Abstract
Objective
To determine plasma pharmacokinetics of penciclovir following oral and rectal administration of famciclovir to young Asian elephants (Elephas maximus).
Animals
6 healthy Asian elephants (5 females and 1 male), 4.5 to 9 years old and weighing 1,646 to 2,438 kg.
Procedures
Famciclovir was administered orally or rectally in accordance with an incomplete crossover design. Three treatment groups, each comprising 4 elephants, received single doses of famciclovir (5 mg/kg, PO, or 5 or 15 mg/kg, rectally); there was a minimum 12-week washout period between subsequent famciclovir administrations. Serial blood samples were collected after each administration. Samples were analyzed for famciclovir and penciclovir with a validated liquid chromatography–mass spectroscopy assay.
Results
Famciclovir was tolerated well for both routes of administration and underwent complete biotransformation to the active metabolite, penciclovir. Mean maximum plasma concentration of penciclovir was 1.3 μg/mL at 1.1 hours after oral administration of 5 mg/kg. Similar results were detected after rectal administration of 5 mg/kg. Mean maximum plasma concentration was 3.6 μg/mL at 0.66 hours after rectal administration of 15 mg/ kg; this concentration was similar to results reported for humans receiving 7 mg/kg orally.
Conclusions and Clinical Relevance
Juvenile Asian elephants are susceptible to elephant endotheliotropic herpesvirus. Although most infections are fatal, case reports indicate administration of famciclovir has been associated with survival of 3 elephants. In Asian elephants, a dose of 8 to 15 mg of famciclovir/kg given orally or rectally at least every 8 hours may result in penciclovir concentrations that are considered therapeutic in humans.
Asian elephants (Elephas maximus) are susceptible to a unique infection caused by EEHV.1,2 Since 1983, there have been at least 50 deaths in Asian elephants in North America, Europe, Asia, and Israel caused by this virus.3,4 Asian elephants that died of the virus ranged in age, although juvenile and young adult elephants were most frequently affected. Most elephants were < 7 years old. The EEHV has a tropism for capillary endothelial cells of the heart, liver, and tongue. Affected elephants usually have an acute onset of lethargy with a reluctance to move. Initial clinical signs also include intermittent anorexia, decreased amounts of feces, colic-like behavior, edema of the head, and cyanosis of the tip of the tongue.5 Postmortem findings include extensive myocardial hemorrhages, hydropericardium, and mesenteric and serosal petechiae throughout the peritoneal cavity.1
Although EEHV infections are typically fatal, there are confirmed cases in which elephants have survived infection. In 3 cases reported in the literature, survival was associated with administration of famciclovir.6,7 Dosages (5.5 to 8.0 mg/kg, PO, q 8 h) for the elephants that survived were selected without the benefit of elephant-specific pharmacokinetics and were often a direct allometric extrapolation from recommended dosages for humans (7 mg/kg, PO, q 8 h). One of the treated calves refused oral medication and was treated via rectal administration (10.6 mg/kg, q 12 h), in the absence of prior knowledge about rectal absorption of this drug in elephants.6,7
Following oral administration, famciclovir is rapidly deacetylated and oxidized to penciclovir during first-pass metabolism in the intestinal wall and liver.8 The mechanism of action of penciclovir is similar to that of acyclovir in that it interacts with viral DNA polymerase as a chain terminator.9
To our knowledge, no studies of pharmacokinetics of famciclovir in elephants have been reported. Plasma penciclovir concentrations in elephants peaked at 4.4 μg/mL at 1 hour after oral administration of famciclovir and decreased to undetectable concentrations at 8 hours after oral administration of 6.4 mg of famciclovir/kg.1 These results are analogous to therapeutic concentrations described for humans with genital herpes.10 Dosage regimens for famciclovir in elephants have been selected on the assumption that famciclovir administered to elephants would be absorbed, distributed, metabolized, and excreted in approximately the same manner as in humans. The early clinical success and preliminary plasma penciclovir concentrations were considered in the design of early treatment regi mens.6 In humans, the mean ± SD volume of distribution of penciclovir is 1.08 ± 0.17 L/kg (estimated after IV injection of penciclovir), and mean t1/2 is approximately 2 hours (after oral administration of famciclovir).8,10,11 Following conversion of famciclovir to penciclovir in the liver and small intestines of humans, penciclovir is eliminated primarily by the kidneys.12 The purpose of the study reported here was to estimate pharmacokinetics of famciclovir in juvenile Asian elephants after oral and rectal administration of single doses of famciclovir.
