 T/Pad). A central venous catheter (AGB+ Arrow Catheter, Teleflex Medical) was placed in the external jugular vein for administration of fluids, anesthesia and continuous monitoring of central venous pressure (CVP). The ipsilateral common carotid artery was cannulated for measurements of mean arterial blood pressure (MAP). Electrocardiography, pulse oximetry, capnography (Evita XL Capnography module, Draeger Medical) and body temperature were monitored continuously. Arterial blood was sampled at baseline, every 15 min for the first hour after injury and every 30 min thereafter. Samples were analyzed for pH, pCO2, pO2, hemoglobin, sodium, potassium, chloride, glucose and lactate using a blood gas analyzer (Stat Profile pHOx PlusL, Nova Biomedical). Venous blood was obtained at baseline (two samples per animal in 15 min intervals) and every 15 min for the first 60 min after injury, followed by increasing intervals (30-120 min) for the remaining 360 min. Venous blood was collected in lithium heparin tubes (APP Pharmaceuticals). The plasma was separated and stored at -80°C until analyses.
Polytrauma
Polytrauma consisted of bilateral open femur fractures and blunt chest trauma, as described (9, 22, 23). Injuries were produced with a captive bolt gun (Karl Schermer), modified with exchangeable mushroom shaped metal heads (1 and 2.5 inches in diameter). In brief, the bolt gun with the small metal head was placed vertically against the femur and fired while the animal was in supine position and the leg extended. The metal head perforated the skin and produced a 2nd degree complex open femur fracture without injury of major vessels. After both femurs were fractured, the small metal head was exchanged for the large metal head and the bolt gun was fired against the right chest wall in the midaxillary line at the level of the fourth intercostal space with a 45° cephalad trajectory. As confirmed by necropsy, this resulted in lung contusion covering approximately 20-30% of the right lung without producing hemo/pneumothoraces or displaced rib fractures. All injuries were produced within 5 min.
Experimental Groups and Treatment Protocol
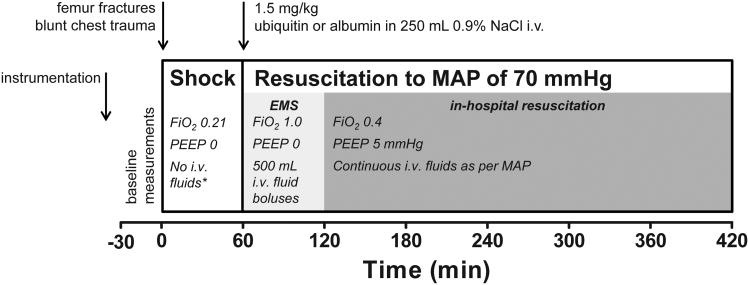
A flowchart of the treatment protocol is shown in Fig. 1. After achieving stable baseline conditions (at least 30 min after instrumentation) polytrauma was produced as described before. To simulate a shock period of 60 min after polytrauma, animals were ventilated with FiO2 of 0.21, PEEP 0 mmHg and no resuscitation was allowed other than the minimum amount of lactated Ringer's solution required for delivery of anesthesia. Immediately following the shock period (t=60 min) animals were randomized to receive 1.5 mg/kg ubiquitin (n=8, Sigma, from bovine erythrocytes) or bovine serum albumin (n=9; control, Sigma) in 250 mL of 0.9% NaCl i.v. with the investigator responsible for the animal care blinded to the randomization assignment. This dose was chosen because it resulted in therapeutically relevant effects in previous animal models (8, 9, 11-13). Bovine serum albumin was used as a protein control from the same species (8, 9, 11, 13).
Fig. 1. Flowchart of the experimental protocol.
MAP: mean arterial blood pressure. i.v.: intravenous. Shock: simulated shock period. EMS: simulated pre-hospital emergency medical services. *: except minimum amount of lactated Ringer's solution required for delivery of anesthesia.
At t=60 min until t=120 min, ventilation was adjusted to FiO2 of 1.0, PEEP 0 mmHg and resuscitation to a MAP of 70 mmHg was performed with warmed lactated Ringer's solution. Within this period, lactated Ringer's solution was administered i.v. in bolus increments of 500mL until the MAP reached 70 mmHg. This was performed to simulate typical human resuscitation regimens during the pre-hospital phase. At t=120 min until the end of the experiment, animals were ventilated with FiO2 0.4, PEEP 5 mmHg and fluid administration was performed continuously as required to maintain the target MAP to simulate in-hospital resuscitation. At the conclusion of the experiment (t=420 min) a saturated KCl solution was infused via the central venous catheter for euthanasia while the animal was under general anesthesia. Immediate necropsy was performed with tissue biopsies of the following structures: left ventricle, directly injured lung from the right (ipsilateral) hemithorax, uninjured lung from the left (contralateral) hemithorax, liver, spleen, kidney, jejunum and gluteal muscle. Tissues were snap frozen and stored in liquid nitrogen until further processing.
Blood Chemistry
Plasma samples were assayed for alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), calcium, creatinine, gamma-glutamyl transpeptidase (GGT), glucose, total protein, total bilirubin, albumin, phosphorus, globulin, albumin/globulin ratio, BUN/creatinine ratio using a veterinary blood chemistry analyzer (DRI-CHEM 7000, Heska).
Complete Blood Counts (CBC)
Blood samples collected with ethylenediamine tetra-acetic acid (EDTA) were used for the analyses of CBC on a hematology analyzer (HemaTrue, Heska).
