1. INTRODUCTION
The advent of adhesive dentistry has substantially improved the quality of restorative dentistry [1,2]. However, dentine bonding is challenging due to the heterogeneous characteristics of the dentine substrate, its low surface energy and variable permeability [3]. Microleakage is defined as the passage of bacteria, fluids, molecules or ions between a cavity wall and the restorative material [4]. Microleakage has been of great concern in restorative dentistry [4], causing discolouration, recurrent/secondary caries [5–7], postoperative sensitivity and pulp pathology [8,9]. In order to suppress growth of bacteria in leaked restorations, an antibacterial monomer, methacryloyloxydodecylpyridinium bromide (MDPB), has been successfully incorporated in a dentine primer [5–7] without adversely affecting its bonding characteristics [7]. Bonding resin containing MDPB has the potential to arrest residual bacteria in the prepared cavity, or inhibit bacterial growth that may penetrate the resin-dentine interface through microleakage.
Nanoleakage, another pattern of leakage, has been described as leakage occurring in the absence of gaps, within the nanometre-sized spaces around unprotected collagen fibrils present in hybrid layers [10–12]. Such leakages may result in hydrolytic breakdown of either the adhesive resin or collagen within the hybrid layer, thereby compromising the durability of the resin–dentine bonds [13].
It has been speculated recently that the auto-degradation of dentine collagen fibrils is responsible for bond degradation within hybrid layers [14,15]. This mechanism is initiated from beneath the bonded interface, with the breakdown of acid-demineralised collagen matrices by host-derived matrix metalloproteinases. The low but persistent endogenous collagenolytic activity was completely inhibited by the use of protease inhibitors [15]. An in vivo study further demonstrated that degradation of dentine hybrid layers could occur as early as six months in caries-affected primary dentine, and that the use of chlorhexidine, even in very low concentrations, inhibited such degradation activities. Hybrid layers that were first treated with chlorhexidine before bonding were uneventfully preserved [16]. If chlorhexidine can be released from dentine adhesives to hybrid layers/collagen matrices for extended periods, the long-term durability of resin-dentine bonds would be improved. Since chlorhexidine possesses antibacterial properties, incorporation of chlorhexidine into bonding agents may be a viable method to inhibit bacteria growth in bonds that exhibit micro/nanoleakage, while simultaneously preserving the integrity of the resin-dentine interfaces by arresting collagen degradation. Thus, it is of interest to examine if the release rate of chlorhexidine would be affected when it is incorporated into bonding agents containing methacrylate resin monomers. Other the other hand, it has been also shown that incorporation of soluble chlorhexidine diacetate in polymers increased water sorption due to osmotic effect of chlorhexidine attracking water within the polymer matrix [17]. Thus, it is possible that water uptake within the polymer matrix would adversely affect the properties of resin adhesives and resin-dentine interfaces.
In this study, we formulated a series of neat, unfilled, non-solvated methacrylate-based experimental resins of increasing hydrophilicity as ranked by their Hoy's solubility parameters. These experimental resins also contained 0.0, 0.2, 1.0 and 2.0 wt% chlorhexidine diacetate (CDA) and were used to examine the mass change and the rate of chlorhexidine release over a 28-day period. The cumulative chlorhexidine release, water sorption and solubility were obtained to analyse correlations among these parameters. Additionally, antibacterial activities of the chlorhexidine-incorporated resins were evaluated by examining the presence of inhibition zones against Streptococcus mutans. The null hypotheses tested were that the degree of hydrophilicity of resin blends has no effect on chlorhexidine release from cured resins, and that the addition of chlorhexidine has no effect on the water uptake/sorption by the resin blends.
2. MATERIALS AND METHODS
2.1. Preparation of CDA-incorporated, thin resin disks
Five comonomer blends were formulated using a base monomer, a diluent comonomer and different hydrophilic comonomers that are representative of currently employed dentine adhesive formulations [18–20]. The composition and the respective Hoy's solubility parameters of the five resin blends (R1, R2, R3, R4 and R5) are shown in Table 1. Resin blends R1 and R2 contain hydrophobic resins that simulate the bonding resin component of three-step etch-and-rinse and two-step self-etch adhesives. Resin blend R3 contains 2-hydroxyethyl methacrylate (HEMA), and represents components present in some two-step etch-and-rinse adhesives. Resin blends R4 and R5 are designed to simulate hydrophilic one-step self-etch adhesives with carboxylic acid functional groups and very hydrophilic one-step self-etch adhesives with phosphate functional groups, respectively.
Table 1.