Materials and Methods
Animals
Six young (4.5- to 9-year-old) Asian elephants (5 females and 1 male) that weighed 1,646 to 2,438 kg at the Ringling Brothers Barnum and Bailey Center for Elephant Conservation, Polk City, Fla, were used in the study. Food was not withheld prior to drug administration, and the elephants had ad libitum access to hay and water. Elephants were assessed as healthy on the basis of results of multiple physical examinations, CBCs, and serum biochemical analyses prior to drug administration by the attending veterinarian. Study procedures were approved by the Kansas State University Animal Care and Use Committee.
Study design
Initially, a complete crossover design for 3 treatments was intended for all 6 elephants. However, because these elephants were young, they were occasionally uncooperative. Thus, a complete crossover design was possible for only 3 elephants. The other 3 elephants were each assigned to a treatment group; thus, each treatment group was composed of 4 elephants. Elephants had not been administered famciclovir for a period of at least 12 weeks prior to the study.
Each of the 3 groups received a single dose of famciclovir (5 mg/kg, PO; 5 mg/kg, per rectum; or 15 mg/kg, per rectum). Famciclovir tabletsa were crushed and suspended in an aqueous solution shortly (< 1 hour) before administration, regardless of the route of administration. Oral administration was achieved by injecting the solution in the pharyngeal region (caudal to the base of the tongue) with a 400-mL nylon large animal dosing syringe with a long, curved metal cannula tip. For rectal administration, fecal material was manually removed from the distal aspect of the rectum. The person administering the drug then reached into the rectum (arm-length depth [approx 0.8 m] to deposit the dose.
Blood samples were collected before (time 0) and 0.17, 0.33, 0.67, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, and 24 hours after administration for both 5 mg/kg doses. Blood samples for the 15 mg/kg dose were collected before (time 0) and 0.25, 0.5, 0.67, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, and 24 hours after administration. Blood samples (10 mL) were collected from auricular veins with an angel wing extension set and placed into evacuated tubes containing potassium oxalate and sodium fluoride. Tubes subsequently were centrifuged to obtain plasma.
Drug assay
Famciclovir and penciclovir concentrations were determined in elephant plasma via a validated liquid chromatography–mass spectrometry assay at the Department of Anatomy and Physiology, College of Veterinary Medicine, Kansas State University. Chromatographic and extraction conditions were slightly modified from those described in another study.13 Analytic standards of famciclovirb and penciclovirb were purchased. All other chemicals used were of high-performance liquid chromatography grade.
Samples were quantitated with a standard curve prepared in famciclovir- and penciclovir-naïve elephant plasma via peak-area ratio and linear-correlation weighted 1/x. Concentrations used to create the standard curve ranged from 0.5 to 15 μg/mL. Quality-control samples were prepared with famciclovir- and penciclovir-naïve elephant plasma in bulk at concentrations of 2.5, 6.0, and 12.5 μg/mL; aliquots (500 μL) were frozen at –70°C until used in assays (extracted in duplicate with each assay). Accuracy and precision were ± 15% of actual values, and recovery was > 80% across the range of concentrations of the assay.