Tissue Wet Weight to Dry Weight Ratios
The ratio of the tissue wet weight to dry weight (W/D) was determined gravimetrically, as described (13, 24).
Tissue Extract Preparation
Snap frozen tissues were homogenized in 1/10 phosphate buffered saline, pH 7.4 (1:5 weight/volume), centrifuged (16,600g, 4°C, 30 min) and supernatants (= extracts) aliquoted, as described (24, 25). All measurements in tissue extracts were standardized to total protein content (26) and are reported per mg of protein.
Enzyme Linked Immunosorbent Assays (ELISA)
Ubiquitin concentrations were measured with an indirect competitive ELISA (lower detection limit (LDL): 11 ng/mL), as described (7, 9). SDF-1α concentrations were measured using a SDF-1α DuoSet ELISA Development kit (R&D Systems), as recommended by the manufacturer (LDL: 9 pg/mL). Interleukin (IL)-6 (R&D Systems; LDL: 10 pg/mL), IL-8 (R&D Systems; LDL: 4.6 pg/mL), IL-10 (Alpco Diagnostics; LDL: 3 pg/mL) and Tumor Necrosis Factor (TNF)α (R&D Systems; LDL: 3.7 pg/mL) were measured with commercially available ELISA kits according to the manufacturers' protocols. Measurements were performed with specimens that had not been thawed previously after all animal experiments were completed.
Western blots
Western blotting was performed as described (3, 6). Anti-phospho-extracellular signal-regulated kinase (ERK)1 (T202/Y204)/ERK2 (T185/Y187) rabbit-IgG, anti-phospho-Akt pan (S437) rabbit IgG and anti-ERK1/2 rabbit IgG (all from R&D Systems) were used in combination with anti-rabbit horseradish peroxidase linked whole antibody (GE Amersham).
Lipid peroxidation assay
Malondialdehyde (MDA) in combination with 4-hydroxyalkenals (4-HAE) were measured as an indicator of lipid peroxidation in the lung extracts using a commercially available assay (No. FR 22, Oxford Biomedical Research, Oxford, MI), as described (13).
Data Analyses and Statistics
Data are presented as mean ± standard deviation (SD) or median with interquartile range (25th/75th percentile), as appropriate. The plasma elimination half-life of ubiquitin (t1/2) was calculated as described (9). Normal distribution was assessed with the Kolmogorov-Smirnov test. Normally distributed data were analyzed with Student's t-test or two-way repeated measures (mixed model) analysis of variance and Bonferroni post-hoc test to correct for multiple testing. Data that did not pass the normality test (alpha=0.05) were analyzed with the Mann-Whitney U test or Wilcoxon matched-pairs signed rank test, as appropriate. Data analyses were calculated with the GraphPad Prism program (GraphPad Software). A two-tailed p<0.05 was considered significant.
Results
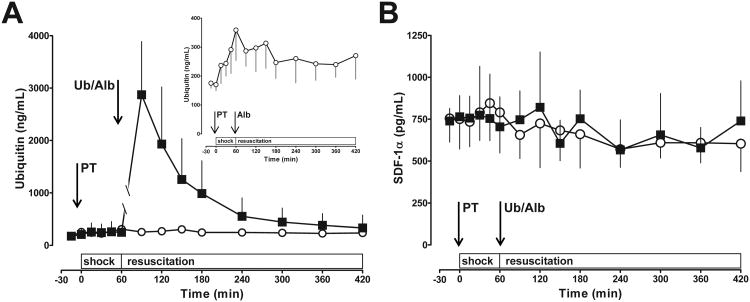
After i.v. administration of ubiquitin, ubiquitin plasma concentrations increased sixteen-fold to 2873±1015 ng/mL at t=90 min and decreased with t1/2 of 60 min (Fig. 2A). Endogenous plasma ubiquitin increased two-fold in the albumin group with peak levels of 359±210 ng/mL (Fig. 2A, insert). SDF-1α plasma concentrations were not affected by polytrauma or ubiquitin treatment (Fig. 2B)
Fig. 2. Ubiquitin (A) and SDF-1α (B) plasma concentrations after polytrauma.
Data are mean ± SD. Arrows indicate the time points of polytrauma (PT) and drug administration (Ub – ubiquitin; Alb – albumin). Black squares: Ubiquitin treatment, n = 8. Open circles: Albumin treatment (= control), n = 9. Shock: shock period (t = 0 – 60 min). Resuscitation: Resuscitation period (t = 60 – 420 min). Insert in A: Endogenous ubiquitin plasma levels in the control group.
