Summary
An antibody raised against isolated paired helical filaments (PHF) was used to identify tangle-bearing (PHF+) neurons in autopsy brain tissue from six Alzheimer disease (AD) patients and six age-matched controls (AMC). A comparison of the levels of polyadenylated messenger RNA [poly(A)+ mRNA] in PHF+ and PHF− neurons of similar cross-sectional area in temporal and parietal lobe and cerebellum from four AD and four AMC brains was made by analysis of in situ hybridization of [3H] polyuridylate [poly(U)] to intracellular poly(A)+ mRNA. In PHF+ neurons, the level of poly(A)+. mRNA was approximately two-thirds that in similar-sized PHF− neurons in either AD or AMC. The level of poly(A)+ mRNA in PHF− neurons in regions of the brain that have more of the histopathologically defined effects in AD was similar to that in regions with less effects.
Keywords: Paired helical filament, Tangle-bearing neurons, Alzheimer disease, Polyadenylated messenger RNA
The intracellular accumulation of neurofibrillary tangles in certain cortical and subcortical neurons and the presence of neuritic plaques with β-amyloid cores are characteristic of the neuropathology of Alzheimer disease (AD) (Khatchaturian, 1985). Morphologically similar neural lesions appear with age, in older individuals with Down syndrome (Ellis et al., 1974; Ball, 1977; Wisniewski et al., 1985; Mann, 1988; Rumble et al., 1989; Woody et al., 1989), and certain other neurodegenerative diseases (Wisniewski et al., 1979). Neurofibrillary tangles are composed of paired helical filaments (PHF) (Kidd, 1963; Wisniewski et al., 1976) and accumulate in the cytoplasm of neurons that eventually die, leaving only the tangles referred to as “ghost” tangles (Rasool et al., 1984). It seems obvious that the presence of PHF in a cell would have detrimental effects on its function, perhaps including protein synthesis. The purpose of the present study was to determine whether such effects include compromises in the levels of total mRNA in PHF-containing neurons.
One intracellular constituent affected by PHF accumulation is ribosomal RNA (rRNA). In many brain regions, the level of rRNA, assessed by nucleolar volume assay, is lower in tangle-bearing neurons than in tangle-free neurons of the same size class (Dayan and Ball, 1973; Mann et al., 1981a; Mann and Yates, 1981). The amount of total intracellular RNA in tangle-free neurons of prefrontal cortex in AD is similar to that in the same regions of brain from controls (Uemura and Hartman, 1978), whereas neurons with tangles have approximately one-half the RNA content of tangle-free neurons (Uemura and Hartman, 1979). Some reports show that only tangle-bearing neurons are depleted of RNA in AD (Dayan and Ball, 1973) while others report variable RNA losses (Mann et al., 1977). Using biopsy tissue, decreases in nucleolar volume in all cortical neurons have been demonstrated in AD (Mann et al., 1981a,b; Mann and Yates, 1981). The use of biopsy material rather than postmortem material may have eliminated potential artifacts that result in selective postmortem RNA degradation in AD tissue (Ilaria et al., 1985; Morrison et al., 1986, 1987a,b; Morrison, 1988).
Recently, the total RNA content within neurons in different brain regions of AD and AMC brains has been directly measured. Although the number of neurons containing tangles in different hippocampal regions does not correlate with the extent of regional RNA loss, azure B RNA staining shows that the total RNA content of hippocampal pyramidal cells is less in AD than in controls (Doebler et al., 1987a). Variable degrees of RNA loss have also been detected in neurons in regions other than hippocampus in AD brain (Doebler et al., 1987b). Using azure B staining, Neary and associates (1986) found less RNA in pyramidal cells of layers III (22%) and V (25%) in biopsies from AD cortex compared to control, indicating a strong correlation between neurofibrillary tangle frequency and lower levels of RNA. In addition, AD hippocampus cells containing lower levels of RNA contain Alz-50 immunoreactive product (Rhoads et al., 1987; Wolozin et al., 1986), suggesting that RNA levels decrease as a consequence of pathological processes in neurons in AD.
Total RNA can be isolated from postmortem AD and control brain, and the levels of specific mRNAs can be determined using either in vitro translation (Gilbert et al., 1981; Marotta et al., 1981; Morrison and Griffin, 1981; Morrison et al., 1983; Ilaria et al., 1985) or hybridization (Johnson et al., 1986). An overall decrease in total RNA and/or mRNA content has been reported in AD cortex (Sajdel-Sulkowska et al., 1983; Guillemette et al., 1986; Taylor et al., 1986, 1987; McLachlan et al., 1988), suggesting that the RNA content of several cell types in these regions in AD brain is decreased. We and others have reported that there is little or no difference in mRNA levels in corresponding regions of AD and control brain (Morrison et al., 1986, 1987a,b; Johnson and Finch, 1987; May et al., 1987; Morrison, 1988).
To characterize AD neuropathology at the molecular level and provide baseline information necessary for the measurement of the relative levels of specific mRNAs in different brain regions and cell types, we combined two techniques—immunohistochemistry, using an antibody raised against PHF, and in situ hybridization, using radiolabeled polyuridylate, i.e., [3H]poly(U). Two questions were addressed: (a) is the presence of PHF correlated with alterations in the levels of total hybridizable poly(A)+ mRNA in neurons of AD cortex; and (b) is the intracellular content of poly (A)+ mRNA in cortical and cerebellar neurons that do not contain PHF similar in AD and age-matched controls (AMQ)?