Composition and Hoy’s solubility parameters of the five experimental resins blends used in the study
| Resin Blend |
Composition | Wt% | Hoy’s solubility parameters (MPa−1) | |||
|---|---|---|---|---|---|---|
| δd | δp | δh | δt | |||
| Resin 1 | Bis-GMA-E | 70.00 | 15.4 | 10.6 | 6.2 | 19.7 |
| TEGDMA | 28.75 | |||||
| CQ | 0.25 | |||||
| EDMAB | 1.00 | |||||
| Resin 2 | Bis-GMA | 70.00 | 15.6 | 12.1 | 8.6 | 21.5 |
| TEGDMA | 28.75 | |||||
| CQ | 0.25 | |||||
| EDMAB | 1.00 | |||||
| Resin 3 | Bis-GMA | 70.00 | 15.4 | 12.0 | 9.9 | 22.5 |
| HEMA | 28.75 | |||||
| CQ | 0.25 | |||||
| EDMAB | 1.00 | |||||
| Resin 4 | Bis-GMA | 40.00 | 16.2 | 12.6 | 9.3 | 23.5 |
| HEMA | 28.75 | |||||
| TCDM | 30.00 | |||||
| CQ | 0.25 | |||||
| DMABA | 1.00 | |||||
| Resin 5 | Bis-GMA | 40.00 | 15.8 | 14.4 | 10.8 | 23.9 |
| HEMA | 28.75 | |||||
| 2MP | 30.00 | |||||
| CQ | 0.25 | |||||
| EDMAB | 1.00 | |||||
Abbreviations:- 2MP: Bis[2-(methacryloyloxy)ethyl] phosphate; Bis-GMA: bisphenol A diglycidyl ether dimethacrylate; Bis-GMA-E: ethoxylated bisphenol A diglycidyl ether dimethacrylate; CQ: camphorquinone; EDMAB: ethyl N,N-dimethyl-4-aminobenzoate; HEMA: 2-hydroxylethyl methacrylate; DMABA: dimethylaminobenzoic acid; TEGDMA: triethylene-glycol dimethacrylate; TCDM: di(hydroxyethylmethacrylate) ester of 5-(2,5-dioxotetrahydrofurfuryl)-3-methyl-3-cyclohexene-1,2-dicarboxylic anhydride; δd dispersion component; δp polar component; δh hydrogen bonding component; δt total cohesive energy density value
Experimental chlorhexidine-incorporated resin blends were prepared by adding CDA (Sigma-Aldrich, St. Louis, MO, USA) to the neat comonomer blends, according to the method described by Anusavice et al. [21]. Four groups chlorhexidine-incorporated resins were created from each neat resin blend by directly adding 0.0 wt%. 0.2 wt%, 1.0 wt% and 2.0 wt% CDA powder to the neat. The freshly prepared resin-CDA mixtures were ultra-sonicated to ensure homogeneity. Six specimen disks (12 ± 0.1 mm diameter, 0.7 ± 0.1 mm thickness) were fabricated from each CDA-incorporated resin blend using a Teflon split ring mould between two glass slabs, that were covered with polyethylene films to eliminate oxygen inhibition layers on the cured surfaces [18]. After all visually apparent bubbles were carefully removed, the resin was first light-cured over the centre of the disk for 40 s, and further light-cured on its opposite surface for 40 s using a quartz-tungsten-halogen light-curing unit (Optilux 500, Demetron Research Corporation, Danbury, CT, USA) operated at 600 mW/cm2. To improve polymerisation, disks were placed inside a composite inlay processing chamber (Dentacolor® XS, Heraeus Kulzer GmbH & Co. KG, Wehrheim, Germany) and light-cured for an additional 3 min.
2.2. Analysis of weight change and water sorption/solubility
Each polymerised resin disk was placed in a desiccator containing silica gel at 37 °C and repeatedly weighed in an analytical balance (Model AD6, Perkin Elmer, Shelton, CT, USA) until a constant mass (M1) was obtained. The thickness and diameter of each disk were measured using a digital calliper (Mitutoyo Corporation, Tokyo, Japan), rounded to the nearest 0.01 mm, from which the volume (V) of each disk was calculated. Each disk was individually immersed at 37 °C in 5 mL of deionised water inside a sealed container. During appropriate time intervals of 3, 12, 24, 48, 72, 96, 120, 144, 168, 240, 288, 336, 508, and 672 h (28 days), the specimen was retrieved from the solution and blot-dried with filter paper. The mass increase/decrease was recorded, from which the mass change was calculated as a percentage of the mass of M1. The specimen was immediately re-immersed in the deionised water after measurement. The maximum mass (M2) was recorded during the 28 day experiment. After 28 days of water storage, each specimen was dried in the desiccator and weighed daily until a constant mass (M3) was obtained. Water sorption and solubility over the 28-day period of water storage were calculated using the following formulae: Water sorption = (M2−M1)/V, Water solubility = (M1−M3)/V [18, 20].
2.3. Analysis of chlorhexidine release
A series of CDA solutions containing 0.1, 0.2, 0.5, 1.0, 2.0 and 4.0 mg/dl CDA was used to obtain a linear relationship between absorbance peak height and CDA concentration using a spectrophotometer (DU530 Life Science UV/Vis Spectrophotometer, Beckman, Fullerton, CA, USA). The maximum absorbance peak of chlorhexidine was confirmed at 260 nm. New CDA-incorporated resin disks were prepared and stored in deionised water as previously described. At appropriate time intervals, the absorbance peak height of the storage solutions at 260 nm were obtained and converted to the quantities of chlorhexidine released based on the established linear calibration. Since the peak of absorbance of HEMA is 210 and 230 nm, diffusion of HEMA from R3–5 was speculated to increase the UV absorbance at 260 nm. Thus, the UV absorbance at 260nm of controls (0% CDA incorporated disks) was subtracted from those produced from CDA-incorporated disks. The rate of chlorhexidine release per unit area of the resin disk was calculated between consecutive measurements. The cumulative release was also obtained for 28-day and represented as mg of chlorhexidine release per g of sample [17].
2.4. Measurement of inhibitory effect on Streptococcus mutans
Streptococcus mutans was cultured in Columbia agar (EMD Chemicals Inc., Gibbstown, NJ, USA) supplemented with 5% defibrinated horse blood. Culturing was performed inside an anaerobic chamber at 37 °C. Isolated colonies were retrieved after 48 h and suspended in 5 mL of Brain Heart Infusion broth (Difco, Detroit, MI, USA). The concentration of the bacteria suspension was adjusted with the spectrophotometer at 660 nm to the optimal density exhibited by a bacterial concentration of 1 × 108 CFU/mL [5]. Twenty microlitre aliquots of the adjusted bacterial suspension were inoculated on sterile Petri dishes (LP Italiana, Spa, Italy) containing a BHI agar using a sterile, automated spiral platter (Autoplate 4000, Exotech Inc., Gaithersburg, MD, USA).