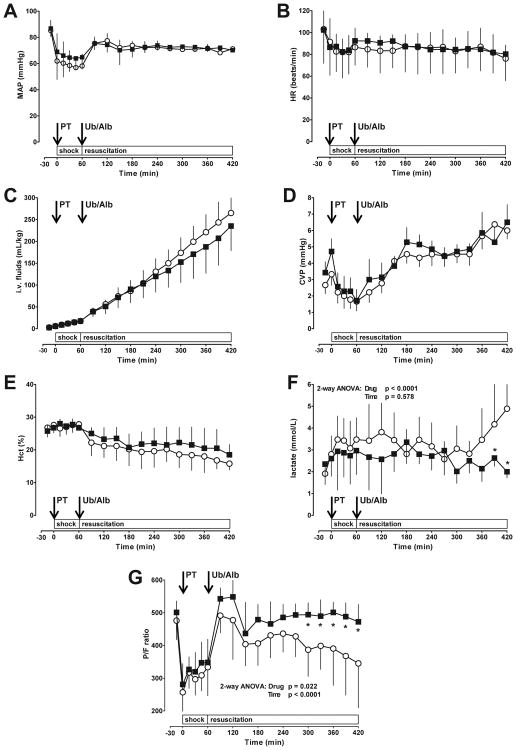
Physiological parameters at baseline and during the shock period did not differ between animals of the ubiquitin and albumin groups (Fig. 3). During the shock period MAP decreased by 25±9 mmHg and the heart rate by 20±17 beats/min (Fig. 3A/B). Animals receiving albumin after polytrauma required 265±17 mL/kg of i.v. fluids to maintain a MAP of 70 mmHg (Fig. 3C). The fluid requirements to maintain the target MAP decreased slightly to 235±20 mL/kg among animals treated with ubiquitin (2-way ANOVA: Time: p<0.001; Drug: p=0.0073; post-tests: p>0.05 vs. albumin for all time points). There were no differences in central venous pressures and hematocrit values between the groups (Fig. 3D/E).
Fig. 3. Physiological responses to polytrauma.
Data are mean ± SD. Arrows indicate the time points of polytrauma (PT) and drug administration (Ub – ubiquitin; Alb – albumin). Black squares: Ubiquitin treatment, n = 8. Open circles: Albumin treatment (= control), n = 9. A. Mean arterial blood pressure (MAP, mmHg). B. Heat rate (beats/min). C. I.v. fluid requirements to maintain MAP of 70 mmHg (mL/kg). D. Central venous pressure (CVP, mmHg). E. Hematocrit (%). F. Arterial lactate concentrations (mmol/L). G. Ratio of arterial oxygen concentration to the fraction of inspired oxygen (P/F). *: p<0.05 vs. control (2-way ANOVA/Bonferroni post-hoc).
In the albumin group arterial blood lactate levels showed a biphasic response, with an initial peak of 3.8±1.3 mmol/L at t=120 min and an upward projecting slope with lactate concentrations of 4.8±2.3 mmol/L at the conclusion of the observation period (Fig. 3F). Blood lactate concentrations in the ubiquitin group reached peak levels of 3.3±0.9 mmol/L at t=180 min and returned to baseline at the end of the experiment (Fig. 3F, p<0.05 vs. albumin at t=390-420 min).
In both treatment groups, the ratio of arterial oxygen concentration to the fraction of inspired oxygen (P/F) decreased during the shock period below 300 and recovered when ventilated with FiO2 1.0 (t=60-120 min) during the simulated pre-hospital phase (Fig. 3G). Subsequently, P/F ratios declined continuously during the final 150 min of the simulated in-hospital resuscitation phase with albumin treatment. In contrast, with ubiquitin treatment, P/F ratios remained constant until conclusion of the experiment (p<0.05 vs. albumin group at t=300-420 min).
There were no differences in leukocyte counts or any of the other routine blood chemistry parameters between groups at baseline or at the conclusion of the experiment (Tab. 1). Furthermore, plasma TNFα and IL-10 levels were similar in both groups throughout the experiment (data not shown).
Table 1. Blood chemistry and leukocyte counts.
| Baseline | t = 420 min | |||
|---|---|---|---|---|
| Alb | Ub | Alb | Ub | |
| ALP (U/L) | 140±13 | 147±20 | 115±18 | 127±19 |
| ALT (U/L) | 35.8±11 | 38.0±4.8 | 25.2±5.2 | 28.3±6.1 |
| GGT (U/L) | 32.4±5.4 | 35.0±4.4 | 26.2±2.6 | 27.0±3.2 |
| Bilirubin (mg/dL) | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 |
| Glucose (mg/dL) | 109±20 | 91.2±33.4 | 78.8±11.5 | 78.5±14.9 |
| Protein (g/dL) | 5.1±0.9 | 5.0±0.5 | 3.4±0.9 | 3.3±0.2 |
| Albumin (g/dL) | 3.0±0.4 | 3.2±0.4 | 2.0±0.7 | 2.1±0.2 |
| Globulin (mg/dL) | 2.0±0.6 | 1.8±0.2 | 1.3±0.2 | 1.3±0.2 |
| BUN mg/dL | 6.7±1.5 | 8.1±2.5 | 8.8±2.0 | 11.2±4.6 |
| Creatinine (mg/dL) | 0.8±0.1 | 1.0±0.1 | 0.7±0.1 | 0.9±0.3 |
| Ca2+ (mg/dL) | 9.3±0.3 | 9.2±0.5 | 8.7±2.0 | 11.2±4.6 |
| Phosphorus (mg/dL) | 9.2±0.4 | 9.1±0.6 | 9.5±0.5 | 9.8±0.8 |
| Leukocytes (×103/μL) | 16±4 | 15±6 | 15±4 | 13±4 |
Data are mean ± SD. Alb: Albumin group (n = 9). Ub: Ubiquitin group (n = 8). There were no statistically significant differences between the groups at baseline and at the end of the experiment (t=420 min).