MATERIALS AND METHODS
Tissue Preparation
Brain tissue was collected at autopsy from six clinically diagnosed AD patients (mean age of 75 years, range of 63 to 86 years) and six AMC (mean age of 71 years, range of 56 to 94 years). Postmortem intervals of AD tissue ranged from 4 to 52 h (mean interval of 19.5 h) and of AMC from 12.5 to 83 h (mean interval of 27.7 h). The brains were fixed in 10% neutral buffered formalin and routinely processed for paraffin embedding. The clinical diagnosis of AD (McKhann et al., 1984) was confirmed by neuropathological examination (Khachaturian, 1985) of silver-stained tissue sections (Sevier and Munger, 1965); the presence of neurofibrillary tangles in the hippocampus and seven or more neuritic plaques per 1.4 mm diameter 100× microscopic field in affected neocortex (Tomlinson et al., 1970) was confirmatory of AD. The number of plaques and tangles in all brain areas was negligible in each AMC. For immunohistochemical and in situ hybridization analyses, paraffin blocks containing superior temporal cortex, parietal cortex, and cerebellum were sectioned at 10 µm and mounted on gelatin-coated microscope slides. These brain regions were chosen because they have previously been shown to have different degrees of PHF accumulation (Hirano and Zimmerman, 1962; Hyman et al., 1984).
Combined In Situ Hybridization and Immunohistochemistry
Paraffin-embedded human brain tissue sections were rehydrated and processed for in situ hybridization followed by immunohistochemistry using modifications of procedures that we have previously described (Griffin, 1987, 1988).
In Situ Hybridization
Each section was permeabilized with 0.2 N HCl, 0.1% Triton X100, and 40 µg/ml of proteinase K, acetylated with acetic anhydride, hybridized with 0.9 µCi of [3H]poly(U) (New England Nuclear, Boston, MA) for 1 h at 50°C, treated with 50 µg of ribonuclease A in 4 × SSC (1 × SSC = 0.15 M NaCl; 0.05 M Na citrate, pH 8.0) for 30 min at 37°C to degrade single-stranded RNA, and washed in two changes, 20 min each, of 2 × SSC, containing 0.1% Triton X100, followed by 30 min in 1 × SSC at room temperature. As negative controls, adjacent sections were treated with 100 µg of ribonuclease A in 0.5 × SSC for 30 min at 37°C either before or after hybridization in order to degrade intracellular RNA.
Immunohistochemistry
Lyophilized whole rabbit serum obtained from animals that were immunized with a highly purified isolate of SDS-insoluble PHF (according to the method of Ihara et al., 1983) derived from AD brain (ICN, Lisle, IL, U.S.A.) was reconstituted with sterile distilled water and used to identify PHF-containing neurons. The immunoreactivity of this PHF antiserum can be neutralized by AD but not AMC brain extract (ICN, personal communication). Endogenous peroxidase activity was blocked in hybridized sections by treatment with 0.03% hydrogen peroxide (H2O2) in 100% methanol. Nonspecific binding of the secondary antibody was blocked by incubation with 20% nonimmune goat serum (NGS). The sections were incubated overnight, at room temperature, with PHF antibody (1:800). Subsequently, the sections were incubated with goat-anti-rabbit IgG (1:50) followed by rabbit peroxidase–antiperoxidase IgG (1:200) for 30 min each at room temperature. All antibodies were diluted in 2% NGS. The chromogen used to label the immunoreactive product was diaminobenzidine tetrahydrochloride (0.044% in ammonium acetate, pH 5.5, containing 0.003% H2O2; Sigma, St. Louis, MO, U.S.A.). As negative controls, adjacent tissue sections were similarly treated, except that nonimmune rabbit serum (ICN) replaced the primary antibody.
After hybridization and immunohistochemistry, the slides were dipped in Kodak NTB2 emulsion (Eastman Kodak, Rochester, NY, U.S.A.). The emulsion was exposed for 20 days at 4°C, developed in Kodak developer for 2 min at 17°C, fixed in Kodak fixer for 2 min at 17°C, and washed in cold, running water for 20 min. The sections were stained with hematoxylin and eosin.
Analyses
Autoradiographic grains were counted above PHF+ and PHF− neurons of similar cross-sectional area (approximately 300 µm2) in tissue sections from the parietal and temporal lobe of AD and AMC brains; cerebellar Purkinje cells and cells in the deep cerebellar nuclei were also examined. Each cell chosen for analysis contained a visible nucleolus. To control for background, grain counts over cells in RNase pre- and posthybridization-treated sections were subtracted from experimental values. The mean number of autoradiographic grains per cell ± SEM was determined for 120 neurons in each brain. Significance was determined at p < 0.05 using the Student’s t test.
RESULTS
Eight of the 12 brains examined yielded positive in situ hybridization results. Very few autoradiographic grains were detected over cells in brain tissue sections from two AD patients (aged 75 and 76 years, postmortem interval of 4 and 10 h, respectively) and two AMC (aged 56 and 69 years, postmortem interval of 83 and 14 h, respectively). The detection of fewer grains was likely due to RNA degradation in both AD and AMC brains. We have previously shown a similar percentage of degradation in RNA isolates from AD and AMC brains (Morrison et al., 1987a,b; Morrison, 1988). All brains analyzed, with the exception of one AD brain collected 52 h postmortem, had postmortem intervals ≤21 h; six were within 18 h.
