Abstract
Slow-release, IL-1-impregnated pellets implanted in rat cerebral cortex to simulate chronic overexpression of IL-1 significantly increased relative tissue levels of tau mRNA and of tau immunoreactivity in neuronal cell bodies and in swollen dystrophic neurites that also overexpressed phosphorylated and nonphosphorylated neurofilament epitopes. In addition, rats with IL-1-impregnated pellets, but not control rats or those with sham pellets, showed focal immunoreactivity for hyperphosphorylated tau epitopes present in paired helical filaments. Our results are consistent with an important driving role for IL-1 in the pathogenesis of Alzheimer-type neuronal and neuritic changes.
Keywords: hyperphosphorylation, interleukin-1, neurofibrillary tangles, neurofilaments, tau
Neurofibrillary changes, in the form of neuritic plaques, neuropil threads, and neurofibrillary tangles, are key histological features of Alzheimer’s disease. These changes are characterized by alterations in neuronal and neuritic cytoskeletal elements, including neurofilaments, microtubules, and microtubule-associated proteins. Tau is one such microtubule-associated protein that promotes microtubule assembly, stabilizes growing axons, and is a major constituent of the paired helical filaments (PHFs) that are present in neurofibrillary tangles and other neurofibrillary alterations of Alzheimer’s disease (3, 11). These neurofibrillary alterations are also characterized by abnormal hyperphosphorylation of both neurofilaments and of tau protein (17). Overexpression of interleukin-1 (IL-1), a pleiotropic, proinflammatory cytokine (2, 14) is a prominent and consistent feature of Alzheimer’s disease (4, 6, 7, 12), and this overexpression is intimately related to neurofibrillary alterations both within β-amyloid plaques (4) and within tangle-bearing neurons (15) in Alzheimer’s disease. In this study, we achieved chronic elevations of intracerebral IL-1 levels by means of implanted, slow-release pellets impregnated with IL-1. Using this model, we provide evidence for an important driving role for IL-1 in the pathogenesis of Alzheimer-type neuronal and neuritic changes.
Pellets impregnated with IL-1β (100 ng of recombinant mouse IL-1β (Sigma Chemical Co., St. Louis)) and control pellets (without IL-1β impregnation) were obtained from Innovative Research of America (Sarasota, FL). These pellets were 1.5 mm in diameter and designed for controlled, slow release of IL-1 over a 21-day period. Forty male Sprague–Dawley rats, weighing 264 ± 6 g, were randomly assigned to three groups. Sixteen rats received implants of IL-1β-containing pellets (IL-1), 14 rats received pellets without interleukin-1 impregnation (sham), and 10 rats served as unoperated (normal) rats. The pellets were implanted 2.8 mm caudal to bregma; 4.5 mm right of the midline, and 2.5 mm deep to the pial surface. Twenty-one days following pellet implantation, rat brains were processed either for immunohistochemistry or for mRNA analysis, as previously described (1, 5, 10).
Immunohistochemical labeling was performed on paraffin tissue sections as previously described (5). Primary antibodies SMI 32 and SMI 31 are monoclonal antibodies (Sternberger-Monoclonals Inc., MD) that recognize nonphosphorylated and phosphorylated epitopes, respectively, of 200-kDa neurofilament sub-units. Monoclonal anti-Tau antibody (Zymed Laboratories Inc., South San Francisco, CA) recognizes a rat tau epitope, independent of phosphorylation state. Polyclonal anti-PHF antibody (ICN Biomedicals Inc., OH) recognizes hyperphosphorylated tau, immunoreacting with neurofibrillary tangles in neurons and neurites. Immunoreactivity, using the above antibodies, in hippocampal tissue sections was analyzed using a CCD video camera with a PowerMac computer. For statistical comparisons of protein expression, immunoreactive area × mean immunoreactive intensity was computed in five fields (0.18 mm2/field) of hippocampus adjacent to the implant site or in an analogous region of contralateral hippocampus or of normal rats.
Reverse transcription of 1-μg samples of total RNA extracted from a 5-mm cube of cerebral tissue from the midline, including the implant site, was performed using an Advantage RT-for-PCR kit (Clontech, Palo Alto, CA), according to the manufacturer’s instructions. Amplification was performed using Advantage cDNA polymerase mix (Clontech). For tau, the forward primer was 5′ AGA CCA CAC CCA GCC CAA AGA CTC C 3′, and the reverse primer was 5′ GGC CAA AGA GGC GGA CAC TTC ATC 3′, creating a 791-bp amplimer (1). For glyceraldehyde-3-phosphate dehydrogenase (G3PDH) amplification, the forward primer was 5′ ACC ACA GTC CAT GCC ATC AC 3′, and the reverse primer was 5′ TCC ACC ACC CTG TTG CTG TA 3′, creating a 452-bp amplimer (Ransom Hill Bioscience, Inc., Ramona, CA). Equal volumes of reaction mixture from each sample were loaded onto 1.5% agarose gels, and fluorescent images were digitally captured for analysis. For semiquantitative estimates of the levels of tau mRNA, tau signal was normalized relative to signal from corresponding G3PDH mRNA using NIH Image 1.6 software.
