Abstract
Background and Purpose
Deferoxamine reduces neuronal death in a piglet model of intracerebral hemorrhage (ICH). This study examined the effect of deferoxamine on perihematomal white matter edema in piglets.
Methods
ICH was induced by an injection of autologous blood into the right frontal lobe of piglets. In the first part of study, the time course of edema formation was determined. In the second part, the effects of deferoxamine on ICH-induced white matter edema, tumor necrosis factor-alpha (TNF-α) and receptor-interacting protein kinase 1 (RIPK1) were examined.
Results
ICH resulted in marked brain edema and increased TNF-α and RIPK1 levels in white matter. Systemic treatment with deferoxamine markedly reduced white matter TNF-α and RIPK1 levels and attenuated white matter edema after ICH.
Conclusions
Deferoxamine reduces white matter edema and TNF-α and RIPK1 levels after ICH in piglets suggesting deferoxamine is a potential effective therapeutic agent for ICH patients.
Keywords: brain edema, cerebral hemorrhage, deferoxamine, TNF-alpha, receptor-interacting protein kinase 1 (RIPK1), piglets
Introduction
Intracerebral hemorrhage (ICH)-induced white matter injury is an important factor in patient outcomes. Approximately 50% human brain tissue is white matter. However, white matter injury after ICH has not been well studied.
Iron has a key role in brain edema formation following ICH 1. Perihematomal brain edema develops immediately after an ICH and peaks several days later. Edema formation following ICH elevates intracranial pressure and may result in herniation 2. Perihematomal edema is mainly located within white matter. However, the role of iron in ICH-induced white matter edema has not been studied.
ICH causes brain cell death including necrosis/necroptosis, apoptosis and autophagy1. Necroptosis, also called programmed necrosis, is associated with the kinase activities of receptor-interacting protein kinase 1 (RIPK1). Tumor necrosis factor-alpha (TNF-α), via activation of RIPK1, can induce necroptosis3. Necroptosis contributes to brain injury in both ischemic and hemorrhagic stroke 3, 4.
In the present study, we examined the time course of white matter edema formation and the effect of deferoxamine on perihematomal white matter edema in the piglet model of ICH. The effects of deferoxamine on brain TNF-α and RIPK1 were also examined.
Materials and Methods
Animal preparation and intracerebral blood infusion
The animal protocols for this study were approved by the University of Michigan Committee on the Use and Care of Animals. Male piglets, body weight of 8 to 10 kg, were obtained from Michigan State University (East Lansing, MI). All surgical procedures were performed with the use of aseptic techniques. Pigs were sedated with Telazol (6 mg/kg, I.M.) and Xylazine (2.2 mg/kg, I.M.) for induction of anesthesia and endotracheal intubation. Piglets were inhaled with 2.0% isoflurane delivered via nose cone. The isoflurane concentration was maintained at 1.0–1.5% during the surgical procedures. Core temperature was maintained at 37.5 ± 0.5°C by a warm water blanket (Gaymar, Orchard Park, NY). The right femoral artery was inserted with a polyethylene catheter (PE-160) to obtain blood for injection and to monitor arterial blood pressure and arterial blood gases.
A 1.5 mm cranial burr hole was drilled at a point 11mm right of the sagittal suture and 11mm anterior to the coronal suture. An 18.5 mm long 18-gauge sterile plastic catheter was placed stereotaxically into the center of the right frontal cerebral white matter at the level of the caudate nucleus. First, 1 ml of autologous arterial blood was infused over 10 minutes with an infusion pump. Then, a second 1.5 ml blood was infused over 10 minutes after a 5 minutes break. Sham-operated pigs had the same procedure except blood injection5.
There were two parts to this study using a total of 51 piglets. First, piglets had two injections of total 2.5ml autogolous blood injected into the right front lobe and were euthanized at days 1, 3, 7 and 14 (n=4 to 6) after the brains had been frozen in situ. Control piglets had a needle insertion (n=4) and were euthanized at day 3. Second, piglets had an ICH or a sham operation, and were treated with vehicle (saline, n=9 for ICH and n=3 for sham) or DFX (50 mg/kg, i.m. at 2 hours post ICH and given every 4 h for two doses and then every 12 h up to 72 hours, n=10 for ICH, n=3 for sham) for 3 days. Brains were used to determine brain water content and Western blot analyses.
Brain in situ freezing and sampling
Brains were frozen in situ by decanting liquid nitrogen into a 12 oz. bottomless foam cup adhered to the head with Dow-Corning high vacuum grease (Dow Corning, Midland, MI), as described previously 6. The duration time of this process usually took about 1 hour and the head was removed after the injection of potassium chloride to stop the heart beating. The frozen head was then cut with a band saw into 5 mm thick coronal sections. Tissues were sampled from the points of interest.
Brain water content
White matter tissue adjacent to the hematoma including superficial gyral white matter and deep white matter was sampled. Sampled brain tissue was placed on foils and weighed on an electronic analytical balance (model AE 100, Mettler Instrument Co.) to obtain the wet weight (WW). Samples were then dried in a gravity oven at 100°C for 24 hours to obtain the dry weight (DW). Brain water content (%) was calculated as ((WW-DW)/WW)*100 %.