PHF+ neurons were heavily distributed in superior temporal cortex in all AD brains examined. No PHF-immunoreactive cells were observed in the cerebella of AD brains or in the regions examined in the AMC brains. PHF+ neurons had fewer autoradiographic silver grains than did PHF− neurons in all four AD brains examined, indicating that the levels of poly(A)+ mRNA hybridized in PHF+ neurons are reduced (Fig. 1). We have previously shown that the presence of intracellular immunoreactive product does not interfere with the detection of 3H-autoradiographic grains (Griffin, 1988).
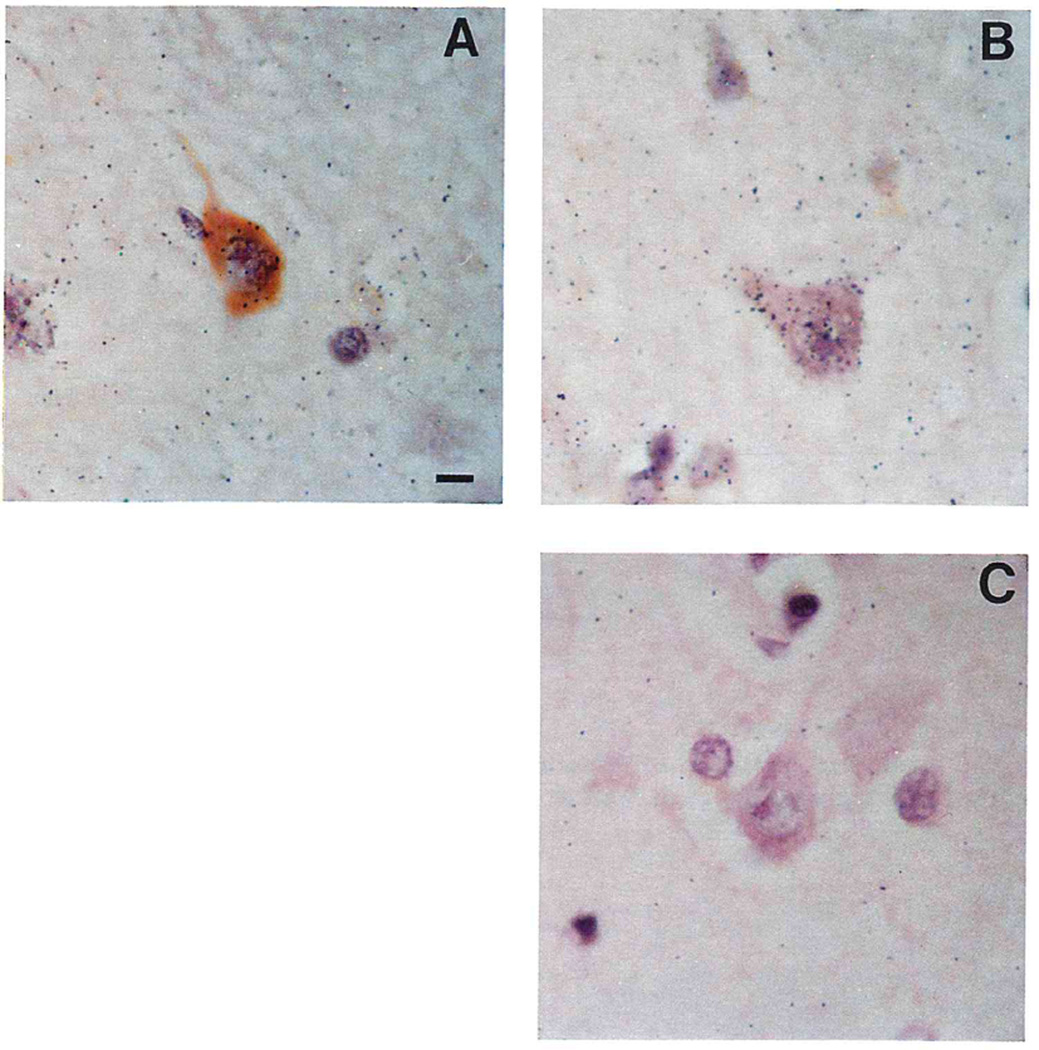
FIG. 1.
Photomicrographs illustrating combined in situ hybridization and immunohistochemistry on 10 µm thick, formalin-fixed, paraffin-embedded sections of superior temporal gyrus from Alzheimer disease (AD) and age-matched control (AMC) brains. Photomicrographs of (A) a neuron with (brown–orange) and without (pink) PHF immunoreactivity in AD superior temporal cortex in situ hybridized with [3H]poly(U); (B) PHF-negative (PHF−) neuron in situ hybridized with [3H]poly(U); and (C) PHF-negative neuron after pretreatment with ribonuclease A in low salt buffer. The bar (A) represents approximately 10 µm.
