Abstract
Serum from 21 healthy, captive Asian elephants (Elephas maximus) was evaluated by measured and calculated osmolality. Serum osmolality results for this population of Asian elephants had a median of 261 mOsm/kg and an interquartile interval of 258–269 mOsm/kg when measured by freezing point osmometry and a median of 264 mOsm/kg and an interquartile interval of 257–269 mOsm/kg when measured by vapor pressure osmometry. These values are significantly lower than values reported in other mammalian species and have important diagnostic and therapeutic implications. Calculated osmolality produced unreliable results and needs further study to determine an appropriate formula and its clinical application in this species. A 16-hr water deprivation test in 16 Asian elephants induced a small, subclinical, but statistically significant increase in measured serum osmolality. Serum osmolality, blood urea nitrogen, and total protein by refractometer were sensitive indicators of hydration status. Serum osmolality measurement by freezing point or vapor pressure osmometry is a useful adjunct to routine clinical tests in the diagnostic evaluation of elephants.
Keywords: Elephants, Elephas maximus, osmolality, osmolarity, water deprivation
Introduction
In the field of veterinary medicine, osmometry refers to the study of solute concentrations in physiological solutions. The use of clinical osmometry has received growing attention, particularly in critical care medicine and assessment of metabolic disorders. Serum osmolality is a measure of the amount of solute particles present in the liquid portion of the blood, expressed as mOsm/kg.20 The major solutes contributing to serum osmolality in mammals are sodium (Na+), potassium (K+), chloride (Cl−), bicarbonate (HCO3−), blood urea nitrogen (BUN), and glucose (Glu).20 Minor contributions are made by cations and anions including calcium (Ca2+), magnesium (Mg2+), phosphorus (P) in various anionic forms, and serum proteins (primarily albumin and globulins).20 Serum osmolality is valuable in determining patient hydration status, electrolyte balance, and response to fluid therapy. It can also be used to evaluate renal function and states of ketoacidosis and to differentiate causes of polyuria and polydipsia.9,12 Comparison of measured serum osmolality to direct calculation of serum osmolality is a rapid method of screening for unidentified solutes, such as those causing certain toxicities (e.g., ethylene glycol).9
Serum osmolality can be directly measured or estimated by calculation when the concentrations of the contributing solutes in the serum have been determined.20 The 2 commercially available, commonly used measurement methods are freezing point osmometry and vapor pressure osmometry.9 Both determine serum osmolality by linear extrapolation after comparing the colligative properties of a serum sample to those of pure water, the physiological solvent. Freezing point osmometers evaluate freezing point depression and can be altered artifactually by highly viscous solutions (e.g., high protein or lipid content), nonhomogeneous solutions, additional solvents, or the presence of nonsoluble particulate matter that can act as a crystalline nidus during freezing.8,22 Vapor pressure osmometers evaluate serum dew point temperature depression, which is an explicit function of the serum vapor pressure.22 Vapor pressure osmometry measurements can be artifactually altered by the presence of volatile solutes or additional solvents, high amounts of multiple solvents, or high lipid content.8 These result in multiphase vapor pressure readings or reduction of vapor pressure formation by reducing solution surface exposure (K. Thomas, personal communication, 2009).8
In the absence of osmometers, equations have been validated in domestic species to estimate serum osmolality based on routine serum biochemistry values.20 Solute measurements are converted to units of mOsm/kg and summated. Generally, these formulas are a variation on the following: osmolality = cations + anions + Glu + BUN.20 Given the major contributors to osmolality and that serum must remain electrically neutral, one of the most common formulas used in domestic mammals is osmolality = 2(Na+ + K+) + Glu + BUN.20 A value slightly less than 2 is sometimes used in the formula to account for incomplete ionic dissociativity.16 Since calculated osmolality is an approximation, the value is rarely exactly equal to the measured osmolality. Typically, an osmol gap (measured osmolality minus calculated osmolality) of 4–6 units is considered clinically insignificant.20 Calculated osmolality may be artifactually altered in states of pseudohyponatremia, which affects osmol gap values.20 The best formula for accurately determining calculated osmolality is species specific and dependent on clinical context.1,9 Thus, simple extrapolation of a formula from one species to another is not appropriate, and formulas should be validated against measured osmolality in individual species.
Normal measured serum osmolality values vary by species (Table 1) with most mammals having values near 300 mOsm/kg.20 Serum osmolality is expected to increase with a decrease in hydration level.20 In large healthy animals, a 24-hr water deprivation test can be performed to induce a temporary state of mild, subclinical dehydration with little risk to health.18 Serum osmolality has not been scientifically evaluated in elephant species in healthy, dehydrated, or other states. The goals of phase 1 of the current study were to evaluate methods for measuring and calculating serum osmolality in Asian elephants (Elephas maximus), to develop normal serum osmolality values for the population, and to compare serum osmolality values with those of other species. The goal of phase 2 of this study was to determine whether mild dehydration induced by 16-hr water deprivation could cause a statistically significant increase in serum osmolality in the Asian elephant. The study was intended to provide information useful in the clinical management of elephants.