Data analysis
Pharmacokinetic variables were determined for each elephant via noncompartmental analysis.14,15,c Parameters calculated for plasma were AUC0–Tlast, Cmax, and Tmax, which were obtained directly from the data. The AUC0–Tlast was calculated from time 0 to the last quantifiable concentration via the linear trapezoidal rule. All values were reported as median, range, and mean ± SD, with the exception of t1/2, which was reported as the harmonic mean.
Results
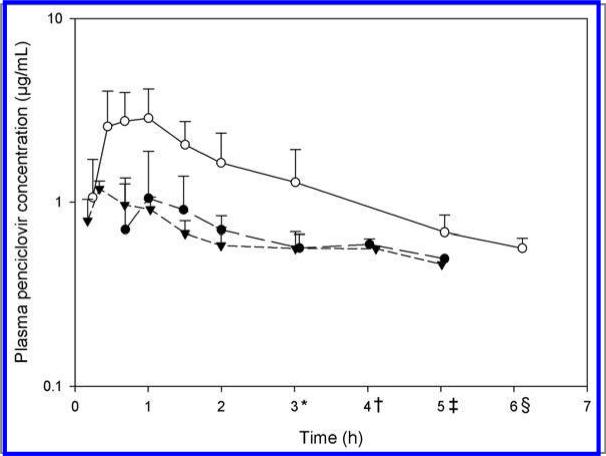
All elephants remained clinically normal during the duration of the study. No apparent adverse effects were detected in any of the 6 elephants. Famciclovir was tolerated well for both routes of administration and underwent rapid biotransformation to the active compound penciclovir (ie, no plasma samples had quantifiable concentrations of famciclovir). After oral administration of famciclovir, we did not detect quantifiable concentrations of penciclovir or famciclovir before 0.67 hours. However, after rectal administration of both doses, penciclovir was quantifiable at the first time point (0.17 and 0.25 hours for 5 and 15 mg/kg, respectively) in all elephants (Figure 1).
Figure 1.
Mean ± SD plasma penciclovir concentrations in young (4.5- to 9-year-old) Asian elephants (Elephas maximus) at various times after oral administration (5 mg/kg [black circles]) and rectal administration (5 mg/kg [black inverted triangles] and 15 mg/kg [white circles]) of famciclovir. Six elephants were included in the study; values reported represent results for 4 elephants/treatment. *Represents results for 3 elephants for the 5 mg/kg oral treatment and 5 mg/kg rectal treatment. †Represents results for 2 elephants for the 5 mg/kg oral treatment and 1 elephant for the 5 mg/kg rectal treatment. ‡Represents results for 1 elephant for the 5 mg/kg oral treatment and 1 elephant for the 5 mg/kg rectal treatment. §Represents results for 1 elephant for the 15 mg/kg rectal treatment.
Plasma penciclovir concentration versus time curves were plotted for the 3 treatments (Figure 1). Pharmacokinetic variables were summarized (Table 1). Only the AUC0–Tlast, which was reported as the percentage of area under the curve extrapolated to infinity, was > 20% in some elephants. After oral administration of a single dose of 5 mg/kg, Cmax was 1.3 μg/mL, with a Tmax of 1.1 hours. The same dose administered rectally resulted in a Cmax of 1.2 μg/mL, with a Tmax of 0.34 hours. Rectal administration of a single dose of 15 mg/kg yielded a t1/2 of 2.6 hours, with a C of 3.6 μg/ml and Tmax of 0.66 hours.
Table 1.
Pharmacokinetic values for penciclovir after oral and rectal administration of a single dose of famciclovir to young (4.5- to 9-year-old) Asian elephants (Elephas maximus).