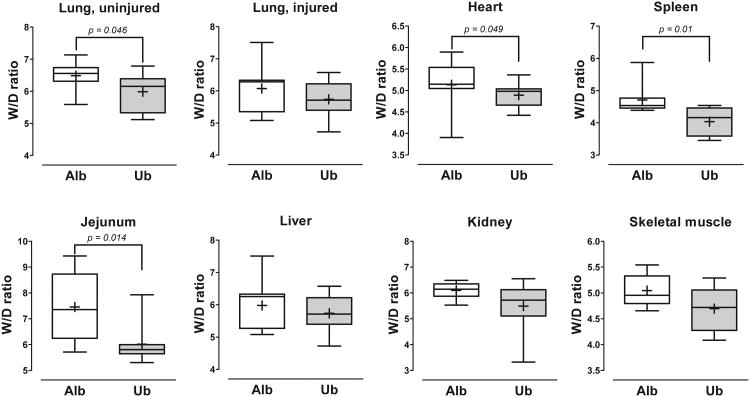
At the end of the experiment, W/D ratios of the uninjured contralateral lung, heart, spleen and jejunum were significantly lower in animals treated with ubiquitin, as compared with albumin treatment (Fig. 4). Although W/D ratios of the directly injured lung, liver, kidney and skeletal muscle were also lower with ubiquitin treatment, these differences did not reach statistical significance for the individual organs/tissues. Nevertheless, when the relative changes of W/D ratios of the sum of all organs/tissues (albumin group=1) were calculated as a parameter of the global organ/tissue effects of the treatment, the median W/D ratio was 0.922 (0.81/0.98) (25th/75th percentile) with ubiquitin treatment (p<0.0001 vs. albumin).
Fig. 4. Wet weight to dry weight (W/D) ratios of individual organs.
Boxes extend from the 25th to 75th percentile; the horizontal line shows the median; the cross indicates the mean. Error bars show the minimum and maximum. Alb: Albumin group (open boxes; n = 9). Ub: Ubiquitin group (grey boxes; n = 8). Significant differences between the groups are indicated.
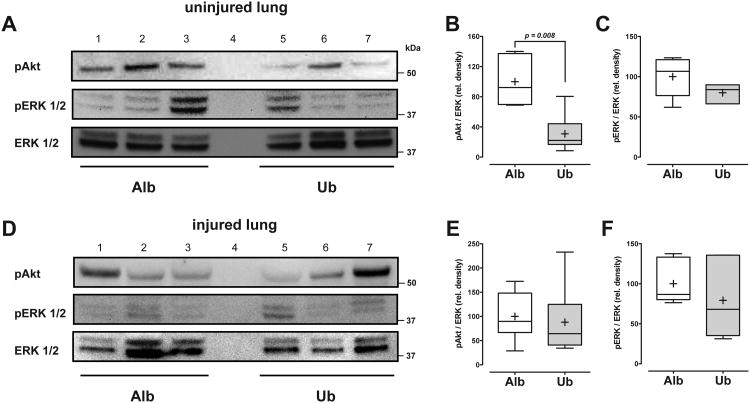
Because ubiquitin treatment improved arterial oxygenation and reduced W/D ratios of the contralateral uninjured lung, we then sought to evaluate whether exogenous ubiquitin also affects cellular signaling events in the lung, which have been shown previously to be modulated by CXCR4 activation in cell systems (6). As shown in Fig. 5A/B, we detected in Western blotting experiments with extracts from uninjured contralateral lungs that phosphorylation of ERK1/2 was slightly (p=0.09) and of Akt significantly reduced with ubiquitin treatment (p=0.008), as compared with albumin treatment. Differences in the phosphorylation status of ERK1/2 and Akt between the groups were not detectable in Western blotting experiments with extracts from directly injured lungs (Fig. 5C/D).
Fig. 5. Phosphorylation of ERK1/2 and Akt in lung extracts.
A. Extracts (100 μg/lane) from uninjured lungs were analyzed by Western blotting with anti-phospho (p) Akt, anti-pERK1/2 and anti-ERK1/2 (protein loading control). Each lane contains a lung extract from an animal after albumin (Alb; lanes 1-3) or ubiquitin (Ub, lanes 5-7) treatment (lane 4: empty). The migration positions of standard proteins are shown on the right. B. and C. Desitometric quantification of the chemiluminescence signals of the ratio between pAkt to ERK1/2 (B) and pERK1/2 to ERK1/2 (C) in extracts from uninjured lungs, as in A. N = 6 animals per group (Alb: Albumin treatment – open boxes = 100%; Ub: Ubiquitin treatment – grey boxes). Boxes extend from the 25th to 75th percentile; the horizontal line shows the median; the cross indicates the mean. Error bars show the minimum and maximum. Significant differences between the groups are indicated. D. Extracts (100 μg/lane) from injured lungs were analyzed by Western blotting with anti-phospho (p) Akt, anti-pERK1/2 and anti-ERK1/2 (protein loading control). Each lane contains a lung extract from an animal after albumin (Alb; lanes 1-3) or ubiquitin (Ub, lanes 5-7) treatment (lane 4: empty). The migration positions of standard proteins are shown on the right. The higher variation of the ERK signal at equal total protein loading among the specimens, as compared to A., can be explained by variations of the proportion of blood that is present in injured lungs. B. and C. Desitometric quantification of the chemiluminescence signals of the ratio between pAkt to ERK1/2 (B) and pERK1/2 to ERK1/2 in extracts from injured lungs, as in D. N = 6 animals per group (Alb: Albumin treatment – open boxes = 100%; Ub: Ubiquitin treatment – grey boxes). Boxes extend from the 25th to 75th percentile; the horizontal line shows the median; the cross indicates the mean. Error bars show the minimum and maximum.