Three CDA-incorporated resin disks from each group were additionally fabricated, immersed in deionised water and retrieved from water storage at 24, 72, 168 and 336 h. Ultraviolet irradiation was performed for 30 min on each disk surface to sterilise the disks, They were then placed on the Streptococcus mutans-inoculated Petri dishes. The plates were incubated anaerobically at 37 °C for 48 h. After incubation, inhibition zones around the resin disks were examined to evaluate their antibacterial effect. The apparent diameters of the inhibition zones around the resin disks were determined with the Leica QWin image analysis software (Leica Microsystems Inc., Bannockburn, IL, USA). The actual dimension of the inhibition zone was calculated by subtracting the diameter of the resin disk from the apparent inhibition zone diameter.
2.5. Statistical analyses
The mass change and rate of chlorhexidine release were analysed with a three-way ANOVA, using the resin material, CDA concentration and the storage time as independent factors. Since the water sorption and solubility data were not normally distributed and there were inequalities in the variances, nonparametric statistical tests were employed. The data were analyzed using the Kruskal-Wallis rank test and subjected to Two-Independent–Sample tests using Mann-Whitney U test for each group.
The normally distributed data derived from the measurement of Streptococcus mutans inhibition zones were analysed with a three-way ANOVA using the resin material, CDA concentration and the storage time as the three factors. Post hoc multiple comparisons were performed using Tukey’s test.
Additionally, the correlation between water sorption and the cumulative release of chlorhexidine was evaluated by means of linear regression analysis. The correlations between the cumulative release of chlorhexidine and Hoy’s solubility parameters for hydrogen bonding (δh) were also analysed. For all analyses, statistical significance were set at α = 0.05.
3. RESULTS
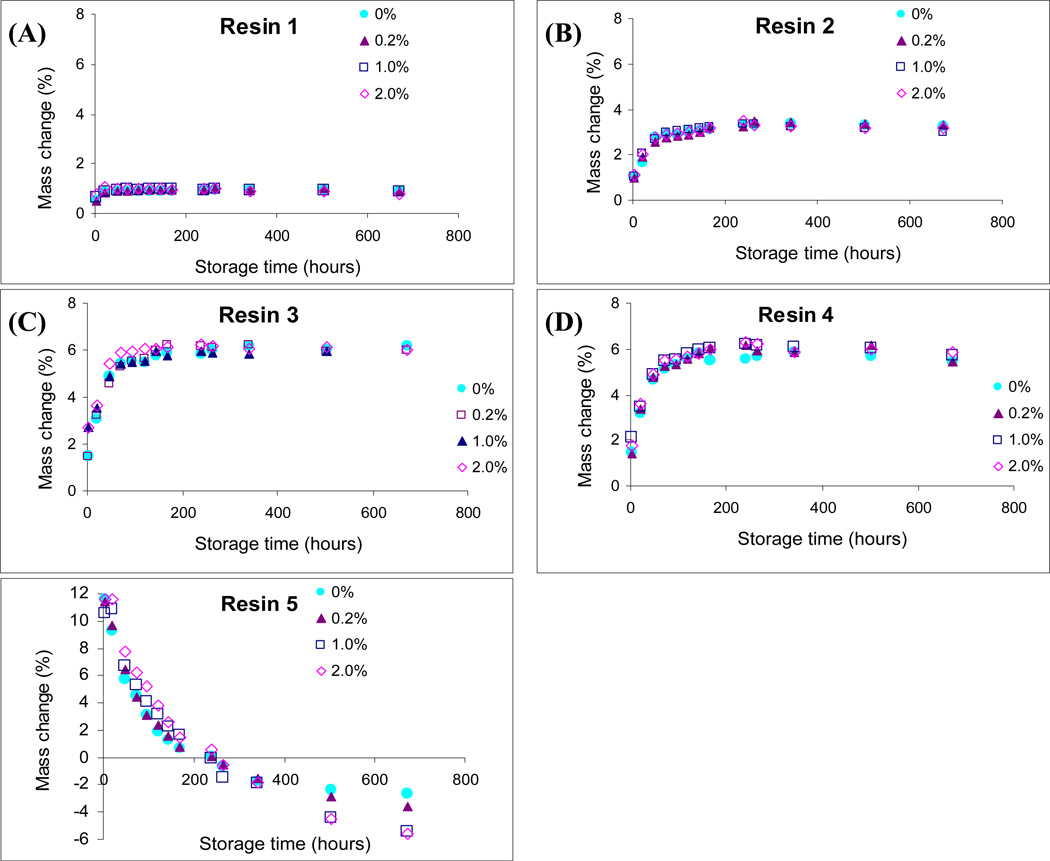
Fig. 1 shows the percentage mass changes of the resin disks after immersion in deionised water for 28 days. Three-way ANOVA showed that the three factors, resin material, CDA concentration and storage time significantly affected the changes in specimen mass (p < 0.0001). There was a significant interaction between resin material and CDA concentration, between resin material and storage time and between CDA concentration and storage time (p < 0.0001). Incorporation of CDA had no significant effect on mass changes in R1– R4. For R5, the greatest mass increase was observed within 1 day, followed by gradual decrease in mass degrease over time. The 1.0% and 2.0% CDA-incorporated R5 specimens significantly decreased in mass at 21-day and 28-day, compared with the 0.0 % and 0.2 % CDA-incorporated R5, although the incorporation of CDA seemed to increase mass change up to 168 h.