| Dose and route | Variable | Mean ± SD | Median | Range |
|---|---|---|---|---|
| 5 mg/kg, PO | AUC0–Tlast (h•μg/mL) | 2.20 ± 0.68 | 2.30 | 1.3–2.90 |
| Cmax (μg/mL) | 1.30 ± 0.77 | 1.20 | 0.58–2.30 | |
| Tmax (h) | 1.10 ± 0.63 | 0.84 | 0.68–2.00 | |
| t1/2 (h) | 1.60* | 3.80 | 0.55–7.10 | |
| 5 mg/kg, per rectum | AUC0–Tlast (h•μg/mL) | 2.40 ± 1.10 | 2.10 | 1.30–3.90 |
| Cmax (μg/mL) | 1.20 ± 0.12 | 1.20 | 1.00–1.30 | |
| Tmax (h) | 0.34 ± 0.02 | 0.34 | 0.32–0.35 | |
| t1/2 (h) | 2.20* | 2.50 | 1.50–3.30 | |
| 15 mg/kg, per rectum | AUC0–Tlast (h•μg/mL) | 8.50 ± 3.50 | 8.80 | 4.30–12.00 |
| Cmax (μg/mL) | 3.60 ± 1.40 | 4.10 | 1.60–4.70 | |
| Tmax (h) | 0.66 ± 0.24 | 0.61 | 0.43–0.98 | |
| t1/2 (h) | 2.60* | 2.40 | 2.10–3.60 |
Values reported represent results for 4 elephants/treatment.
Value reported is the harmonic mean.
Discussion
The study reported here provided estimated systemic penciclovir concentrations following administration of a single dose of famciclovir (5 mg/kg orally or 5 and 15 mg/kg rectally) to Asian elephants. Although the oral and rectal routes of administration had similar extents of exposure, the oral route was associated with slightly slower detection of quantifiable systemic concentrations and more variation in plasma concentrations of penciclovir. In part, the latter 2 findings may have been attributable to the fact that oral administration was provided to elephants in the fed state, although low numbers of animals may also have contributed to these findings.
The median AUC0–Tlast of penciclovir was similar after both oral and rectal administration of famciclovir at a dose of 5 mg/kg. The lack of detectible famciclovir concentrations was attributable to the fact that the drug was absorbed well and then rapidly metabolized to penciclovir or that famciclovir was metabolized prior to absorption. The rectal route had faster absorption, with a Tmax ranging from 0.34 to 0.66 hours after rectal administration, compared with 1.1 hours after oral administration. The harmonic mean t1/2 was 1.6 hours and Cmax was 1.3 μg/mL after oral administration of famciclovir to elephants. These results are similar to those reported in humans, in which oral administration of 500 mg (approx 7 mg/kg) of famciclovir yields an apparent t1/2 of approximately 2 hours with a Cmax of 3.3 μg/mL. On the basis of results of the present study and previous reports,1,6,7 a dose of 8 to 15 mg/kg administered orally or rectally every 8 hours to Asian elephants should result in drug concentrations similar to those considered therapeutic in humans. Unfortunately, to the authors’ knowledge, there is currently no information regarding therapeutic plasma concentrations for the treatment of EEHV. Once this information is available, it is likely that dosing recommendations for famciclovir will require adjustment.
The recovery of a young elephant from EEHV infection following rectal administration of famciclovir provided preliminary clinical evidence that there was an alternative to oral administration.6 The present study provided direct evidence that rectally administered famciclovir was efficiently absorbed in elephants. These findings are similar to those of other studies16,17 that confirmed aqueous suspensions of metronidazole and isoniazid are well absorbed when administered rectally to elephants. Analysis of the data in the study reported here indicated that oral administration may result in more pharmacokinetic variation than does rectal administration; however, because of the low numbers of elephants for each treatment, this finding should not be overinterpreted. Additional research is needed to determine whether rectal administration is preferable to oral administration. Having the option to administer famciclovir rectally may be clinically valuable because herpesvirus infections typically affect young elephants, and medicating a poorly trained, sick, and generally uncooperative elephant is a challenge.18 Many healthy elephants are reluctant to voluntarily take medication orally, even when it is mixed in food. Elephants can be trained to accept a gag and to allow administration of medication into the caudal aspect of the oropharynx.18,19 However, even well-trained animals may refuse oral administration of medication when they are sick or anorectic. Thus, rectal administration may offer an important alternative for elephants that are reluctant to accept oral administration of medications or unable to consume the medication because of edema of the face and tongue. Many trained elephants are accustomed to rectal palpation for reproductive evaluations, and even untrained elephants can be safely restrained inside a chute during the procedure.