Table 1.
Reported normal values for serum osmolality in various species.*
| Species | Osmometry | n | Mean | SD |
|---|---|---|---|---|
| Dog (Canis lupus familiaris) | FPO and VPO | 100 | 303 | 6 |
| Cat (Felis silvestris catus) | FPO and VPO | 100 | 324 | 16 |
| Horse (Equus ferus caballus) | FPO and VPO | 100 | 292 | 5 |
| Manatee (Trichechus manatus)15 | VPO | 6 | 310.2 | 2.2 |
| Rat (Rattus norvegicus)5 | FPO | 16 | 294 | 1.4 |
| Hispaniolan Amazon parrot (Amazona ventralis)1 | VPO | 20 | 326 | 6.9 |
Values are reported in mOsm/kg. SD = standard deviation; FPO = freezing point osmometer; VPO = vapor pressure osmometer. Dog, cat, and horse values were obtained by personal communication with the University of Florida, Veterinary Diagnostic Laboratory, Clinical Pathology Service.
Materials and methods
All procedures were approved via IACUC 201004899 through the University of Florida (UF). Elephants were owned and housed by the Ringling Brothers and Barnum & Bailey Center for Elephant Conservation.
Phase 1
The sampled elephants were 11–67 years of age and consisted of 2 bulls, 1 castrated male, and 18 females, all of whom were considered healthy based on serial physical examinations and normal behavior. Twelve to 15 ml of venous blood was collected using standard phlebotomy technique from a pinna vein of the 21 adult Asian elephants.13 Blood was collected into Vacutainera red-top glass 10-ml tubes containing no serum separator gel. After visible clotting occurred and within 1 hr of collection, the blood was centrifugedb (10 min; 2,479 × g). The serum was separated from the cellular component and frozen in CryoVialsc for subzero storage. Serum was provided to all laboratories in a frozen state and was thawed on the day of testing. Serum was submitted to the UF Veterinary Diagnostic Laboratories (UFVDL) Clinical Pathology Service. Serum osmolality was measured by 2 methods: vapor pressure osmometryd (VPO) and freezing point osmometrye (FPO). In accordance with the manufacturer’s instructions for the osmometers, on each day of use, the vapor pressure osmometer was calibrated with 3 concentrations of standard salt solution, and the freezing point osmometer was calibrated with 2 concentrations of standard salt solution. Both osmometers were tested on each day of use with 2 levels of human assayed controls. In addition, feline, canine, and equine serum samples (n = 20 of each) were evaluated throughout the elephant testing period to ensure that normal domestic species samples were generating FPO and VPO results in agreement with established laboratory reference intervals on both osmometers (data not published). On the elephant samples, serum chemistry panels were performedf concurrently to obtain individual serum solute values, which included total protein (TP) by the biuret reaction method and albumin by the bromocresol green dye–binding reaction method. Serum protein electrophoresis panels were performedg to measure individual serum protein constituents that contribute to osmolality. The panels consisted of albumin, alpha-1 globulins (α1), alpha-2 globulins (α2), beta globulins (β), and gamma globulins (γ). In the protein electrophoresis, TP was determined by refractometry, and absolute individual protein fractions were determined by calculation using electrophoretogram-generated percentages.
For further analysis of the elephant serum, 2 mathematical formulas were used to generate calculated osmolality values from the serum chemistry panel results. Solute unit of measurement conversions were performed based on reported atomic and molecular weights and conversion units that are considered uniform across species.20 The first formula, referred to as the simple formula (SFO), was based on the common equation previously mentioned, where SFO osmolality = 1.95(Na+ + K+) + Glu + BUN. The value of 1.95 was used to force the mean SFO to closely match the mean of the osmometer measurements. This value is reasonable to account for incomplete ionic dissociativity and is close to values reported in other species.16,20 A second formula, referred to as the complete formula (CFO), was generated in an attempt to more accurately reflect all the measured solute concentrations, where CFO osmolality = Na+ + K+ + Cl− + Mg2+ + P + Ca2+ + HCO3− + BUN + Glu + creatinine + total bilirubin + triglycerides. This formula makes an inherent assumption of complete ionic dissociativity. Protein was excluded because it is considered a minor contributor to osmolality, and appropriate protein unit conversion factors, which vary by species, have not been determined for the Asian elephant.20
Phase 2
Several weeks after phase 1, a water deprivation test was performed independently. One castrated male and 15 female elephants (ages 16–67 years) were selected from the previously described group based on elephant availability and behavioral compliance for consecutive blood draws under time constraints. This group typically received water ad libitum twice daily: once in the morning when shifting from barn to pasture and once in the evening when returning to the barn for the night. As previously described, venous blood samples were collected at 2 time points into Vacutainer red-top glass 10-ml tubes containing no serum separator gela and Vacutainer green-top plastic 3.0-ml tubes each containing 51 USP units of lithium heparin.a Red-top tubes were filled prior to green-top tubes. The initial blood samples (T0) were collected in the evening after the elephants were given free access to water and were moved into the barn. Water was withheld after the T0 sampling. The second blood samples (T1) were taken an average of 16 hr 34 min (minimum 15 hr 42 min; maximum 17 hr 33 min) post T0, which was after the animals were shifted to pasture. After the T1 sampling, the elephants returned to receiving water as usual. The sampled elephants, therefore, had missed 1 routine water administration at T1. Packed cell volume (PCV) and TP (refractometer) were measured on each blood sample from the green-top tubes using standard techniques.20 Serum was separated from the cellular component, frozen, and submitted to the UFVDL Clinical Pathology Service for testing as previously described. Based on results from phase 1, only 1 osmometry method was deemed necessary for comparison between time points. Because it is the more common method, FPO was performed along with concurrently run serum chemistry panels to obtain solute values for SFO.20
Statistical analysis
Calculations were performed using MATLAB v. R2008b.h All statistical tests were performed at the 95% confidence interval with a P value < 0.05 considered significant. All comparison tests were 2-tailed. Due to the low number of samples, results are presented as medians with interquartile intervals where appropriate.