Fig.1.
Changes in mass of the chlorhexidine diacetate (CDA)-incorporated resin blends (R1–R5) over 28 days of water storage. Symbols represent mean values of mass changes as percentages of their initial mass (n = 6). A. CDA-incorporated R1. B. CDA-incorporated R2. C. CDA-incorporated R3. D. CDA-incorporated R4. E. CDA-incorporated R5.
Water sorption and solubility varied significantly among materials. The results of the Kruskal-Wallis rank test and Mann-Whitney U test are summarised in Tables 2 and 3. There was no effect of CDA concentration on water sorption (p > 0.05). The most hydrophilic resin blend, R5, showed the highest water sorption (172.8–184.6 µg/mm3), while the most hydrophobic resin blend, R1, exhibited the lowest water sorption (15.4–15.9 µg/mm3). There was no significant difference between R3 and R4. The highest solubility was observed in the most hydrophilic resin blend R5 (227.6–279.5 µg/mm3), followed by the most hydrophobic resin blend R1 (4.4–7.6 µg/mm3). Solubility, in general, increased with the incorporation of CDA into the resin blends. The 1.0 % and 2.0 % groups prepared from R2, R3 and R4 showed significantly higher solubility than the 0 % and 0.2 % groups prepared from those resins (p < 0.05).
Table 2.
Water sorption (µg/mm3) data derived from the five chlorhexidine diacetate-incorporated resin blends (CDA)
| CDA% | 0% | 0.2% | 1.0% | 2.0% |
|---|---|---|---|---|
| Resin 1 | 15.38a(2.97) | 14.77a(2.40) | 15.82a(1.50) | 15.92a(2.30) |
| Resin 2 | 52.57b(4.32) | 52.57b(5.08) | 50.76b(2.66) | 52.87b(2.81) |
| Resin 3 | 91.75c(13.97) | 95.31c(6.46) | 92.49c(8.46) | 94.32c(8.44) |
| Resin 4 | 91.08c(8.64) | 95.62c(4.96) | 92.17c(5.94) | 96.01c(4.57) |
| Resin 5 | 172.80d(5.97) | 173.26d(11.73) | 164.35d(18.98) | 184.55d(21.41) |
Values are means (standard deviations). Groups with the same superscripts are not significantly different (p > 0.05).
Table 3.
Solubility (µg/mm3) data derived from the five chlorhexidine diacetate-incorporated resin blends (CDA)
| CDA% | 0% | 0.2% | 1.0% | 2.0% |
|---|---|---|---|---|
| Resin 1 | 4.36ab(2.50) | 6.28a(1.13) | 6.55a(1.10) | 7.58a(3.39) |
| Resin 2 | −5.04ce(3.57) | −3.56c(1.18) | 0.76d(1.42) | 3.81b(1.97) |