Allometric scaling is a mathematical conversion based on body mass that uses a dose established for one species to estimate a dose for another species. Allometric scaling of pharmacokinetic variables is of interest to zoological veterinarians because elephants and other large animals are at the high end of the body weight scale, and this method can provide an estimate for designing therapeutic regimens when species-specific pharmacokinetic information is not available. Adhering to the principles of metabolic scaling, extrapolation of a dose for humans would be expected to yield a lower dose for a much heavier elephant. Allometric scaling for conversion of famciclovir administration was used in the treatment of 1 elephant.6 Thus, the human dose of 7 mg/kg was used to estimate a dose of 4.06 mg/kg for that elephant.6 However, not all drugs are scalable.14 Specifically, drugs that are extensively metabolized by the liver are considered poor candidates for allometric scaling because of considerable interspecific heterogeneity in phase I and phase II drug metabolic reactions.14,20,21 On the basis of results of the present study, it appears that famciclovir doses obtained via allometric scaling are not supported by the current data and that famciclovir doses should not be determined via allometric scaling, as was presumed in the case reports.
For the study reported here, we did not detect clinically relevant differences in plasma penciclovir concentrations, compared with the plasma penciclovir concentrations reported for humans after famciclovir administration. The results of the present study support oral or rectal administration of a dose of 8 to 15 mg of famciclovir/kg every 8 hours for the treatment of EEHV in Asian elephants.
Acknowledgments
Supported in part by National Institutes of Health-National Center for Research Resources Clinical and Translational Science Awards to the University of Florida (KL2 RR029888 and UL1 RR029890), Feld Entertainment Inc, and the Kansas State University College of Veterinary Medicine.
The authors thank Gary Jacobson for assistance with drug administration and sample collection.
ABBREVIATIONS
- AUC0–Tlast
Area under the plasma concentration–versus–time curve from 0 to the last quantifiable time point
- Cmax
Maximum plasma concentration
- EEHV
Elephant endotheliotropic herpesvirus
- t1/2
Apparent terminal half-life
- Tmax
Time of maximum plasma concentration
Footnotes
Presented in part as an oral presentation at the Conference of the American Association of Zoo Veterinarians, Minneapolis, October 2003.
Famvir, SmithKline Beecham Pharmaceuticals, Philadelphia, Pa.
Moravek Biochemicals, Brea, Calif.
WinNonlin, version 5.3, Pharsight Corp, Cary, NC.
References
- 1.Richman LK, Montali RJ, Cambre RC, et al. Clinical and pathological findings of a newly recognized disease of elephants caused by endotheliotropic herpesviruses. J Wildl Dis. 2000;36:1–12. doi: 10.7589/0090-3558-36.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Richman LK, Montali RJ, Garber RL, et al. Novel endotheliotropic herpesviruses fatal for Asian and African elephants. Science. 1999;283:1171–1176. doi: 10.1126/science.283.5405.1171. [DOI] [PubMed] [Google Scholar]
- 3.Hayward GS, Latimer E, Richman LK, et al. International Elephant Foundation; Fort Worth, Tex: 2009. Elephant endotheliotrophic herpesvirus progress report 3. [Google Scholar]