Phase 1
Lilliefors tests were used to evaluate data for normality, and a P value ≥ 0.05 was interpreted as indicating a normal distribution. The elephant FPO results and VPO results were compared using a paired t-test. The elephant FPO and VPO means were compared to the reported reference means of the comparison species listed in Table 1 using a 1-sample t-test. All other comparisons were performed using a Wilcoxon signed-rank test. For the elephant FPO and VPO comparisons, variance was evaluated with the 2-tailed chi-square test. All other evaluations of variance were performed with the Ansari–Bradley equality of variance test. Pearson product-moment correlation coefficient (r) was used to assess correlations. Linear relationships between elephant osmometer results, chemistry panel solute values, and protein electrophoresis values were evaluated using Deming regression, simple least squares, total least squares, and constrained least squares. Intercept and slope estimates are reported with confidence intervals established using the jack-knife method.
Phase 2
A Lilliefors test for normality was performed. Because not all data were normally distributed, Wilcoxon signed-rank tests were used for comparing the T0 and T1 values for PCV, TP (biuret), TP (refractometer), BUN, creatinine, FPO, and SFO.
Results
Phase 1
For the sampled population of healthy, captive Asian elephants, the measured serum osmolality values generated a median of 261 mOsm/kg and an interquartile interval of 258–269 mOsm/kg for freezing point osmometry and a median of 264 mOsm/kg and an interquartile interval of 257–269 mOsm/kg for vapor pressure osmometry. Serum osmolality results by each method are shown in Table 2. There was no significant difference between the FPO and VPO median values (P = 0.40). The elephant FPO and VPO measurements were consistent with normal distributions (FPO P = 0.35 and VPO P = 0.50), and no significant difference was observed between elephant FPO and VPO mean values (P = 0.60) or variances (P = 0.80). The elephant osmometer results were significantly lower than the reported species listed in Table 1 when compared with the reported reference means (all comparisons P < 10−9). In fact, the reported reference intervals for the other species listed in Table 1 did not even overlap with the interquartile interval for the elephant FPO and VPO values.
Table 2.
Asian elephant (Elephas maximus) serum osmometry results in the current study.*
| Osmometry | n | Min. | 25% | Median | 75% | Max. | Mean | SD |
|---|---|---|---|---|---|---|---|---|
| FPO | 21 | 250 | 258 | 261 | 269 | 295 | 264 | 11 |
| VPO | 21 | 244 | 257 | 264 | 269 | 291 | 263 | 10 |
| SFO | 21 | 204 | 262 | 267 | 269 | 285 | 264 | 16 |
| CFO | 21 | 199 | 253 | 255 | 260 | 274 | 254 | 14 |
Results from phase 1 where n = 21. Values are reported in mOsm/kg. SD = standard deviation; FPO = freezing point osmometer; VPO = vapor pressure osmometer; SFO = simple formula osmolality (calculated); CFO = complete formula osmolality (calculated); Min. = minimum value obtained; Max. = maximum value obtained; 25% and 75% refer to percentage quartile values.