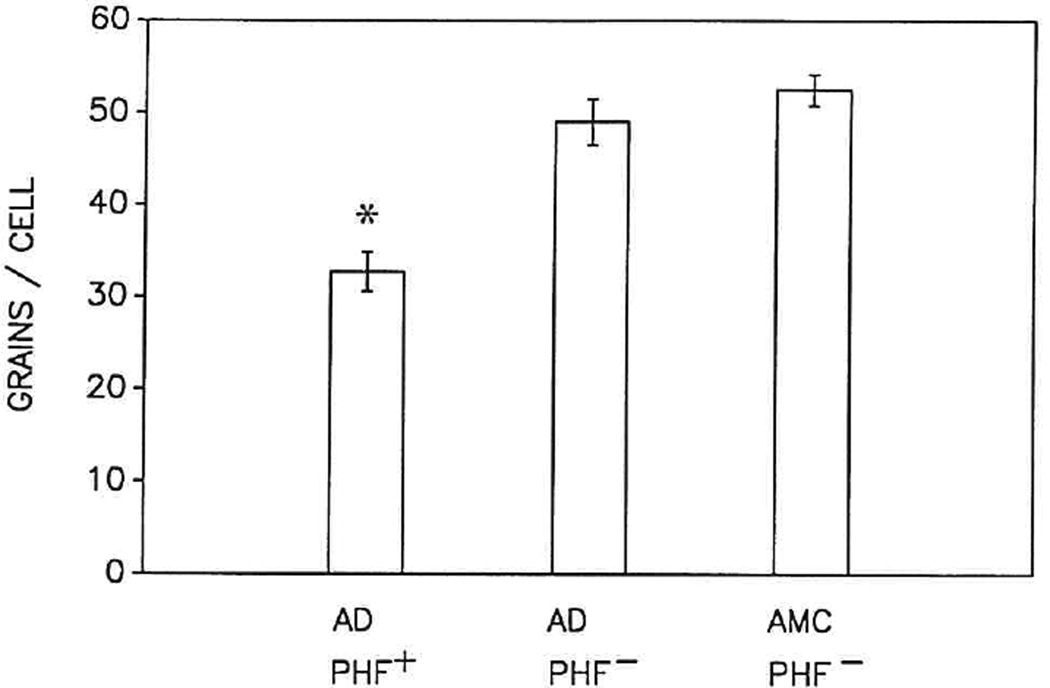
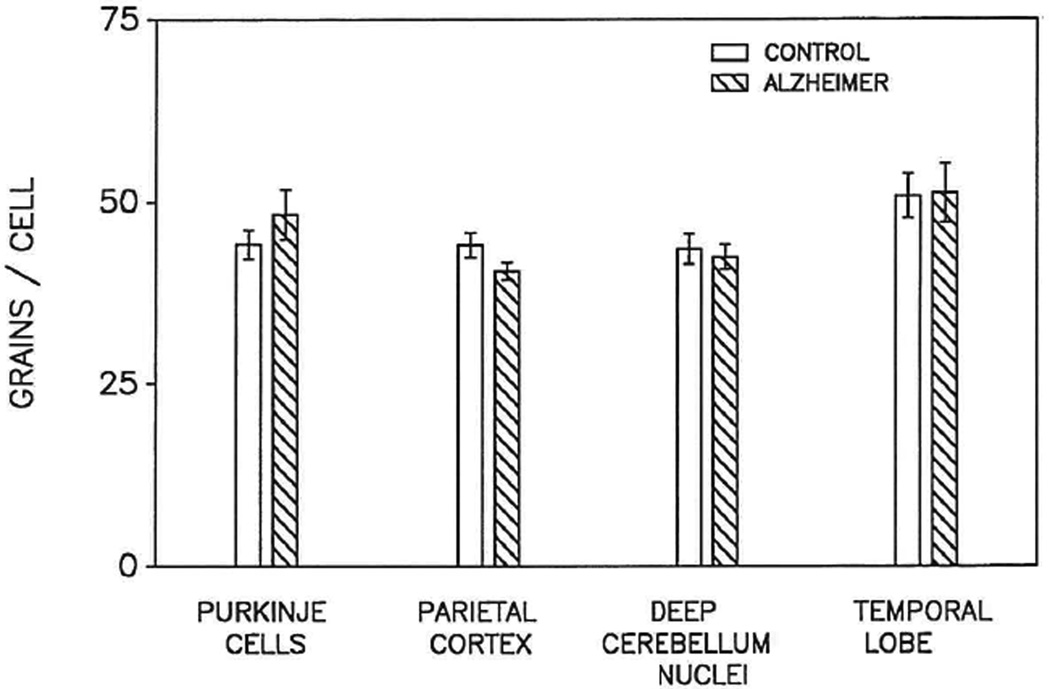
Despite inherent variability in several parameters, including postmortem interval, the SEM grain counts over comparable neurons from AD and AMC did not vary from the mean by more than 5–10% (Figs. 2 and 3). The number of grains over PHF+ neurons (32.7 ± 2.1, mean ± SEM) was approximately two-thirds that over PHF− neurons (49.0 ± 2.5; p ≤ 0.05) in AD cortex (Fig. 2). The number of grains over PHF− neurons in more affected regions of AD brain, e.g., parietal cortex and temporal lobe, as well as in affected regions, e.g., cerebellum, was similar to the number over PHF− neurons in AMC brain (52.5 ± 1.7) (Figs. 2 and 3).