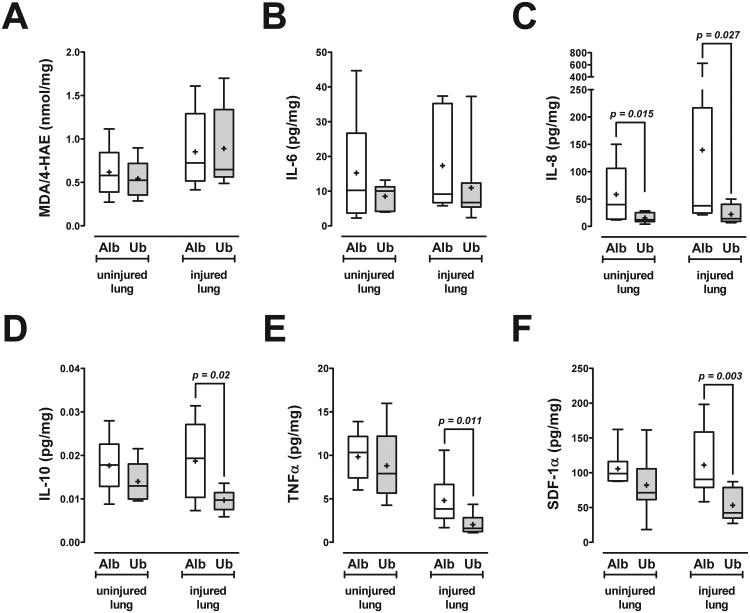
Furthermore, we measured tissue levels of MDA/4-HAE and a selected panel of pro- and anti-inflammatory cytokines/chemokines as surrogate markers of tissue injury and inflammation in lung extracts (Fig. 6). In the albumin group, levels of MDA/4-HAE, IL-6, IL-8, IL-10 and SDF-1α were similar in extracts from lungs ipsi- and contralateral to the injury (p>0.05 for all). With albumin treatment, TNFα levels were significantly lower in the directly injured lung (3.9 (2.8/6.7) pg/mg (median with 25th/75th percentile)), as compared to the uninjured, contralateral lung (10.3 (7.4/12.2) pg/mg (median with 25th/75th percentile), p=0.0028; Fig. 6E). While ubiquitin treatment did not affect levels of MDA/4-HAE in extracts from directly injured and contralateral lungs, it reduced IL-8 levels in extracts from the contralateral lung and IL-8, IL-10, TNFα and SDF-1α levels in extracts from directly injured lungs, as compared with albumin treatment.
Fig. 6. Surrogate markers of tissue injury and inflammation in lung extracts.
Data are expressed per mg of total protein. Boxes extend from the 25th to 75th percentile; the horizontal line shows the median; the cross indicates the mean. Error bars show the minimum and maximum. Significant differences between the groups are indicated. Alb: Albumin treatment (open boxes; n = 9). Ub: Ubiquitin treatment (grey boxes; n = 8). A. Malondialdehyde (MDA)/4-hydroxyalkenals (4-HAE); nmol/mg). B. IL-6; pg/mg. C. IL-8; pg/mg. D. IL-10; pg/mg. E. TNFα; pg/mg. F. SDF-1α; pg/mg.
Discussion
In the present study, we provide an initial assessment of the therapeutic efficacy of ubiquitin in a model of blunt polytrauma. The injuries in our model were chosen to mimic the most common injury pattern in patients (19, 20). In the absence of hemorrhagic shock, animals showed a 30% reduction of MAP during the shock phase and an increase of arterial lactate concentrations. The transient decrease in heart rate despite the presence of relative hypotension during the shock phase likely reflects concomitant cardiac contusion after blunt chest trauma (27). Furthermore, animals were fluid dependent to maintain hemodynamics and arterial oxygenation decreased continuously during resuscitation in the control group. The persistent fluid requirements and increases in arterial lactate levels suggest that animals were not stabilized within 6 hours of resuscitation. This documents that the blunt injuries were able to induce a relevant systemic response. Although fluid requirements of the injured pigs were higher than the expected resuscitation volumes in humans, the injuries in our model created a condition that closely resembled typical clinical characteristics of a polytrauma patient.
While the majority of endogenous plasma ubiquitin probably originates from its passive release from cells undergoing physiological turnover under normal conditions and from damaged cells and tissues after trauma, the cognate CXCR4 ligand SDF-1α is a constitutively and abundantly expressed chemokine (2, 28, 29). The plasma levels of endogenous ubiquitin and of SDF-1α that we detected at baseline, and of ubiquitin after polytrauma, are consistent with previous findings in animals and humans (3, 7, 9, 30-32). The determined plasma concentrations of ubiquitin after i.v. administration and its systemic half-life of 60 min are in agreement with the pharmacokinetic properties that we described previously (8, 9, 11).
Our findings from the present study suggest that ubiquitin treatment improves metabolic homeostasis, reduces third spacing of fluids into tissues and preserves arterial oxygenation during resuscitation following blunt extremity and chest trauma. Ubiquitin treatment has been shown to improve resuscitation and reduce organ injury in models of extremity trauma plus hemorrhage (9), fluid percussion brain injury plus hemorrhage (11), isolated brain injuries (12), lung ischemia reperfusion injury (13), brain ischemia reperfusion injury (14) and now in a polytrauma model with blunt chest trauma. In the present study ubiquitin treatment did not result in any adverse effects or acute toxicity, as assessed by its effects in routine blood chemistry parameters. This finding is in agreement with previous animal studies, in which ubiquitin treatment for up to two weeks was well tolerated and did not result in noticeable side effects (10). Collectively, these data advocate development of ubiquitin as a protein therapeutic and justify further studies that are required for a transition into the clinical arena, such as assessment of its long term effects on outcomes and dose-response experiments with escalating dosing regimens to assess the toxicity and pharmacological side effect profile.