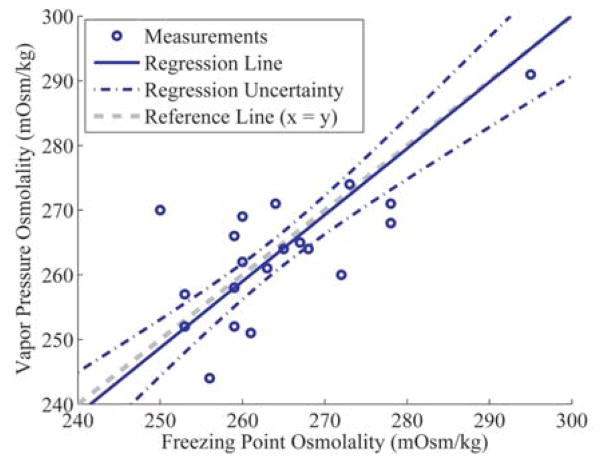
The elephant FPO and VPO results exhibited a statistically significant correlation (r = 0.71). Assuming a linear relationship, when Deming regression analysis was used to express elephant VPO in terms of FPO, where VPO = a + b(FPO), an estimated intercept (a) of 6 ± 61 and a slope (b) of 0.97 ± 0.23 were obtained. These values indicated no significant constant bias (a) or proportional bias (b) between the FPO and VPO measurements. However, relatively large confidence intervals existed for the intercept and slope estimates, which is reflected in the regression uncertainty lines in Figure 1. These confidence intervals were directly related to the sizeable distances between the osmometer measurements and the illustrated reference line. As a result of this uncertainty, there is no obvious or significant trend to explain the observed differences between corresponding FPO and VPO measurements.
Figure 1.
Deming regression of Asian elephant (Elephas maximus) serum freezing point osmometer (FPO) and vapor pressure osmometer (VPO) results, where VPO = a + b(FPO). The elephant samples obtained an intercept (a) of 6 ± 61 and a slope (b) of 0.97 ± 0.23. Linear bounds represent the uncertainty in the Deming regression line estimate.
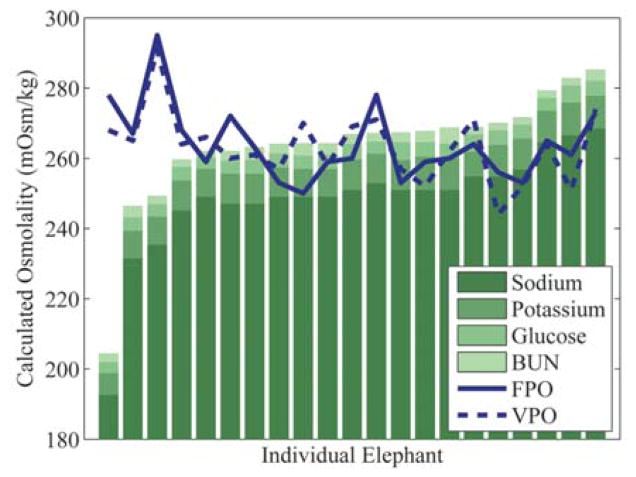
In contrast to the elephant osmometer measurements, the elephant SFO and CFO results (Table 2) were not normally distributed (SFO and CFO P < 0.001). No significant differences in variance were observed when FPO and VPO measurements were compared with SFO and CFO measurements (all comparisons P > 0.32). As expected, the SFO mean and median were in agreement with both the FPO and VPO mean and median values because the dissociativity coefficient of 1.95 was chosen for this purpose. Interestingly, the CFO results were significantly lower than the FPO and VPO results (CFO vs. FPO P = 0.0496; CFO vs. VPO P = 0.03). Furthermore, neither the SFO nor CFO results significantly correlated with the measured osmolality values (SFO to FPO r = −0.28; SFO to VPO r = −0.40; CFO to FPO r = −0.24; CFO to VPO r = −0.39), as shown in Figure 2. The only significant correlation between chemistry panel values and osmometer values was a negative correlation between total serum calcium and FPO (Ca2+ to FPO r = −0.47). Most serum solutes had a slightly negative, but nonsignificant, correlation with both FPO and VPO.
Figure 2.
Asian elephant (Elephas maximus) serum calculated osmolality bar graph with overlaid serum osmometer results. Elephants are sorted on the x-axis in ascending simple formula osmolality (SFO) value. BUN = blood urea nitrogen; FPO = freezing point osmometer values; VPO = vapor pressure osmometer values. Calculated osmolality values shown were generated by SFO. Bars exhibit proportional contribution of solutes used in the SFO formula.
Phase 2
From the T0 to T1 time of water deprivation, there was a significant increase in FPO, BUN, and TP (refractometer). Results with corresponding P values are shown in Table 3, and individual elephant values are shown in Figures 3–5. A significant increase in FPO variance (P < 0.001) also occurred. The values for SFO, PCV, TP (biuret), and creatinine exhibited no significant change between time points.