| Resin 3 | −8.00e(2.33) | −6.32e(2.26) | 0.70d(1.64) | 3.54b(1.51) |
| Resin 4 | −6.97e(2.49) | −6.93e(0.87) | 1.01d(1.94) | 3.94b(1.45) |
| Resin 5 | 227.61f(25.08) | 243.66f(21.76) | 253.13f(22.20) | 279.46f(14.43) |
Values are means (standard deviations). Groups with the same superscripts are not significantly different (p > 0.05). Negative values indicate increase in mass due to water uptake.
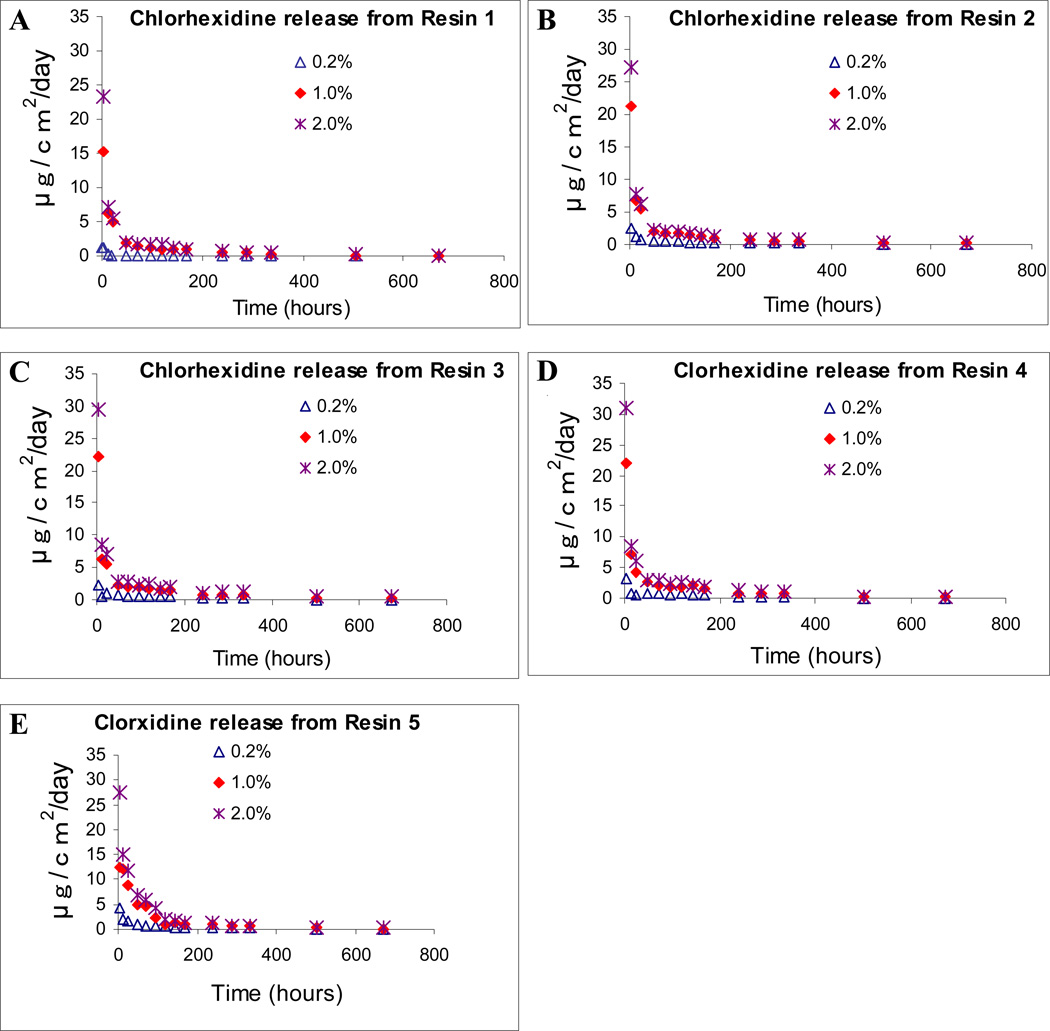
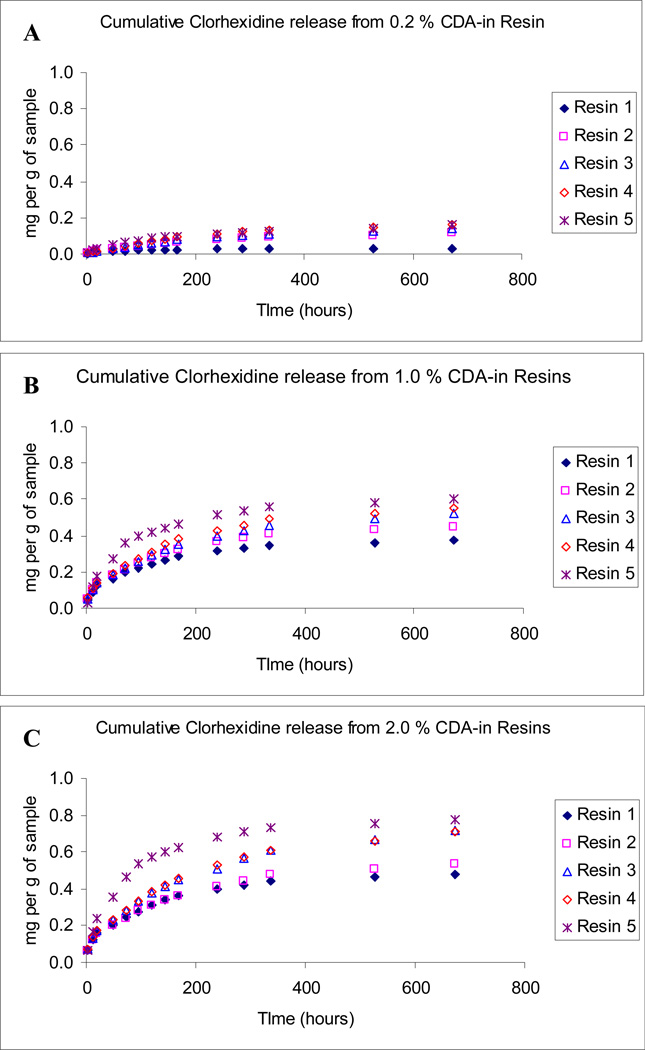
The rate of chlorhexidine release and cumulative release from polymerised resin blends are shown in Figs. 2 and 3. The specimens prepared from control neat resin blends R1 and R2 (without any CDA) did not release any detectable amount of chlorhexidine. Three-way ANOVA showed that the three factors, resin material, CDA concentration and storage time significantly affected the chlorhexidine release rates (p < 0.0001). There was a significant interaction between resin material and CDA concentration, between resin material and storage time and between CDA concentration and storage time (p < 0.0001). Specimens prepared from R1, regardless of the CDA concentrations, exhibited the lowest CDA release. In general, after an initially high release of chlorhexidine for 48 h, the mean release rate declined rapidly, and then reduced gradually over time. After 28 days of water storage, the final cumulative chlorhexidine release from R1–R5 were 0.030–0.1585 mg per g of sample that contained 0.2 % CDA (Fig. 3A), 0.3737–0.6017 mg per g of sample that contained 1.0 % CDA (Fig. 3B) and 0.480–0.7768 mg per g of sample that contained 2.0 % CDA (Fig. 3C).
Fig.2.
Chlorhexidine release rates from CDA-incorporated resin disks containing 0% (control), 0.2%, 1.0% and 2.0% CDA. A. CDA-incorporated R1. B. CDA-incorporated R2. C. CDA-incorporated R3. D. CDA-incorporated R4. E. CDA-incorporated R5.
Fig.3.
Cumulative chlorhexidine release after 28 days of water storage, expressed as mg per g of samples that contain different CDA concentrations. A. 0.2 % CDA-incorporated resin blends. B. 0.1 % CDA-incorporated resin blends. C. 2.0 % CDA-incorporated resin blends.