FIG. 2.
Bar graph representing the mean ± SEM of autoradiographic silver grains above PHF-immunoreactive neurons (PHF+) and nonimmunoreactive (PHF−) neurons of similar cross-sectional area (approximately 300 µm2) in tissue sections of superior temporal gyrus from Alzheimer disease (AD; n = 4) and age-matched control (AMC; n = 4) brains. Grains were counted above 20 PHF+ for comparison to 20 PHF− neurons in the same AD section as well as 20 from AMC. The ranges in the number of grains were as follows: 17–49 per PHF+ cell in AD; 33–75 per PHF− cell in AD; and 43–72 per PHF− cell in AMC. The asterisk (*) denotes difference (p ≤ 0.05) in the number of autoradiographic grains above PHF+ neurons compared to PHF− neurons in either AD or AMC.
FIG. 3.
Bar graph representing the mean ± SEM of autoradiographic silver grains above 20 neurons in tissue sections from postmortem parietal and temporal lobes and cerebellum from each of four patients who had Alzheimer disease (AD) for comparison to equal numbers of neurons of comparable size (approximately 300 µm2) in the same brain regions from four similar-aged individuals (AMC). The range of grains per cell was 39 to 104.
DISCUSSION
The detection of fewer autoradiographic grains over PHF+ neurons compared to PHF− neurons of comparable size in AD cortex following in situ hybridization of [3H]poly(U) indicates that intracellular accumulation of PHF is associated with a reduction (33%) in cellular poly(A)+ mRNA content. Although this reduction in poly(U)–poly(A) hybrids in PHF+ neurons could theoretically result from a reduction in length of the poly(A) attached to mRNAs (Morrison and Lingrel, 1976), our previous findings (Griffin et al., 1984, 1985) suggest that it is more likely the consequence of a reduction in the levels of total poly(A)+ mRNAs in neurons compromised by PHF. It follows that the levels of individual mRNAs in these cells are likely to be reduced by a similar magnitude, solely as a consequence of PHF accumulation (Palmer et al., 1988). The presence of other intracellular inclusions such as granulovacuolar degeneration and pigment may, in addition to their documented effect on rRNA levels (Mann, 1978; Mann and Sinclair, 1978; Mann and Yates, 1979; Mann et al., 1978, 1982), contribute to decreased mRNA levels; their effects were not addressed here.
We conclude that the presence of neurons containing PHF does not compromise the total poly(A)+ mRNA content of neighboring neuronal populations since PHF− cells in AD brains have levels of poly(A)+ mRNA similar to those in PHF− neurons in AMC brains. The poly(A)+ mRNA levels in neurons in the cerebellar cortex and deep cerebellar nuclei (regions of AD brain that have relatively few plaques and tangles) were not different from the poly(A)+ RNA levels of similar cell types in AMC brains. Moreover, in temporal cortex, despite the presence of PHF+ neurons, there was no significant difference in poly(A)+ mRNA content in PHF− neurons of similar size in AD patients and AMC. Because the total number of PHF+ neurons is small compared to the total number of neurons even in the most severely affected regions of AD brain, it is not surprising that the total levels of mRNAs representing all brain cell types would not be detectably reduced (Morrison et al., 1986; 1987a,b; Johnson and Finch, 1987; May et al., 1987; Morrison, 1988).
If specific mRNAs are preferentially synthesized in PHF+ neurons, then the overall cortical levels of those mRNAs and their translation products may be significantly reduced. Somatostatin-like immunoreactivity is decreased in cortex (Davies et al., 1980; Davies and Terry, 1981), particularly in temporal lobe (Rosser et al., 1980; Beal et al., 1986), where there is a high concentration of PHF-containing cells (Hyman et al., 1984). In addition, somatostatin immunoreactivity has been detected in plaques (Morrison et al., 1985; Armstrong et al., 1989) and in tangle-bearing cells in AD temporal lobe (Roberts et al., 1985), suggesting that degenerative processes occur in a population of somatostatin-containing neurons. The correlation, if any, between PHF content in neurons and a decrease in the levels of somatostatin mRNA is unknown, but a decrease in somatostatin mRNA in tangle-bearing cells could contribute to a reduction in somatostatin. Our findings of decreased total poly(A)+ mRNA in tangle-bearing neurons support the idea that the presence of PHF in a neuron decreases its capacity to synthesize specific gene products. This idea could be tested using in situ hybridization to determine the levels of a specific mRNA relative to the levels of total poly(A)+ RNA (Griffin and Morrison, 1985; Griffin et al., 1985; Griffin 1987, 1988) in PHF+ and PHF− neurons in AD cortex.
Acknowledgment
We would like to thank the donors and their families for the gift of postmortem tissue, and Nancy Tyler, Jennie Ratliff, Cheryl Beisert, Laura Stanley, and Marie LeCroy for their help with these investigations, which were supported in part by NIH AG05537 and NIH AG08013.