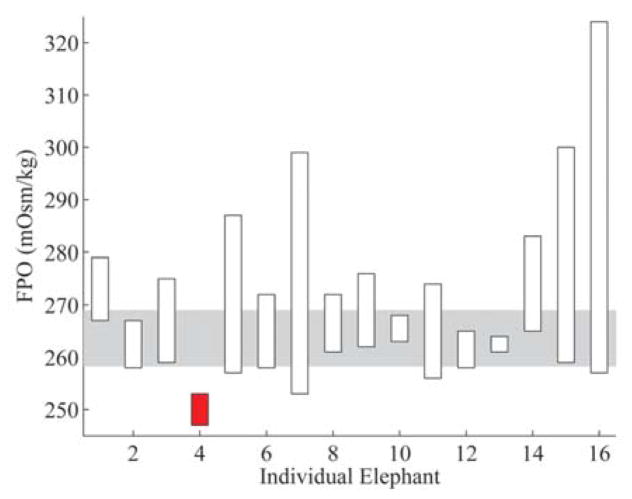
Figure 3.
Freezing point osmolality (FPO) results from the water deprivation test. Elephants are listed on the x-axis and FPO values are on the y-axis. Corresponding results are displayed for each elephant as bars showing the difference between the T0 and T1 FPO values. Unfilled bars indicate elephants with an increase in FPO and filled bars indicate elephants with a decrease in FPO over the water deprivation period. The shaded area represents the interquartile interval obtained from phase 1 results.
Figure 5.
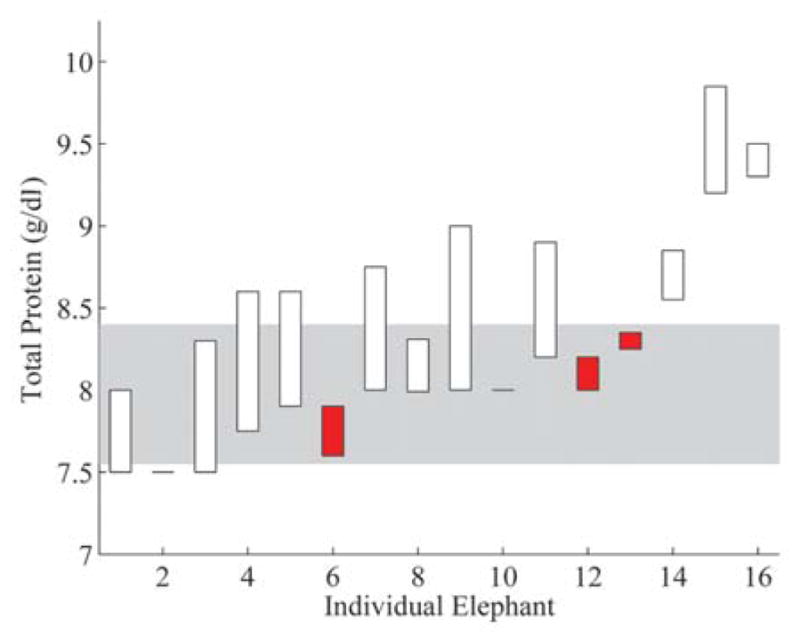
Total protein by refractometer results from the water deprivation test. Elephants are listed on the x-axis in the same order as Figure 3 and total protein (refractometer) values are on the y-axis. Corresponding results are displayed for each elephant as bars showing the difference between the T0 and T1 total protein values. Unfilled bars indicate elephants with an increase in total protein and filled bars indicate elephants with a decrease in total protein over the water deprivation period. The shaded area represents the interquartile interval obtained from phase 1 results.
Discussion
For the Asian elephants in the current study, the serum osmolalities generated a median of 261 mOsm/kg by freezing point osmometry and 264 mOsm/kg by vapor pressure osmometry. The osmolality values obtained were significantly lower than reported reference intervals from dogs, cats, horses, manatees, rats, and Hispaniolan Amazon parrots. In fact, the osmolality levels measured in these healthy, normally hydrated, adult Asian elephants would be considered critically low, even potentially fatal, in other reported mammalian species.2,4 Proper interpretation of serum osmolality values in Asian elephants has important clinical implications, including avoiding erroneous diagnosis of hypo-osmolar disorders in this species. Due to the poor correlations with measured FPO and VPO values and the wide reference intervals, the calculated osmolality formulas assessed in the present study are of little clinical value. If further investigation determines that an appropriate calculated osmolality formula for Asian elephants exists, it likely requires unique ionic dissociativity coefficients.