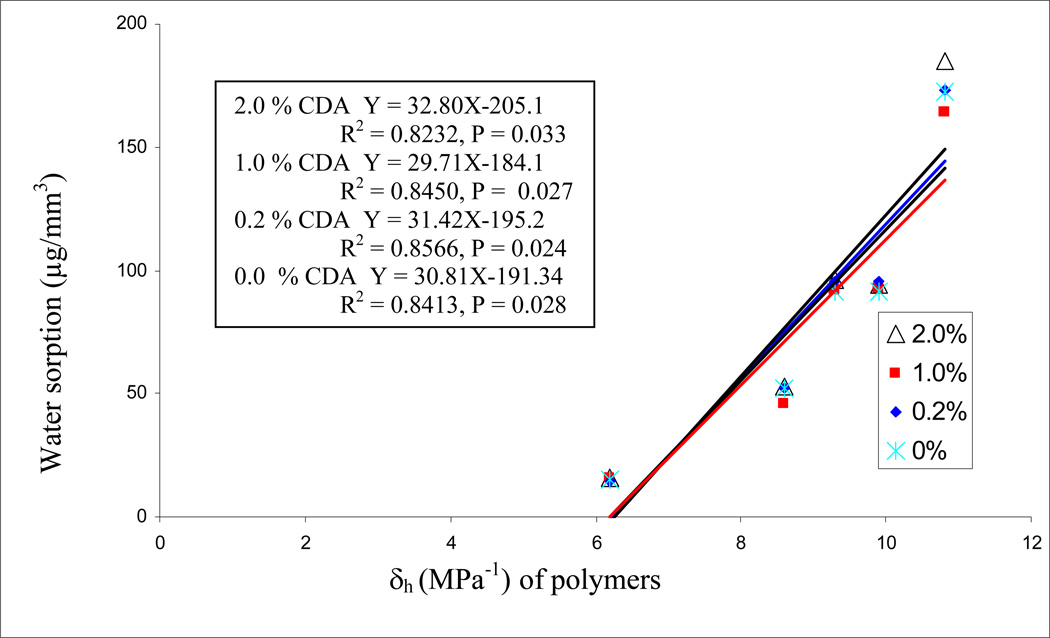
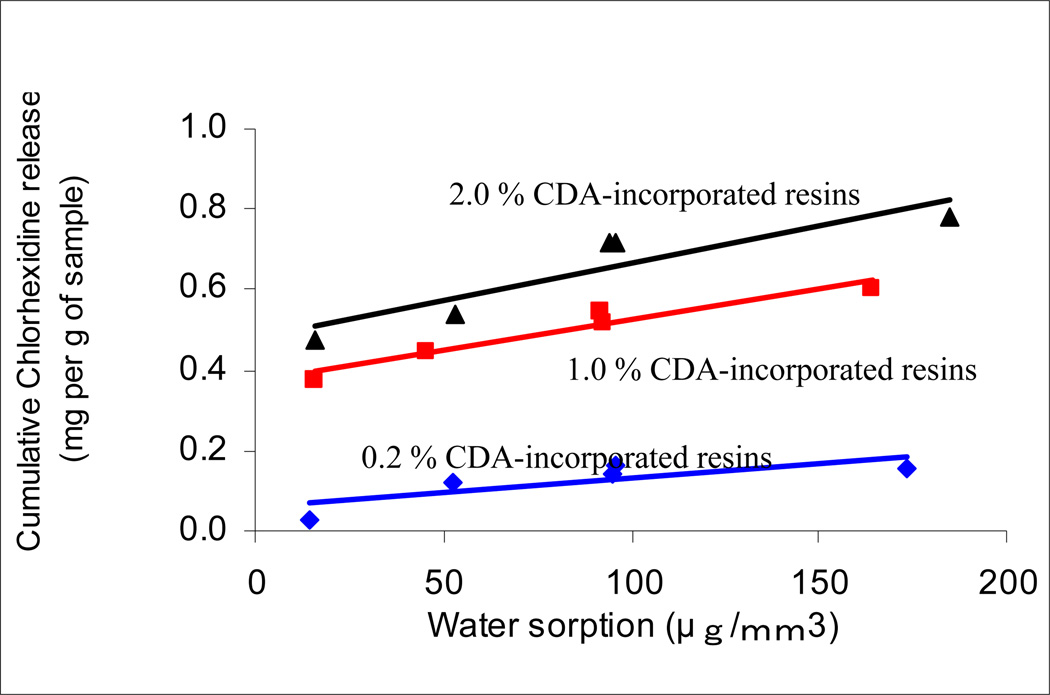
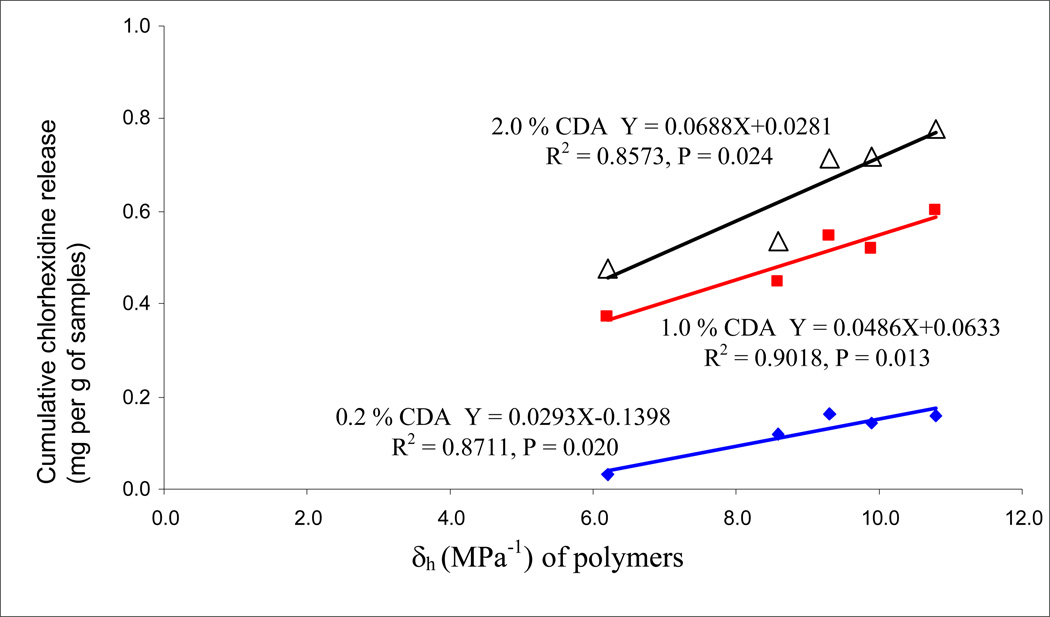
Regression analysis of the water sorption data of the five experimental resins versus their Hoy’s solubility parameters (δh) are shown in Fig. 4. The high significant R2 values were obtained; the higher hydrogen bonding of the resins, the higher was the water sorption. The presence or absence of CDA did not change the relationships. When the cumulative release of chlorhexidine was plotted against the water sorption of the same resins (Fig. 5), significant positive linear correlations were observed with R = 0.962 (p = 0.009) for 1.0% CDA-incorporated resins, and R = 0.908 (p = 0.033) for the 2.0% CDA-incorporated resins, while not significant correlation were found with R = 0.810 (p = 0.097) for the 0.2% CDA-incorporated resins. When cumulative chlorhexidine release was plotted against the Hoy’s δh values of the resins, highly significant correlations were obtained (Fig. 6). When similar regression analyses were done on resin solubility, no significant correlations could be made with Hoy’s solubility parameters (the result not shown).
Fig. 4.
Regression analysis of water sorption of resins 1–5 with 0, 0.2, 1.0 or 2.0 wt% CDA, versus the Hoy’s solubility parameter (δh).