In contrast to the therapeutic efficacy of exogenous ubiquitin, which has been documented in several disease models and species, the molecular mechanisms through which exogenous ubiquitin mediates its physiological effects remain poorly defined. In line with previous studies on tissue cytokine expression, our findings from the present study on cytokine/chemokine levels in lung extracts also suggest that ubiquitin treatment modulates the local inflammatory response (13, 16). Interestingly, these effects were more pronounced in the lung ipsilateral of the mechanical impact. In context of the assumption that extracellular ubiquitin functions as an endogenous opponent of damage-associated molecular pattern molecules (2), it might be speculated that these findings reflect attenuation of the inflammatory response that is induced by the local release of alarmins from the mechanically damaged lung tissue.
Recently, we identified extracellular ubiquitin as a natural agonist of CXCR4 (4-6, 33). Although CXCR4 is known to play pleiotropic roles in the immune system (34), its regulation and function during the inflammatory response to trauma is unknown. The finding that tissue levels of the cognate CXCR4 ligand SDF-1α were significantly reduced in extracts from lungs ipsilateral of the injury after ubiquitin treatment suggests that activation of CXCR4 with ubiquitin results in a negative feedback loop that controls the expression of SDF-1α.
The affinity of SDF-1α for CXCR4 (Kd 1.5 - 54 nM (35-40)) is higher than the affinity of ubiquitin (Kd ∼ 100 nM (4, 6)). Nevertheless, SDF-1α plasma concentrations were not affected by polytrauma and ubiquitin concentrations exceeded those of SDF-1α by more than 500-fold post injury. In addition, previous studies showed that plasma SDF-1α is rapidly inactivated (41, 42). This suggests that extracellular ubiquitin functions as the biologically relevant CXCR4 agonist in the systemic circulation after polytrauma. Furthermore, the effects of exogenous ubiquitin on lung W/D ratios and arterial oxygenation that have been described in the present and previous studies correspond well with the high expression of CXCR4 in the lung (8, 11, 13, 29).
We have shown previously that activation of CXCR4 with ubiquitin and with SDF-1α lead to increased phosphorylation of ERK1/2 and Akt in the human monocytic cell line THP-1 (6). Increased phosphorylation of ERK1/2 and Akt occurred within 5-10 min and returned to baseline levels within 30 min (6). However, we were unable to detect increased phosphorylation of ERK1/2 and Akt in lung extracts at 6h after ubiquitin treatment. A possible explanation is that we have missed the time period of transient activation of these signaling pathways. The reduced phosphorylation of Akt that we detected in lungs contralateral from the injury after ubiquitin treatment could then be interpreted as a compensatory down-regulation of Akt phosphorylation following initial activation. Alternatively, CXCR4 mediated effects on Akt and ERK could be differentially regulated among different cell types and depend on the local inflammatory environment. In order to address these questions, detailed time course analyses of the ERK and Akt phosphorylation status in various organs and tissues would be required. However, such experiments are not feasible in a large animal polytrauma model and beyond the scope of our study.
In conclusion, the present study provides evidence that ubiquitin treatment during the early resuscitation period is advantageous and attenuates physiological consequences that occur during the inflammatory response to blunt polytrauma in a large animal model. In combination with the beneficial effects of exogenous ubiquitin in previous animal models, these data provide a strong rationale for future studies on the pharmacological properties, therapeutic window and long term effects of ubiquitin.
SDF-1α, a protease resistant bioengineered form of SDF-1α, a SDF-1α-IgG fusion protein and a SDF-1α mimetic peptide have also been shown to result in beneficial effects in models of acute infectious and sterile inflammation in vivo (43-46). Thus, the currently available data on the therapeutic properties of the CXCR4 agonists ubiquitin and SDF-1α point towards CXCR4 as a new drug target for the treatment of trauma patients.
Acknowledgments
The authors thank Casey E. Hegger for technical help.
This research was made possible by grants that were awarded and administered by the U.S. Army Medical Research & Materiel Command (USAMRMC) and the Telemedicine & Advanced Technology Research Center (TATRC), at Fort Detrick, MD, under Contract Numbers W81XWH-05-1-0585 and W81XWH1020122. The views, opinions and/or findings contained in this research are those of the author(s) and do not necessarily reflect the views of the Department of Defense and should not be construed as an official DoD/Army position, policy or decision unless so designated by other documentation. No official endorsement should be made.
This work was also supported, in part, by the National Institute of Health Grant T32 GM008750 and the Dr. Ralph and Marian Falk Medical Research Trust.
The therapeutic use of ubiquitin has been patented (US patent #7,262,162) and M.M. is an inventor. M.M. has not received any income related to the patent or patent application.
Footnotes
The authors have not disclosed any potential conflicts of interest
This research has been presented, in part, at the 34th Annual Conference on Shock, Norfolk, Virginia, June 11-14, 2011.