Statistical significance of differences in protein and mRNA expression between IL-1 pellet-implanted rats, inert pellet-implanted controls (sham), and unoperated controls (normal) was assessed using ANOVA followed by Fisher’s test.
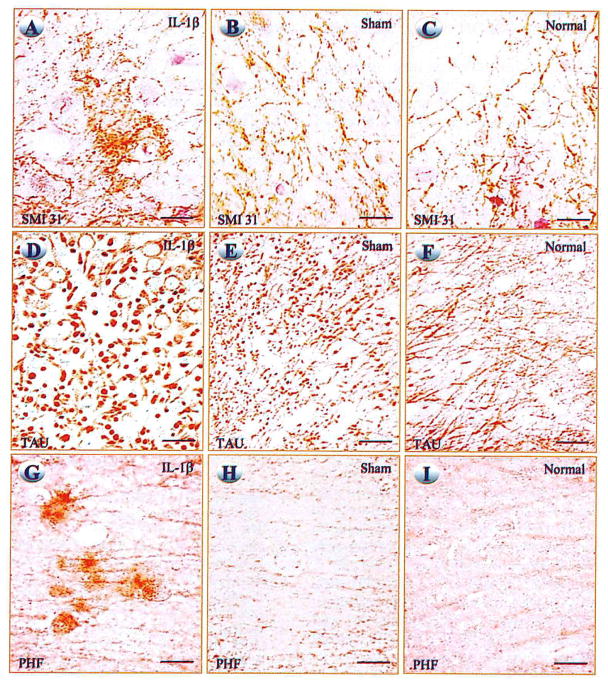
Neurons and neurites in CA2 and CA3 regions of hippocampus of rats receiving cerebral IL-1β pellet implants showed greater phosphorylated neurofilament immunoreactive intensity, and the neurites appeared to be larger than those in sham and normal rats (Figs. 1A–1C). Quantification of immunoreactivity showed increases of 2.5- to 4-fold for phosphorylated neurofilament antigen (P < 0.005) and 4-fold for non-phosphorylated neurofilament antigen (P < 0.03), relative to either values from sham or normal rats. Neuronal cell bodies in CA2 and CA3 of IL-1 pellet-implanted rats showed increased immunoreactivity for tau protein, and surrounding neurites were enlarged and intensely tau-immunoreactive (Figs. 1D–1F). Tau immunoreactive intensities were significantly increased in both ipsilateral (3.3-fold, P < 0.05) and contralateral (2.8-fold, P < 0.05) hippocampus of IL-1 pellet-implanted rats, relative to values for either sham or normal rats. Rats receiving IL-1 pellets also had higher cerebral levels of tau mRNA (relative to G3PDH mRNA) both ipsilateral and contralateral to pellet implantation, relative to values from corresponding regions of either sham (2.0-fold or better, P < 0.001) or normal (2.3-fold or better, P < 0.0005) rats. PHF immunoreactivity was apparent within both neuronal cell bodies and neurites in both ipsilateral and contralateral hippocampus of IL-1 pellet-implanted rats, but not in sham or normal rats (Figs. 1G–1I).
FIG. 1.
Photomicrographs of hippocampus showing immunoreactivity for phosphorylated neurofilament (SMI-31; A–C), for tau (D–F), and for paired helical filament (PHF; G–I) in rats carrying IL-1β pellets (A, D, G), sham (inert) pellets (B, E, H), and normal (unoperated) (C, F, I) rats. Phosphorylated neurofilament, Tau, and PHF protein immunoreactivity is increased in perikarya and processes of neurons in hippocampus of IL-1β pellet-implanted rats. Bars, 15 μm.
We have previously shown IL-1-induced increases in neuronal expression of βAPP and astrocytic expression of S100β in vivo, using an IL-1 injection technique (16). However, the transient nature of the increases in IL-1 levels using this technique may be of relatively limited value in assessing neuropathological consequences arising from chronic IL-1 overexpression, such as that noted in neurodegenerative diseases (6). In contrast, the pellet implantation technique used here provides chronically elevated intracerebral levels of cytokine (10) and thus provides a model for the chronic IL-1 elevations seen in Alzheimer’s disease and Down’s syndrome (7).
IL-1 is known to increase phosphorylation of proteins in several cell types, including olfactory neuroepithelial cells grown from biopsies of human donors (18). The IL-1 signal transduction pathway is thought to involve activation of protein kinases (8, 13) and possibly inhibition of a protein phosphatase (9). We have shown early and progressive association of IL-1-immunoreactive microglia with β-amyloid deposits in Alzheimer’s disease, suggesting a role for this neurotrophic cytokine in the pathogenesis of dystrophic neurites, containing hyperphosphorylated tau, in these plaques (4). We have also described progressive association of IL-1-immunoreactive microglia with evolving tau-containing neurofibrillary tangles in Alzheimer’s disease (15). These results, together with the effects of IL-1 on expression of hyperphosphorylated tau, shown here, suggest an important role for IL-1 in the pathogenesis of these characteristic and diagnostic neurofibrillary changes in Alzheimer’s disease. These results demonstrate that IL-1 can induce expression of proteins associated with neurodegenerative events and are consistent with the idea (6, 12) that the chronic, sustained overexpression of IL-1 drives a cascade of cellular and molecular events that culminates in the neurofibrillary changes of Alzheimer’s disease.