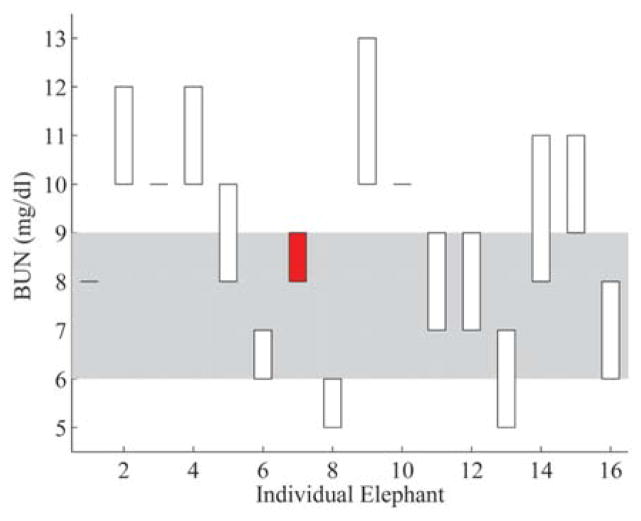
The 16-hr water deprivation test successfully induced a measureable, significant change in FPO from T0 to T1 (Fig. 3). Additionally, BUN and TP (refractometer) values had statistically significant changes from T0 to T1 (Figs. 4, 5), but the values for SFO, PCV, TP (biuret), and creatinine exhibited no significant change. The subtlety of the changes in 3 parameters and the lack of changes in the remaining parameters indicate that a statistically significant but subclinical degree of dehydration occurred. These findings suggest that measured serum osmolality, BUN, and TP (refractometer) appear to be early indicators of change in hydration status for Asian elephants. When compared with previously determined normal values for an individual elephant, measured serum osmolality may be a useful adjunct for detecting minor changes in hydration that are not clinically apparent. Additionally, paired serum osmolality measurements may be useful in assessing patient response to fluid and electrolyte therapy, nonisotonic diuresis, or changes in husbandry.
Figure 4.
Blood urea nitrogen (BUN) results from the water deprivation test. Elephants are listed on the x-axis in the same order as Figure 3, and BUN values are on the y-axis. Corresponding results are displayed for each elephant as bars showing the difference between the T0 and T1 BUN values. Unfilled bars indicate elephants with an increase in BUN, and filled bars indicate elephants with a decrease in BUN over the water deprivation period. The shaded area represents the interquartile interval obtained from phase 1 results.
The correlation between the elephant FPO and the VPO readings (r = 0.71) was somewhat lower than expected. The significance of this is unknown, but the fact that the elephant FPO and VPO results had a relatively narrow range may have magnified the influence of the inherent measurement error. Other potential causes include presence of unique serum solutes or solute interactions that affected osmometer readings. The same osmometer method should be used when measured osmolality values with high precision are desired or when comparing osmolality values for minor changes.
The biological significance of the Asian elephants having a lower serum osmolality than the comparison species, including another Afrotherian, is unknown. This may be a vestige of proboscidean ancestry, which recent evidence indicates may have had an aquatic component contributing to unique anatomical and physiological features.7,23 In addition to decreased rigidity of erythrocytes, this may be a feature aiding peripheral circulation in a large body.24 Alternatively, elephants do not always have immediate access to water in the wild, and a lower osmolality may allow physiological room for water deprivation.11 Studies of wild elephant populations, elephants in states of illness, and other Afrotherians will improve understanding of this physiological parameter, its biological context, states of alteration, and clinical applications.
The effects of fluid therapy on serum osmolality in elephants also need investigation. Elephants are often given free water by oral and rectal routes, whereas commercially available intravenous fluids are more often reserved for neonates and juveniles, critically ill cases, and anesthetic procedures.6,10,14,19 The commercial fluids are similar to the extracellular fluid composition of most mammals and include 0.9% sodium chloride (308 mOsm/kg), Plasmalytei (312 mOsm/kg), Normosol-Rj (296 mOsm/kg), and others.3 Based on the current study, it is important to recognize that such fluids are hyperosmotic compared with adult Asian elephant serum. The clinical significance of this fact needs to be investigated; until then, clinicians should consider coupling any commercial fluids with orally or rectally administered free water to replace water deficits and reduce any potential deleterious effects of hyperosmotic fluid administration.
The absence of a clear, linear relationship between the elephant measured osmolalities and either the chemistry panel or protein electrophoresis solute values may simply indicate that a successful regression over this small of a measurement interval may not be possible. The expected strong correlations between many of the chemistry panel measurements may also have interfered with regression analysis. Additionally, there may be an unaccounted serum characteristic that is causing a dominant influence on the chemistry panel or osmometers. The significance of the negative correlation of Ca2+ with FPO is unknown. Calcium is known to interact with serum proteins, although the level of interaction varies with species. In the healthy animal, total Ca2+ is comprised of approximately 51% ionized Ca2+ in the horse and 56% ionized Ca2+ in the dog.17,21 The remaining Ca2+ binds to negatively charged sites on proteins, with roughly 80% bound to albumin and 20% to globulins in domestic species.20 Binding is charge dependent and, consequently, affected by serum pH.20 An increase in Ca2+-protein binding could reduce the dissociativity coefficient and affect osmolality values. Study of ionized Ca2+ and its relationship to measured serum osmolality would help clarify this relationship in the Asian elephant.