Fig.5.
Regression analyses of the cumulative chlorhexidine release for 28 days against the water sorption of resins (R1–R5). The cumulative chlorhexidine release represents as mg per g of samples. Equations for 0.2%, 1.0% and 2.0% are Y = 0.0007X + 0.00578 (R2 = 0.6557, p = 0.097), Y = 0.0015X + 0.3734 (R2 = 0.926, p = 0.009) and Y = 0.0019X + 0.4789 (R2 = 0.8243 p = 0.0033), respectively.
Fig. 6.
Regression analysis of cumulative chlohexidine release from the five experimental resins (R1–5) containing 0.2, 1.0 or 2.0 wt% CDA plotted versus the Hoy’s δh values for the resins.
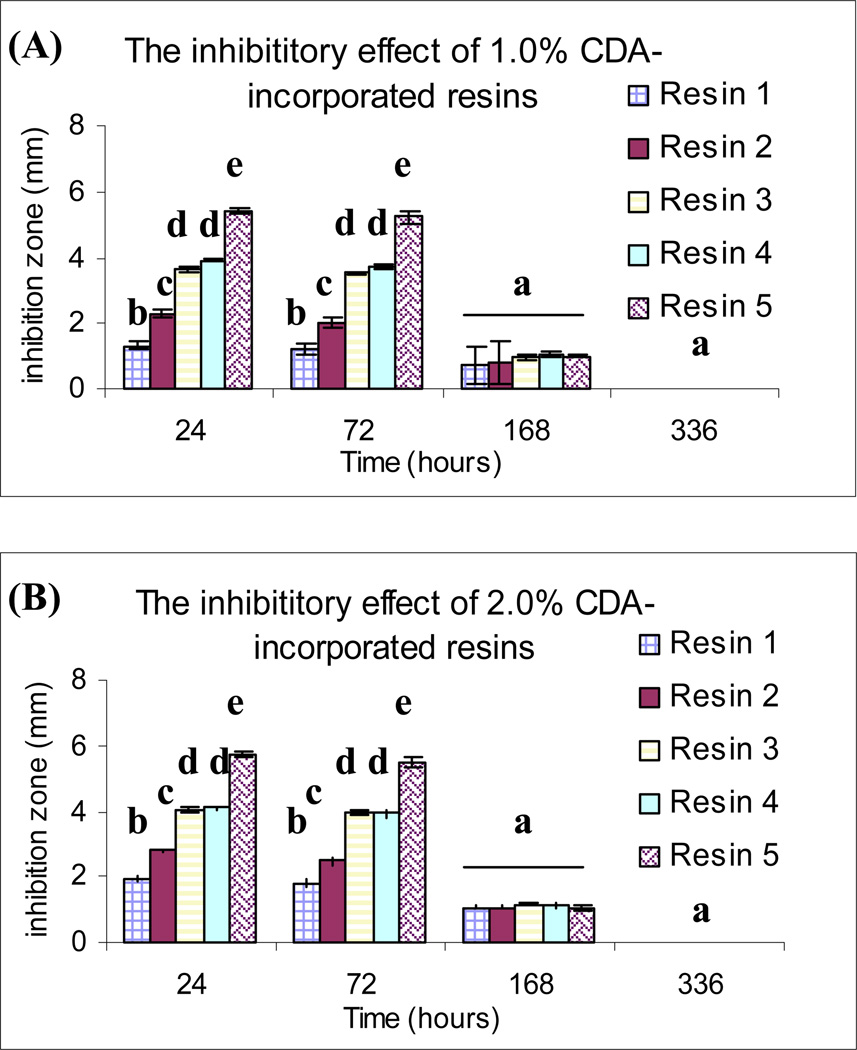
The inhibitory effect of chlorhexidine released from the resin disks against growth of Streptococcus mutans are summarised in Fig. 7. No inhibition zones were produced by either of the specimens containing 0 % (control) and 0.2 % CDA at all times. Among the 1.0 % and 2.0 % CDA-incorporated specimens that were retrieved at 24 and 72 h of water storage, the inhibition zones were significantly larger for R5, while those observed for R1 were the smallest (p < 0.05). After 168 h of water immersion, inhibition zones showed decreased diameter and no significant differences between resin types and CDA concentrations were further observed.