References
- 1.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 2.Majetschak M. Extracellular ubiquitin: immune modulator and endogenous opponent of damage-associated molecular pattern molecules. Journal of leukocyte biology. 2011;89(2):205–219. doi: 10.1189/jlb.0510316. [DOI] [PubMed] [Google Scholar]
- 3.Majetschak M, Krehmeier U, Bardenheuer M, et al. Extracellular ubiquitin inhibits the TNF-alpha response to endotoxin in peripheral blood mononuclear cells and regulates endotoxin hyporesponsiveness in critical illness. Blood. 2003;101(5):1882–1890. doi: 10.1182/blood-2002-03-0918. [DOI] [PubMed] [Google Scholar]
- 4.Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. The Journal of biological chemistry. 2010;285(20):15566–15576. doi: 10.1074/jbc.M110.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saini V, Marchese A, Tang WJ, et al. Structural Determinants of Ubiquitin-CXC Chemokine Receptor 4 Interaction. The Journal of biological chemistry. 2011;286(51):44145–44152. doi: 10.1074/jbc.M111.298505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saini V, Staren DM, Ziarek JJ, et al. The CXC Chemokine Receptor 4 Ligands Ubiquitin and Stromal Cell-derived Factor-1{alpha} Function through Distinct Receptor Interactions. The Journal of biological chemistry. 2011;286(38):33466–33477. doi: 10.1074/jbc.M111.233742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majetschak M, Zedler S, Hostmann A, et al. Systemic ubiquitin release after blunt trauma and burns: association with injury severity, posttraumatic complications, and survival. J Trauma. 2008;64(3):586–596. doi: 10.1097/TA.0b013e3181641bc5. discussion 596-588. [DOI] [PubMed] [Google Scholar]
- 8.Majetschak M, Cohn SM, Nelson JA, et al. Effects of exogenous ubiquitin in lethal endotoxemia. Surgery. 2004;135(5):536–543. doi: 10.1016/j.surg.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Majetschak M, Cohn SM, Obertacke U, et al. Therapeutic potential of exogenous ubiquitin during resuscitation from severe trauma. J Trauma. 2004;56(5):991–999. doi: 10.1097/01.ta.0000127770.29009.5a. discussion 999-1000. [DOI] [PubMed] [Google Scholar]
- 10.Earle SA, El-Haddad A, Patel MB, et al. Prolongation of skin graft survival by exogenous ubiquitin. Transplantation. 2006;82(11):1544–1546. doi: 10.1097/01.tp.0000236057.56721.d0. [DOI] [PubMed] [Google Scholar]
- 11.Earle SA, Proctor KG, Patel MB, et al. Ubiquitin reduces fluid shifts after traumatic brain injury. Surgery. 2005;138(3):431–438. doi: 10.1016/j.surg.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Griebenow M, Casalis P, Woiciechowsky C, et al. Ubiquitin reduces contusion volume after controlled cortical impact injury in rats. Journal of neurotrauma. 2007;24(9):1529–1535. doi: 10.1089/neu.2007.0306. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Covarrubias L, Manning EW, 3rd, Sorell LT, et al. Ubiquitin enhances the Th2 cytokine response and attenuates ischemia-reperfusion injury in the lung. Crit Care Med. 2008;36(3):979–982. doi: 10.1097/CCM.0B013E318164E417. [DOI] [PubMed] [Google Scholar]
- 14.Ahn HC, Yoo KY, Hwang IK, et al. Ischemia-related changes in naive and mutant forms of ubiquitin and neuroprotective effects of ubiquitin in the hippocampus following experimental transient ischemic damage. Exp Neurol. 2009;220(1):120–132. doi: 10.1016/j.expneurol.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Xing J, Guan XD. Masters Thesis. Sun Yat-sen University; China: 2007. Effects of exogenous ubiquitin on serum NO, NOS and lung injury in the early stages after cecal ligation and puncture in mice. [Google Scholar]
- 16.Goelz L, Casalis PA, Thomale UW, et al. The effect of ubiquitin on immune response after controlled cortical impact injury. The Journal of trauma. 2011;70(5):1104–1111. doi: 10.1097/TA.0b013e3181e9c2f8. [DOI] [PubMed] [Google Scholar]
- 17.Singh M, Roginskaya M, Dalal S, et al. Extracellular ubiquitin inhibits beta-AR-stimulated apoptosis in cardiac myocytes: role of GSK-3beta and mitochondrial pathways. Cardiovasc Res. 2010;86(1):20–28. doi: 10.1093/cvr/cvp402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaremko L, Jaremko M, Pasikowski P, et al. The immunosuppressive activity and solution structures of ubiquitin fragments. Biopolymers. 2009;91(6):423–431. doi: 10.1002/bip.21160. [DOI] [PubMed] [Google Scholar]
- 19.Hildebrand F, Giannoudis PV, Griensven M, et al. Management of polytraumatized patients with associated blunt chest trauma: a comparison of two European countries. Injury. 2005;36(2):293–302. doi: 10.1016/j.injury.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Bardenheuer M, Obertacke U, Waydhas C, et al. Epidemiology of the severely injured patient. A prospective assessment of preclinical and clinical management AG Polytrauma of DGU. Der Unfallchirurg. 2000;103(5):355–363. doi: 10.1007/s001130050550. [DOI] [PubMed] [Google Scholar]
- 21.Greenspan L, McLellan BA, Greig H. Abbreviated Injury Scale and Injury Severity Score: a scoring chart. J Trauma. 1985;25(1):60–64. doi: 10.1097/00005373-198501000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Dudkiewicz M, Harpaul TA, Proctor KG. Hemoglobin-based oxygen carrying compound-201 as salvage therapy for severe neuro- and polytrauma (Injury Severity Score = 27-41) Crit Care Med. 2008;36(10):2838–2848. doi: 10.1097/CCM.0b013e318186f6b3. [DOI] [PubMed] [Google Scholar]