In conclusion, interquartile intervals for serum osmolality of a healthy, captive population of Asian elephants have been established as 258–269 mOsm/kg for freezing point osmometry and 257–269 mOsm/kg for vapor pressure osmometry. These values are significantly lower than those reported in other mammalian species. This unique aspect of Asian elephant physiology has important diagnostic and therapeutic implications, particularly in regard to fluid therapy. Calculated osmolality values correlate poorly with measured osmolality, indicating that calculated osmolality should not be used in this species without new insight from further investigation. In the Asian elephants, water deprivation induced a statistically significant increase in measured serum osmolality, BUN, and TP (refractometer), indicating the potential to use paired samples to evaluate subclinical changes in hydration status in response to medical therapies or husbandry changes. Clinicians may use serum osmolality measurement in Asian elephants for a more thorough diagnostic evaluation and to better assess response to medical therapies and husbandry changes.
Table 3.
Results from the Asian elephant (Elephas maximus) water deprivation test.*
| Unit | Phase 1 median | Phase 1 IQI | T0 median | T1 median | ΔT0 T1 | P value | |
|---|---|---|---|---|---|---|---|
| PCV | % | — | — | 43 | 44 | No | 0.23 |
| TP-R | g/dl | 8.2 | 7.6–8.4 | 8.0 | 8.5 | Yes | <0.01 |
| TP-B | g/dl | 8.5 | 8.2–9.1 | 8.3 | 8.7 | No | 0.08 |
| BUN | mg/dl | 8.0 | 6.0–9.0 | 8.0 | 9.5 | Yes | <0.01 |
| Creatinine | mg/dl | 1.7 | 1.48–1.80 | 1.6 | 1.5 | No | 0.06 |
| FPO | mOsm/kg | 262 | 257–269 | 259 | 275 | Yes | <0.01 |
| SFO | mOsm/kg | 267 | 262–269 | 267 | 276 | No | 0.06 |
Phase 1 (n = 21) median and interquartile intervals (IQI) are shown for reader reference. Results from the phase 2 water deprivation test (n = 16) are shown as T0 (initial blood sample) and T1 (second blood sample); Δ = significant change; P values correspond to Wilcoxon signed-rank test results for paired T0 and T1 values. PCV = packed cell volume (not measured in phase 1); TP-R = total protein measured by refractometer; TP-B = total protein measured by biuret method; BUN = blood urea nitrogen; FPO = freezing point osmometer; SFO = simple formula osmolality (calculated). The average time of water deprivation was 16 hr 34 min; “No” and “Yes” indicate whether significant change occurred.
Acknowledgments
The authors gratefully acknowledge the staff of the Ringling Bros. and Barnum & Bailey Center for Elephant Conservation for their assistance in conducting this study; Dr. Carolyn Cray for her input and work with the serum protein electrophoresis tests; Dr. Bob Bonde, Dr. Jim Wellehan, and Dr. Maureen Long for their assistance; Dr. Randy Stewart for valuable input during the initial development of the study design; and the reviewers of the Journal of Veterinary Diagnostic Investigation for their insight and recommendations.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support for this study was funded in part by the NIH/NCRR Clinical and Translational Science Awards to the University of Florida KL2 RR029888 and UL1 RR029890. Additional support was provided by the Batchelor Foundation.
Footnotes
BD, Franklin Lakes, NJ.
Hettich Rotofix 32A Zentrifugen, Andreas Hettich GbmH & Co. KG, Tuttlingen, Germany.
Simport Scientific, Beloeil, Quebec, Canada.
Wescor 5500 Vapor Pressure Osmometer, Wescor Inc., Logan, UT.
Precision Systems 5004 Micro-osmette Automatic Osmometer, Precision Systems Inc., Natick, MA.
Siemens Dimension Xpand Clinical Chemistry Analyzer, Siemens Healthcare Diagnostics, Newark, DE.
University of Miami, Leonard M. Miller School of Medicine, Comparative Pathology Laboratory, Miami, FL.
MathWorks, Natick, MA.
Baxter Healthcare Corporation, Deerfield, IL. j. Abbott Laboratories, North Chicago, IL.