REFERENCES
- Armstrong DM, Benzing WC, Evans J, Terry RD, Shields D, Hansen LA. Substance P and somatostatin coexist within neuritic plaques: implications for the pathogenesis of Alzheimer’s disease. Neuroscience. 1989;31:663–671. doi: 10.1016/0306-4522(89)90431-4. [DOI] [PubMed] [Google Scholar]
- Ball MJ. Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. A quantitative study. Acta Neuropathol (Bed) 1977;37:111–118. doi: 10.1007/BF00692056. [DOI] [PubMed] [Google Scholar]
- Beal MF, Uhl G, Mazurek MF, Kowall N, Martin JB. Somatostatin: alterations in the central nervous system in neurological diseases. In: Martin JB, Barchas JD, editors. Neuropeptides in neurological and psychiatric disease. New York: Raven Press; 1986. pp. 215–257. [PubMed] [Google Scholar]
- Davies P, Katzman R, Terry RD. Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer’s disease and Alzheimer senile dementia. Nature (Lond) 1980;288:279–280. doi: 10.1038/288279a0. [DOI] [PubMed] [Google Scholar]
- Davies P, Terry RD. Cortical somatostatin-like immunoreactivity in cases of Alzheimer’s disease and senile dementia of the Alzheimer type. Neurobiol Aging. 1981;2:9–14. doi: 10.1016/0197-4580(81)90053-1. [DOI] [PubMed] [Google Scholar]
- Dayan AD, Ball MJ. Histometric observations of the metabolism of tangle-bearing neurons. J Nenrol Sci. 1973;19:433–436. doi: 10.1016/0022-510x(73)90040-3. [DOI] [PubMed] [Google Scholar]
- Doebler JA, Markesbery WR, Anthony A, Rhoads RE. Neuronal RNA in relation to neuronal loss and neurofibrillary pathology in the hippocampus in Alzheimer’s disease. J Neuropathol Exp Neurol. 1987a;46:28–39. doi: 10.1097/00005072-198701000-00003. [DOI] [PubMed] [Google Scholar]
- Doebler JA, Markesbery WR, Anthony A, Rhoads RE. In situ analysis of neostriatal RNA and chromatin in Alzheimer’s disease. Neurology. 1987b;37:309–313. doi: 10.1212/wnl.37.2.309. [DOI] [PubMed] [Google Scholar]
- Ellis WG, McCulloch JR, Corley CL. Presenile dementia in Down’s syndrome. Neurology. 1974;24:101–106. doi: 10.1212/wnl.24.2.101. [DOI] [PubMed] [Google Scholar]
- Gilbert JM, Brown BA, Strocchi P, Bird ED, Marotta CA. The preparation of biologically active messenger RNA from human postmortem brain tissue. J Netirochem. 1981;36:976–984. doi: 10.1111/j.1471-4159.1981.tb01689.x. [DOI] [PubMed] [Google Scholar]
- Griffin WST. Methods for hybridization and quantitation of mRNA in individual brain cells. In: Valentino KL, Eberwine JH, Barchas JD, editors. In situ hybridization: applications to neurobiology. New York: Oxford University Press; 1987. pp. 97–110. [Google Scholar]
- Griffin WST. In situ hybridization: Visualizing brain messenger RNA. In: Rosenberg RN, Harding AE, editors. The molecular biology of neurological disease. London: Butterworth & Co., Ltd.; 1988. pp. 34–43. [Google Scholar]
- Griffin WST, Alejos MA, Cox EJ, Morrison MR. The differential distribution of beta tubulin mRNAs in individual mammalian brain cells. J Cell Biochem. 1985;27:205–214. doi: 10.1002/jcb.240270303. [DOI] [PubMed] [Google Scholar]
- Griffin WST, Cox R, Morrison MR. Quantitation of mRNAs in individual neurons in human brain. Neurosci Abstr. 1984;10:374. [Google Scholar]
- Griffin WST, Morrison MR. In situ hybridization—visualization and quantitation of genetic expression in mammalian brain. Peptides. 1985;6:89–96. doi: 10.1016/0196-9781(85)90139-1. [DOI] [PubMed] [Google Scholar]
- Guillemette JG, Wong L, Crapper-McLachlan DR, Lewis PN. Characterization of messenger RNA from the cerebral cortex of control and Alzheimer-afflicted brain. J Neurochem. 1986;47:987–997. doi: 10.1111/j.1471-4159.1986.tb00708.x. [DOI] [PubMed] [Google Scholar]
- Hirano A, Zimmerman HM. Alzheimer’s neurofibrillary changes—a topographic study. Arch Neurol. 1962;7:227–242. doi: 10.1001/archneur.1962.04210030065009. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Ihara Y, Abraham C, Selkoe DJ. Antibodies to paired helical filaments in Alzheimer’s disease do not recognize normal brain proteins. Nature (Lond) 1983;304:727–730. doi: 10.1038/304727a0. [DOI] [PubMed] [Google Scholar]
- Ilaria R, Wines D, Pardue S, et al. A rapid microisolation procedure for isolating RNA from multiple samples of human and rat brain. J Neurosci Methods. 1985;15:165–174. doi: 10.1016/0165-0270(85)90053-6. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Finch CE. Cloning of RNA sequences whose prevalence is increased in Alzheimer cortex. Soc Newosci Abstr. 1987;13:1325. [Google Scholar]
- Johnson SA, Morgan DG, Finch CE. Extensive post-mortem stability of RNA from rat and human brain. J Newosci Res. 1986;16:267–280. doi: 10.1002/jnr.490160123. [DOI] [PubMed] [Google Scholar]
- Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neitrol. 1985;42:1097–1104. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kidd M. Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature (Loud) 1963;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Mann DMA. Granulovacuolar degeneration in pyramidal cells of the hippocampus. Ada Nenropathol (Berl) 1978;42:149–151. doi: 10.1007/BF00690983. [DOI] [PubMed] [Google Scholar]
- Mann DMA. The pathological association between Down syndrome and Alzheimer disease. Mech Ageing Dev. 1988;43:99–136. doi: 10.1016/0047-6374(88)90041-3. [DOI] [PubMed] [Google Scholar]
- Mann DMA, Neary D, Yates PO, Lincoln J, Snowden JS, Stanworth P. Neurofibrillary pathology and protein synthetic capability in nerve cells in Alzheimer’s disease. Nenropathol Appl Neurobiol. 1981a;7:37–47. doi: 10.1111/j.1365-2990.1981.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Mann DMA, Neary D, Yates PO, Lincoln J, Snowden JS, Stanworth P. Alterations of protein synthetic capacity in nerve cells in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1981b;44:97–102. doi: 10.1136/jnnp.44.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DMA, Sinclair KGA. The quantitative assessment of lipofuscin pigment, cytoplasmic RNA and nucleolar volume in senile dementia. Neuropaihol Appl Neurobiol. 1978;4:129–135. doi: 10.1111/j.1365-2990.1978.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Mann DMA, Yates PO. The effects of ageing on the pigmented nerve cells of the human locus caeruleus and substantia nigra. Ada Neuropathol (Berl) 1979;47:93–97. doi: 10.1007/BF00717030. [DOI] [PubMed] [Google Scholar]
- Mann DMA, Yates PO. The relationship between formation of senile plaques and neurofibrillary tangles and changes in nerve cell metabolism in Alzheimer type dementia. Mech Ageing Dev. 1981;17:395–401. doi: 10.1016/0047-6374(81)90056-7. [DOI] [PubMed] [Google Scholar]
- Mann DMA, Yates PO, Barton CM. Cytophotometric mapping of neuronal changes in senile dementia. J Neurol Neurosurg Psychiatry. 1977;40:299–302. doi: 10.1136/jnnp.40.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DMA, Yates PO, Hawkes J. The noradrenergic system in Alzheimer and multi-infarct dementias. J Neurol Neurosurg Psych. 1982;45:113–119. doi: 10.1136/jnnp.45.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DMA, Yates PO, Stamp JE. The relationship between lipofuscin pigment and ageing in the human nervous system. J Neurol Sci. 1978;37:83–93. doi: 10.1016/0022-510x(78)90229-0. [DOI] [PubMed] [Google Scholar]
- Marotta CA, Brown BA, Strocchi P, Bird ED, Gilbert JM. In vitro synthesis of human brain proteins including tubulin and actin by purified polysomes. J Neurochem. 1981;36:966–975. doi: 10.1111/j.1471-4159.1981.tb01688.x. [DOI] [PubMed] [Google Scholar]
- May PC, Johnson SA, Masters JN, Lampert-Echells M, Finch CE. Cloning of poly(A) RNA differentially regulated in Alzheimer’s disease from a hippocampal cDNA library. Soc Newosci Abstr. 1987;13:1325. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS–ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;23:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McLachlan DRC, Lukiw WJ, Wong L, Bergeron C, Bech-Hansen NT. Selective messenger RNA reduction in Alzheimer’s disease. Mol Brain Res. 1988;3:255–262. doi: 10.1016/0169-328x(88)90048-4. [DOI] [PubMed] [Google Scholar]
- Morrison MR. Messenger RNA levels in neurological disease. In: Rosenberg RN, Harding AE, editors. The molecular biology of neurological disease. London: Butterworth & Co., Ltd.; 1988. pp. 135–152. [Google Scholar]
- Morrison MR, Griffin WST. The isolation and in vitro translation of undegraded messenger RNAs from human postmortem brain. Anal Biochem. 1981;113:318–324. doi: 10.1016/0003-2697(81)90083-x. [DOI] [PubMed] [Google Scholar]
- Morrison MR, Griffin WST, White CL., III . Brain messenger RNA in Alzheimer’s disease. In: Guiditta A, Kaplan B, Zomzely-Neurath C, editors. Role of RNA and DNA in brain function. Boston: Martinus Nijhoff Press; 1986. pp. 142–159. [Google Scholar]
- Morrison MR, Lingrel JB. Molecular weight characterization of the α and β globin mRNAs. Biochem Biophys Ada. 1976;447:104–109. doi: 10.1016/0005-2787(76)90100-3. [DOI] [PubMed] [Google Scholar]
- Morrison MR, Pardue S, Griffin WST. Altered expression of different tubulin electrophoretic variants during human cortex development. J Newogenet. 1983;1:105–111. doi: 10.3109/01677068309107076. [DOI] [PubMed] [Google Scholar]
- Morrison MR, Pardue S, Maschhoff K, et al. Brain messenger RNA levels and ribonuclease activity in Alzheimer’s disease. Biochem Soc Trans. 1987a;15:133–134. doi: 10.1042/bst0150133a. [DOI] [PubMed] [Google Scholar]