- 23.Baker TA, Romero J, Bach HHt, et al. Systemic release of cytokines and heat shock proteins in porcine models of polytrauma and hemorrhage. Crit Care Med. 2011 Oct 6; doi: 10.1097/CCM.0b013e318232e314. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geng Q, Romero J, Saini V, et al. A subset of 26S proteasomes is activated at critically low ATP concentrations and contributes to myocardial injury during cold ischemia. Biochem Biophys Res Commun. 2009;390(4):1136–1141. doi: 10.1016/j.bbrc.2009.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majetschak M, Patel MB, Sorell LT, et al. Cardiac proteasome dysfunction during cold ischemic storage and reperfusion in a murine heart transplantation model. Biochem Biophys Res Commun. 2008;365(4):882–888. doi: 10.1016/j.bbrc.2007.11.092. [DOI] [PubMed] [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 27.Cane RD, Buchanan N. The electrocardiographic and clinical diagnosis of myocardial contusion. Intensive care medicine. 1978;4(2):99–102. doi: 10.1007/BF01684393. [DOI] [PubMed] [Google Scholar]
- 28.Nagasawa M, Sakimura K, Mori KJ, et al. Gene structure and chromosomal localization of the mouse NMDA receptor channel subunits. Brain Res Mol Brain Res. 1996;36(1):1–11. doi: 10.1016/0169-328x(95)00225-h. [DOI] [PubMed] [Google Scholar]
- 29.Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135(3):561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao Q, Ye S, Oberhollenzer F, et al. SDF1 gene variation is associated with circulating SDF1alpha level and endothelial progenitor cell number: the Bruneck Study. PLoS One. 2008;3(12):e4061. doi: 10.1371/journal.pone.0004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becchi C, Pillozzi S, Fabbri LP, et al. The increase of endothelial progenitor cells in the peripheral blood: a new parameter for detecting onset and severity of sepsis. Int J Immunopathol Pharmacol. 2008;21(3):697–705. doi: 10.1177/039463200802100324. [DOI] [PubMed] [Google Scholar]
- 32.Fox A, Smythe J, Fisher N, et al. Mobilization of endothelial progenitor cells into the circulation in burned patients. Br J Surg. 2008;95(2):244–251. doi: 10.1002/bjs.5913. [DOI] [PubMed] [Google Scholar]
- 33.Saini V, Romero J, Marchese A, et al. Ubiquitin receptor binding and signaling in primary human leukocytes. Communicative & Integrative Biology. 2010;3(6):608–610. doi: 10.4161/cib.3.6.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karin N. The multiple faces of CXCL12 (SDF-1alpha) in the regulation of immunity during health and disease. Journal of leukocyte biology. 2010;88(3):463–473. doi: 10.1189/jlb.0909602. [DOI] [PubMed] [Google Scholar]
- 35.Fricker SP, Anastassov V, Cox J, et al. Characterization of the molecular pharmacology of AMD3100: a specific antagonist of the G-protein coupled chemokine receptor, CXCR4. Biochem Pharmacol. 2006;72(5):588–596. doi: 10.1016/j.bcp.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Hesselgesser J, Liang M, Hoxie J, et al. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J Immunol. 1998;160(2):877–883. [PubMed] [Google Scholar]
- 37.Loetscher P, Gong JH, Dewald B, et al. N-terminal peptides of stromal cell-derived factor-1 with CXC chemokine receptor 4 agonist and antagonist activities. J Biol Chem. 1998;273(35):22279–22283. doi: 10.1074/jbc.273.35.22279. [DOI] [PubMed] [Google Scholar]
- 38.Bernhagen J, Krohn R, Lue H, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13(5):587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 39.Di Salvo J, Koch GE, Johnson KE, et al. The CXCR4 agonist ligand stromal derived factor-1 maintains high affinity for receptors in both Galpha(i)-coupled and uncoupled states. Eur J Pharmacol. 2000;409(2):143–154. doi: 10.1016/s0014-2999(00)00846-3. [DOI] [PubMed] [Google Scholar]
- 40.Hesselgesser J, Halks-Miller M, DelVecchio V, et al. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7(2):112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 41.Villalba S, Salvucci O, Aoki Y, et al. Serum inactivation contributes to the failure of stromal-derived factor-1 to block HIV-I infection in vivo. Journal of leukocyte biology. 2003;74(5):880–888. doi: 10.1189/jlb.0403149. [DOI] [PubMed] [Google Scholar]
- 42.Antonsson B, De Lys P, Dechavanne V, et al. In vivo processing of CXCL12alpha/SDF-1alpha after intravenous and subcutaneous administration to mice. Proteomics. 2010 doi: 10.1002/pmic.201000331. [DOI] [PubMed] [Google Scholar]
- 43.Fan H, Wong D, Ashton SH, et al. Beneficial Effect of a CXCR4 Agonist in Murine Models of Systemic Inflammation. Inflammation. 2011 Jan 28; doi: 10.1007/s10753-011-9297-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanki S, Segers VF, Wu W, et al. Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail. 2011;4(4):509–518. doi: 10.1161/CIRCHEARTFAILURE.110.960302. [DOI] [PubMed] [Google Scholar]
- 45.Hu X, Dai S, Wu WJ, et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116(6):654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meiron M, Zohar Y, Anunu R, et al. CXCL12 (SDF-1alpha) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med. 2008;205(11):2643–2655. doi: 10.1084/jem.20080730. [DOI] [PMC free article] [PubMed] [Google Scholar]