Western blotting
White matter tissue adjacent to the hematoma was sampled for Western blot analysis which was performed as previously described 7. The primary antibodies were mouse anti-TNF-α (1:1,000 dilution, AbDserotec), mouse anti-RIPK1 (1:1,000 dilution, R&D) or mouse anti-beta-actin (1:10,000 dilution, Sigma). The secondary antibody was anti-mouse IgG HRP-conjugated (Bio-Rad).
Statistics
All data in this study are presented as mean ± SD. Data were analyzed by analysis of variance (ANOVA) followed by Scheffe’s post hoc test or Student’s t-test. Significant difference was set at p<0.05 levels.
Results
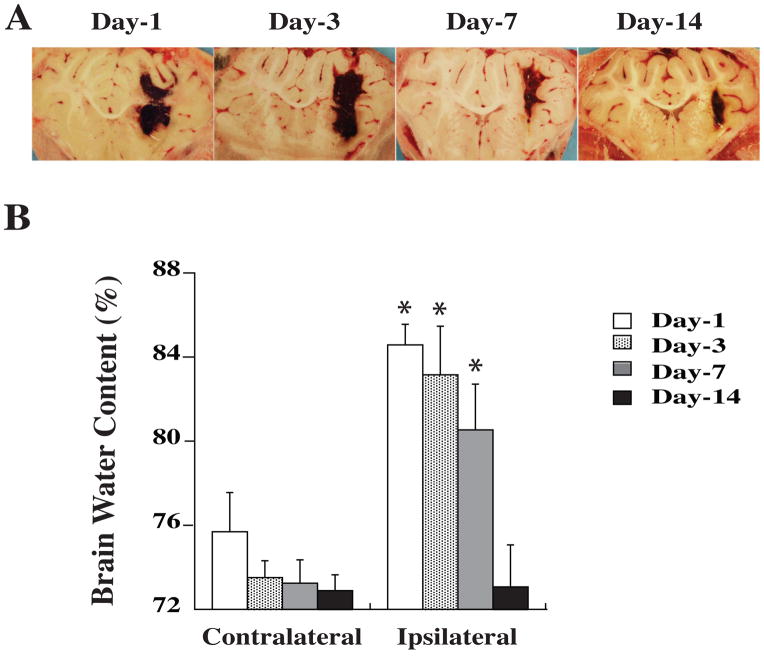
In the time course study, we found that marked brain edema was observed on the first day after whole blood injection. Perihematomal edema was still severe at day 3, declined at day 7 and was resolved at day 14 (Figure 1). Thus, we tested DFX’s effects on brain edema at day 3 since severe brain edema at day 1 after ICH results from clot retraction and thrombin formation.
Figure 1.
Hematoma resolution (A) and brain water content in white matter (B) at different times after ICH. Values are mean ± SD. *p<0.05 vs. the contralateral side.
Intracerebral infusion of 2.5 ml blood caused a marked increase in perihematomal white matter water content (83.2±2.3 vs. 73.5 ± 0.8% in the contralateral side, p<0.05). Systemic administration of 50 mg/kg deferoxamine (i.m.) significantly reduced edema (80.7 ± 2.7 vs. 83.2 ± 2.3% in vehicle-treated group, p<0.05, Figure 2). In addition, deferoxamine treatment (50 mg/kg, i.m.) did not change brain water content in sham-operated piglets (white matter: 74.2 ± 1.8 vs. 73.9 ± 0.4% in vehicle-treated group, n=4, p>0.05).
Figure 2.
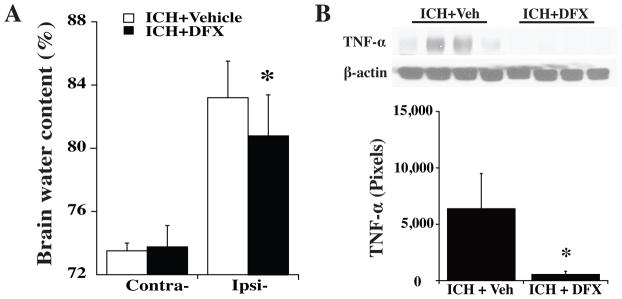
(A) Brain water content in white matter 3 days after ICH with DFX or vehicle (Veh) treatment. (B) TNF-α levels in white matter 3 days after ICH with DFX or vehicle treatment. Values are mean ± SD. *p<0.05 vs. vehicle treatment.