- Morrison MR, Pardue S, Maschhoff K, et al. Messenger RNA analysis in control and Alzheimer’s disease brain: rapid autopsy evaluation. In: Wurtman RJ, Corkin SH, Growdon JH, editors. Alzheimer’s disease: advances in basic research and therapies. Cambridge, MA: Center for Brain Sciences and Metabolism Charitable Trust; 1987b. pp. 505–509. [Google Scholar]
- Morrison JH, Rogers J, Scherr S, Benoit R, Bloom FE. Somatostatin immunoreactivity in neuritic plaques of Alzheimer’s patients. Nature (Lond) 1985;314:90–92. doi: 10.1038/314090a0. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Mann DMA, et al. Alzheimer’s disease: a correlative study. J Neurol Neurosurg Psych. 1986;49:229–237. doi: 10.1136/jnnp.49.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MR, Golde TE, Cohen ML, et al. Amyloid protein precursor messenger RNAs: differential expression in Alzheimer’s disease. Science. 1988;241:1080–1084. doi: 10.1126/science.2457949. [DOI] [PubMed] [Google Scholar]
- Rasool CG, Abraham CA, Anderton BH, Haugh M, Kahn J, Selkoe DJ. Alzheimer’s disease: immunoreactivity of neurofibrillary tangles with antineurofilament and anti-paired helical filament antibodies. Brain Res. 1984;310:249–260. doi: 10.1016/0006-8993(84)90148-3. [DOI] [PubMed] [Google Scholar]
- Rhoads RE, Doebler JA, Markesbery WR, Anthony A, Davies P, Sheff SW. RNA levels and Alz50 immunoreactivity in Alzheimer’s disease hippocampus. J Cell Biochem Suppl. 1987;11:198. [Google Scholar]
- Roberts GW, Crow TJ, Polak JM. Location of neuronal tangles in somatostatin neurons in Alzheimer’s disease. Nature (Lond) 1985;314:92–94. doi: 10.1038/314092a0. [DOI] [PubMed] [Google Scholar]
- Rosser MN, Emson PC, Mountjoy CA. Reduced amounts of immunoreactive somatostatin in the temporal cortex in senile dementia of the Alzheimer type. Neurosci Lett. 1980;20:373–377. doi: 10.1016/0304-3940(80)90177-9. [DOI] [PubMed] [Google Scholar]
- Rumble B, Retallack R, Hilbich C, et al. Amyloid A4 protein and its precursor in Down’s syndrome and Alzheimer’s disease. N Engl J Med. 1989;320:1446–1452. doi: 10.1056/NEJM198906013202203. [DOI] [PubMed] [Google Scholar]
- Sadjel-Sulkowska EM, Coughlin JF, Staton DM, Marotta CA. In vitro protein synthesis by messenger RNA from the Alzheimer’s disease brain. In: Katzman R, editor. Banbury report 15, biological aspects of Alzheimer’s disease. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1983. pp. 193–200. [Google Scholar]
- Sevier AC, Munger BL. Technical note: a silver method for paraffin sections of neural tissue. J Neuropathol Exp Neurol. 1965;24:130–135. doi: 10.1097/00005072-196501000-00012. [DOI] [PubMed] [Google Scholar]
- Taylor GR, Carter GI, Crow TJ, et al. Recovery and measurement of specific RNA species from postmortem brain tissue: general reduction in Alzheimer’s disease detected by molecular hybridization. Exp Mol Pathol. 1986;44:111–116. doi: 10.1016/0014-4800(86)90038-9. [DOI] [PubMed] [Google Scholar]
- Taylor GR, Carter GI, Crow TJ, et al. Recovery and measurement of RNA in Alzheimer’s disease by molecular hybridization. J Neurol Neurosurg Psychiatry. 1987;50:356. doi: 10.1136/jnnp.50.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;l1:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- Uemura E, Hartman HA. RNA content and volume of nerve cell bodies in human brain. I. Prefrontal cortex in ageing normal and demented patients. J Neuropathol Exp Neurol. 1978;37:487–496. doi: 10.1097/00005072-197809000-00004. [DOI] [PubMed] [Google Scholar]
- Uemura E, Hartman HA. Quantitative studies of neuronal RNA on the subiculum of demented old individuals. Brain Res Bull. 1979;4:301–305. doi: 10.1016/s0361-9230(79)80005-2. [DOI] [PubMed] [Google Scholar]
- Wisniewski HM, Jervis GA, Moretz RC, Wisniewski HM. Alzheimer neurofibrillary tangles in diseases other than senile and presenile dementia. Ann Neurol. 1979;5:288–294. doi: 10.1002/ana.410050311. [DOI] [PubMed] [Google Scholar]
- Wisniewski HM, Narang HK, Terry RD. Neurofibrillary tangles of paired helical filaments. J Neurol Sci. 1976;27:173–181. doi: 10.1016/0022-510x(76)90059-9. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Wolozin BL, Pruchnicki A, Dickson DW, Davies PL. Neuronal antigen in the brains of Alzheimer patients. Science. 1986;232:648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Woody RC, Stanley LC, Roberts GW, Griffin WST. β-Amyloid immunoreactivity in hippocampus from fetal, neonatal, infant, adolescent, and adult Down’s syndrome. Ann Neurol. 1989;26:485. [Google Scholar]