Acknowledgments
The authors thank Sue Woodward and Xue Quan Zhou for skilled technical assistance and Pam Free for secretarial support. This research was supported in part by NIH AG 10208 and NIH AG 12411.
References
- 1.Chambers CB, Muma NA. Tau mRNA isoforms following sciatic nerve axotomy with and without regeneration. Mol Brain Res. 1997;48:115–124. doi: 10.1016/s0169-328x(97)00089-2. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 3.Feany MB, Dickson DW. Neurodegenerative disorders with extensive tau pathology: A comparative study and review. Ann Neurol. 1996;40:139–148. doi: 10.1002/ana.410400204. [DOI] [PubMed] [Google Scholar]
- 4.Griffin WST, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease: Significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Griffin WST, Sheng JG, Mrak RE. Senescence-accelerated overexpression of S100β in brain of SAMP6 mice. Neurobiol Aging. 1998;19:71–76. doi: 10.1016/s0197-4580(97)00167-x. [DOI] [PubMed] [Google Scholar]
- 6.Griffin WST, Sheng JG, Royston MC, Gentleman SM, Graham DI, Roberts GW, Mrak RE. Glial-neuronal interactions in Alzheimer’s disease: The potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin WST, Stanley LC, Ling C, White L, Macleod V, Perrot LJ, White CL, III, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Z, Tetsuka T, Baier LD, Morrison AR. Interleukin-1 beta activates c-jun NH2-terminal kinase subgroup of mitogen-activated protein kinases in mesangial cells. Am J Physiol. 1966;270:F634–F641. doi: 10.1152/ajprenal.1996.270.4.F634. [DOI] [PubMed] [Google Scholar]
- 9.Guy GR, Cairns J, Ng SB, Tan YH. Inactivation of a redox-sensitive protein phosphatase during the early events of tumor necrosis factor/interleukin-1 signal transduction. J Biol Chem. 1993;268:2141–2148. [PubMed] [Google Scholar]
- 10.Li Y, Liu L, Kang J, Sheng JG, Barger SW, Mrak RE, Griffin WST. Neuronal-glial interactions mediated by interleukin-1 enhance neuronal acetylcholinesterase activity and mRNA expression. J Neurosci. 2000;20:149–155. doi: 10.1523/JNEUROSCI.20-01-00149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Watanabe A, Titani K, Ihara Y. Hyperphosphorylation of tau in PHF. Neurobiol Aging. 1995;16:365–371. doi: 10.1016/0197-4580(95)00027-c. [DOI] [PubMed] [Google Scholar]
- 12.Mrak RE, Sheng JG, Griffin WST. Glial cytokines in Alzheimer’s disease. Review and pathogenic implications. Hum Pathol. 1995;226:816–823. doi: 10.1016/0046-8177(95)90001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Neill LAJ. Towards an understanding of the signal transduction pathways for interleukin 1. Biochim Biophys Acta. 1995;1266:31–44. doi: 10.1016/0167-4889(94)00217-3. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell NJ. Functions and mechanisms of interleukin 1 in the brain. Trends Pharmacol Sci. 1991;12:430–436. doi: 10.1016/0165-6147(91)90623-z. [DOI] [PubMed] [Google Scholar]
- 15.Sheng JG, Mrak RE, Griffin WST. Glial-neuronal interactions in Alzheimer’s disease: Progressive association of IL-1α+ microglia and S100β+ astrocytes with neurofibrillary tangle stages. J Neuropathol Exp Neurol. 1997;56:285–290. [PubMed] [Google Scholar]
- 16.Sheng JG, Ito K, Skinner RD, Mrak RE, Rovnaghi CR, Van Eldik LJ, Griffin WST. In vivo and in vitro evidence supporting a role for the inflammatory cytokine interleukin-1 as a driving force in Alzheimer pathogenesis. Neurobiol Aging. 1996;17:761–766. doi: 10.1016/0197-4580(96)00104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su JH, Cummings BJ, Cotman CW. Plaque biogenesis in brain aging and Alzheimer’s disease. I. Progressive changes in phosphorylation states of paired helical filaments and neurofilaments. Brain Res. 1996;793:79–87. doi: 10.1016/s0006-8993(96)00811-6. [DOI] [PubMed] [Google Scholar]
- 18.Vawter MP, Basaric-Keys J, Li Y, Lester DS, Lebovics RS, Lesch KP, Kulaga H, Freed WJ, Sunderland T, Wolozin B. Human olfactory neuroepithelial cells: Tyrosine phosphorylation and process extension are increased by the combination of IL-1β, IL-6, NGF, and bFGF. Exp Neurol. 1996;142:179–194. doi: 10.1006/exnr.1996.0189. [DOI] [PubMed] [Google Scholar]