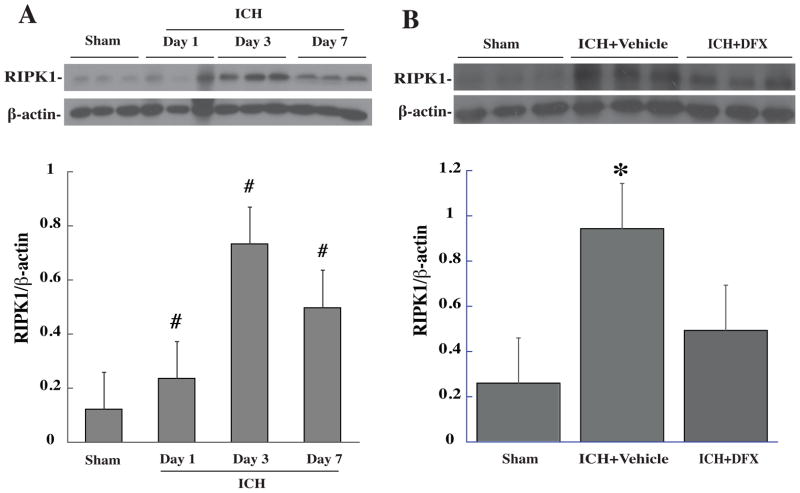
Little TNF-α expression was found in the white matter after needle insertion. We measured TNF-alpha in the white matter at days 1, 3 and 7 following ICH and found that TNF-alpha levels increased significantly at day 1, peaked at day 3 (a 3-fold increase, p<0.05 vs. sham) and then decreased at day 7 after ICH. At day 3, DFX reduced white matter TNF-α levels after ICH (560 ± 279 vs. 6364 ± 3156 pixels in vehicle, *p<0.05, Figure 2). ICH also resulted in RIPK1 upregulation in white matter, which peaked at day 3 after hemorrhage. Systemic treatment of DFX reduced white matter RIPK1 levels after ICH (Figure 3).
Figure 3.
(A) Time course of RIPK1 in white matter after ICH. Values are mean ± SD. #p<0.05 vs. sham. (B) White matter RIPK1 levels after ICH with DFX or vehicle treatment. Values are mean ± SD. *p<0.05 vs. the other groups.
Discussion
The major findings of present study are: 1) DFX attenuated ICH-induced white matter edema; 2) DFX reduced white matter TNF-α levels after ICH; and 3) RIPK1 levels in white matter were upregulated following ICH and reduced by DFX treatment.
Iron has a major role in brain damage following ICH. Brain iron overload causes brain edema in the acute phase. We have demonstrated that the iron chelator, deferoxamine, reduces ICH-induced brain edema and neurological deficits in young 8 and aged rats 9. The dosage and therapeutic time point used in this study for deferoxamine were based on an earlier report in aged rats 9. Clinical data also suggest a role for iron in ICH-induced brain injury. For example, clot lysis is associated with perihematomal edema development. Recent studies show that high levels of serum ferritin, an iron storage protein, are independently associated with poor outcome and severe brain edema in ICH patients 10. Our recent study found that DFX reduces neuronal death in the piglet model of ICH 5. Here, we demonstrated that DFX reduces ICH-induced white matter edema.
Both TNF-α and RIPK1 levels were increased in white matter after ICH. TNF-α is a major pro-inflammatory cytokine. ICH causes significant increases of brain TNF-α in rats, mice and pigs7. Our unpublished data showed that iron can upregulate TNF-α levels in the brain. TNF-α can also induce necroptosis through RIPK13. We have found that DFX reduces TNF-α and RIPK1 levels in white matter after ICH. Necroptosis has been found in ICH4 and future studies should determine whether or not DFX can reduce ICH-induced necroptosis.
Acknowledgments
Funding Sources
Supported by grants NS-052510, NS-073595, NS-079157 and NS-084049 from NIH.
Footnotes
Disclosure
None.
References
- 1.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurology. 2012;11:720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. New England Journal of Medicine. 1986;314:953–958. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- 3.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 4.Zhu X, Tao L, Tejima-Mandeville E, Qiu J, Park J, Garber K, et al. Plasmalemma permeability and necrotic cell death phenotypes after intracerebral hemorrhage in mice. Stroke. 2012;43:524–531. doi: 10.1161/STROKEAHA.111.635672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–2243. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE. Early metabolic alterations in edematous perihematomal brain regions following experimental intracerebral hemorrhage. J Neurosurg. 1998;88:1058–1065. doi: 10.3171/jns.1998.88.6.1058. [DOI] [PubMed] [Google Scholar]
- 7.Hua Y, Wu J, Keep R, Nakamura T, Hoff J, Xi G. Tumor necrosis factor-alpha increases in the brain following intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006;58:542–550. doi: 10.1227/01.NEU.0000197333.55473.AD. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Keep R, Hua Y, Schallert T, Hoff J, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100:672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- 9.Okauchi M, Hua Y, Keep RF, Morgenstern LB, Xi G. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke. 2009;40:1858–1863. doi: 10.1161/STROKEAHA.108.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehdiratta M, Kumar S, Hackney D, Schlaug G, Selim M. Association between serum ferritin level and perihematoma edema volume in patients with spontaneous intracerebral hemorrhage. Stroke. 2008;39:1165–1170. doi: 10.1161/STROKEAHA.107.501213. [DOI] [PubMed] [Google Scholar]