- 4.Reid CE, Hildebrandt TB, Marx N, et al. Endotheliotropic elephant herpes virus (EEHV) infection. The first PCR-confirmed fatal case in Asia. Vet Q. 2006;28:61–64. doi: 10.1080/01652176.2006.9695209. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt DL. Proboscidea (elephants). In: Fowler ME, Miller RE, editors. Zoo and wild animal medicine. 5th ed. Saunders; St Louis: 2003. pp. 541–550. [Google Scholar]
- 6.Schmitt DL, Hardy DA, Montali RJ, et al. Use of famciclovir for the treatment of endotheliotrophic herpesvirus infections in Asian elephants (Elephas maximus). J Zoo Wildl Med. 2000;31:518–522. doi: 10.1638/1042-7260(2000)031[0518:UOFFTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Schaftenaar W, Mensink JMCH, de Boer AM, et al. Successful treatment of a subadult Asian elephant bull (Elephas maximus) infected with the endotheliotropic elephant herpes virus. Proceedings. 40th Int Symp Dis Zoo Wild Anim. 2001:141–146. [Google Scholar]
- 8.Simpson D, Lyseng-Williamson KA. Famciclovir: a review of its use in herpes zoster and genital and orolabial herpes. Drugs. 2006;66:2397–2416. doi: 10.2165/00003495-200666180-00016. [DOI] [PubMed] [Google Scholar]
- 9.De Clercq E. Strategies in the design of antiviral drugs. Nat Rev Drug Discov. 2002;1:13–25. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- 10.Gill KS, Wood MJ. The clinical pharmacokinetics of famciclovir. Clin Pharmacokinet. 1996;31:1–8. doi: 10.2165/00003088-199631010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Novartis Pharmaceuticals Corp. [Jan 1, 2011];FAMVIR (famciclovir) tablets: prescribing information. Available at: www.pharma.us.novartis.com/product/pi/pdf/Famvir.pdf.
- 12.Boike SC, Pue MA, Freed MI, et al. Pharmacokinetics of famciclovir in subjects with varying degrees of renal impairment. Clin Pharmacol Ther. 1994;55:418–426. doi: 10.1038/clpt.1994.51. [DOI] [PubMed] [Google Scholar]
- 13.Fowles SE, Pierce DM. High-performance liquid chromatographic method for the determination of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL-39123) in human plasma and urine. Analyst. 1989;114:1373–1375. doi: 10.1039/an9891401373. [DOI] [PubMed] [Google Scholar]
- 14.Riviere JE. Comparative pharmacokinetics: principles, techniques, and applications. Iowa State University Press; Ames, Iowa: 1999. [Google Scholar]
- 15.Gibaldi M, Perrier D. Pharmacokinetics. Marcel Dekker Inc; New York: 1982. [Google Scholar]
- 16.Gulland FM, Carwardine PC. Plasma metronidazole levels in an Indian elephant (Elephas maximus) after rectal administration. Vet Rec. 1987;120:440. doi: 10.1136/vr.120.18.440. [DOI] [PubMed] [Google Scholar]
- 17.Maslow JN, Mikota SK, Zhu M, et al. Population pharmacokinetics of isoniazid in the treatment of Mycobacterium tuberculosis among Asian and African elephants (Elephas maximus and Loxodonta africana). J Vet Pharmacol Ther. 2005;28:21–27. doi: 10.1111/j.1365-2885.2004.00619.x. [DOI] [PubMed] [Google Scholar]
- 18.Isaza R, Hunter RP. Drug delivery to captive Asian elephants—treating Goliath. Curr Drug Deliv. 2004;1:291–298. doi: 10.2174/1567201043334641. [DOI] [PubMed] [Google Scholar]
- 19.Mikota S, Larsen RS, Montali R. Tuberculosis in elephants in North America. Zoo Biol. 2000;19:393–403. [Google Scholar]
- 20.Mahmood I, Martinez M, Hunter RP. Interspecies allometric scaling. Part I: prediction of clearance in large animals. J Vet Pharmacol Ther. 2006;29:415–423. doi: 10.1111/j.1365-2885.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- 21.Hunter RP, Isaza R. Concepts and issues with interspecies scaling in zoological pharmacology. J Zoo Wildl Med. 2008;39:517–526. doi: 10.1638/2008-0041.1. [DOI] [PubMed] [Google Scholar]