Abbott Laboratories, North Chicago, IL.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Acierno MJ, Mitchell MA, Freeman DM, et al. Determination of plasma osmolality and agreement between measured and calculated values in healthy adult Hispaniolan Amazon parrots (Amazona ventralis) Am J Vet Res. 2009;70:1151–1154. doi: 10.2460/ajvr.70.9.1151. [DOI] [PubMed] [Google Scholar]
- 2.Church D. Electrolyte disorders. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. 6. Saunders Elsevier; Philadelphia, PA: 2004. pp. 236–240. [Google Scholar]
- 3.Dibartola SP, Bateman S. Introduction to fluid therapy. In: DiBartola SP, editor. Fluid, electrolyte, and acid-base disorders in small animal practice. 3. Saunders; St. Louis, MO: 2006. pp. 325–344. [Google Scholar]
- 4.Dibartola SP, Green RA, Autran De Morais HS, Willard MD. Electrolyte and acid-base disorders. In: Willard MD, Tvedten H, Turnwald GH 3rd, editors. Small animal clinical diagnosis by laboratory methods. WB Saunders; Philadelphia, PA: 1999. pp. 94–107. [Google Scholar]
- 5.Dunn FL, Brennan TJ, Nelson AE, Robertson GL. The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest. 1973;52:3212–3219. doi: 10.1172/JCI107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emanuelson K. Neonatal care and hand rearing. In: Fowler ME, Mikota SK, editors. Biology, medicine, and surgery of elephants. Blackwell; Ames, IA: 2006. pp. 233–241. [Google Scholar]
- 7.Gaeth AP, Short RV, Renfree MB. The developing renal, reproductive, and respiratory systems of the African elephant suggest an aquatic ancestry. Proc Natl Acad Sci U S A. 1999;96:5555–5558. doi: 10.1073/pnas.96.10.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grattoni A, Canavese G, Montevecchi FM, Ferrari M. Fast membrane osmometer as alternative to freezing point and vapor pressure osmometry. Anal Chem. 2008;80:2617–2622. doi: 10.1021/ac7023987. [DOI] [PubMed] [Google Scholar]
- 9.Green RA. Perspectives of clinical osmometry. Vet Clin North Am. 1978;8:287–299. doi: 10.1016/s0091-0279(78)50034-8. [DOI] [PubMed] [Google Scholar]
- 10.Isaza R, Hunter RP. Drug delivery to captive Asian elephants—treating Goliath. Curr Drug Deliv. 2004;1:291–298. doi: 10.2174/1567201043334641. [DOI] [PubMed] [Google Scholar]
- 11.Lehnhardt J. Husbandry. In: Fowler ME, Mikota SK, editors. Biology, medicine, and surgery of elephants. Blackwell; Ames, IA: 2006. pp. 45–55. [Google Scholar]
- 12.Lord RC. Osmosis, osmometry, and osmoregulation. Postgrad Med J. 1999;75:67–73. doi: 10.1136/pgmj.75.880.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikota SK. Hemolymphatic system. In: Fowler ME, Mikota SK, editors. Biology, medicine and surgery of elephants. Blackwell; Ames, IA: 2006. pp. 325–345. [Google Scholar]
- 14.Mikota SK. Therapeutics. In: Fowler ME, Mikota SK, editors. Biology, medicine and surgery of elephants. Blackwell; Ames, IA: 2006. pp. 211–231. [Google Scholar]
- 15.Ortiz RM, Worthy GA, MacKenzie DS. Osmoregulation in wild and captive West Indian manatees (Trichechus manatus) Physiol Zool. 1998;71:449–457. doi: 10.1086/515427. [DOI] [PubMed] [Google Scholar]
- 16.Rasouli M, Kalantari KR. Comparison of methods for calculating serum osmolality: multivariate linear regression analysis. Clin Chem Lab Med. 2005;43:635–640. doi: 10.1515/CCLM.2005.109. [DOI] [PubMed] [Google Scholar]
- 17.Schenck PA, Chew DJ, Brooks CL. Fractionation of canine serum calcium, using a micropartition system. Am J Vet Res. 1996;57:268–271. [PubMed] [Google Scholar]
- 18.Schott HC., II . Examination of the urinary system. In: Reed SMB, Warwick M, editors. Equine internal medicine. WB Saunders; Philadelphia, PA: 1998. pp. 830–845. [Google Scholar]
- 19.Steffey E. General anesthesia. In: Fowler ME, Mikota SK, editors. Biology, medicine, and surgery of elephants. Blackwell; Ames, IA: 2006. pp. 110–118. [Google Scholar]
- 20.Stockham S, Scott MA. Fundamentals of veterinary clinical pathology. Wiley-Blackwell; Ames, IA: 2008. pp. 13–15.pp. 124pp. 369–413.pp. 495–557. [Google Scholar]
- 21.van der Kolk JH, Nachreiner RF, Refsal KR, et al. Heparinised blood ionised calcium concentrations in horses with colic or diarrhoea compared to normal subjects. Equine Vet J. 2002;34:528–531. doi: 10.2746/042516402776117692. [DOI] [PubMed] [Google Scholar]
- 22.Wescor Inc. Wescor 5500 vapor pressure osmometer instruction and service manual M2448-4. Wescor Inc; Logan, UT: 1989. [Google Scholar]
- 23.West JB. Snorkel breathing in the elephant explains the unique anatomy of its pleura. Resp Physiol. 2001;126:1–8. doi: 10.1016/s0034-5687(01)00203-1. [DOI] [PubMed] [Google Scholar]
- 24.Windberger U, Plasenzotti R, Voracek T. The fluidity of blood in African elephants (Loxodonta africana) Clin Hemorheol Microcirc. 2005;33:321–326. [PubMed] [Google Scholar]