Fig.7.
Antibacterial effects of CDA-incorporated resin disks against Streptococcus mutans after different periods of water storage, as evaluated by the dimensions of their inhibition zones. The same superscript letters indicate no significant difference (p > 0.05). A. Inhibition zone (mm) produced by 1.0 % CDA-incorporated resin disks. B. Inhibition zone (mm) produced by 2.0 % CDA-incorporated resin disks. No inhibition zones were observed by the 0.0% and 0.2% CDA-incorporated resin disks at any time (not shown).
4. DISCUSSION
Chlorhexidine and other antimicrobial agents have been shown to be released at relatively high rates from various methacrylate polymers [22–24]. A chlorhexidine-releasing HEMA system has been investigated for factors that affect polymerisation rates, water sorption, volumetric stability and chlorhexidine release rates [23]. In that study, chlorhexidine-releasing HEMA-based composites were produced by addition of a hydrophobic urethane dimethacrylate (UDMA) and a relatively hydrophilic triethyleneglycol dimethacrylate (TEGDMA). Clinically acceptable polymerisation rates was achieved by the addition of UDMA and TEGDMA, with increased cross-linking of the comonomers and suppressed water sorption-induced swelling that resulted in controlled chlorhexidine release for extended time periods. When high loads (9–33 wt%) of CDA were added to 70 wt% UDMA/30% TEGDMA resin systems the undissolved CDA acted as fillers [21]. Higher filler loadings was found to reduce the degree of polymerisation, increase the dissolution of organic components, and resulted in faster chlorhexidine release rates [21]. In the current study, CDA powder was dissolved in the neat blends before polymerization.
Experimental CDA-incorporated resin systems were prepared using unfilled resin blends with increasing hydrophilicity to measure mass changes and chlorhexidine release over time. For CDA to be eluted from the neat resin, water must be absorbed into the resin where it can solubilise the CDA. The amount of chlorhexidine released was linearly proportional to the water sorption exhibited by resin blends of increasing hydrophilicity. That is, one can control the rate of CDA release by controlling water sorption that is proportional to the hydrophilicity of the resin. Thus, the first null hypothesis that the degree of hydrophilicity of resin blends has no effect on chlorhexidine release from cured resins has to be rejected. The addition of chlorhexidine to methacrylate-based resins had no significant effect on water sorption. Conversely, solubility of the resin components was increased depending on the concentration of the chlorhexidine being incorporated into the resins. Therefore, the second null hypothesis that the addition of chlorhexidine has no effect on the water uptake/sorption by the resin blends was partially rejected because higher CDA concentrations were associated with higher solubilities. This may have been due to lower percent conversions of monomers to polymers at higher CDA concentrations.
Profiles of drug controlled release usually reveal an initial burst release of short duration that is followed by a longer period of continuous but declining release [25]. As shown in Fig.2, the 1.0 and 2.0 % CDA-incorporated resin disks exhibited an initial burst release. This is probably attributed to the CDA presented on the outer surface of the CDA-incorporated resin. The profiles of chlorhexidine release from resin blends R1–R5, in general, corresponded with the changes in resistance and capacitance of thin resin films prepared from the same five resin blends [26]. The cumulative chlorhexidine release also correlated significantly with water sorption of the resin disks (Fig.5), which is indicative of swelling within the polymer matrix [27]. The time when chlorhexidine release was restrained should correspond to the time when mass change in the resin disks attained an equilibrium level. The greatest cumulative chlorhexidine release was observed from the R5 specimens. This is probably the result of more severe swelling of the polymer network in the R5 resin by water intake. As a result of swelling, water channels were probably enlarged in the polymer [26], through which rapid diffusion of the water molecules was facilitated. Water would slowly elute CDA depending on its solubility in water and its diffusion coefficient through the resin matrix.
An unexpected finding was that the least hydrophilic resin R1 presented higher solubility than the more hydrophilic resins R2, R3 and R4. Generally, polymer networks prepared by free radical polymerisation of dimethacrylates show a spatial heterogeneity in cross-linking density [28]. In more heterogeneous part of the polymer network, the microporous spaces created between the polymer clusters serve as a depot for unreacted monomers. The monomer units of Bis-GMA and Bis-GMA-E have similar chemical structures. However, the former resin molecule contains two hydroxyl groups which can form strong hydrogen bonds that increase the rigidity of the polymer network [28]. Poly-Bis-GMA-E has been shown to create less rigid, more heterogeneous polymer networks, with lower Young’s modulus and higher release of unreacted monomers than poly-Bis-GMA [28,29]. This may account for the higher solubility observed in R1.
The resin disks from R5 exhibited accelerated water solubility and severe crazing and cracking after water immersion for 28 days. Similar irreversible morphologic changes have been reported on R5 resin disks [18]. Crack formation facilitates bulk water movement through a polymer matrix by creating new channels for water diffusion and enhances water uptake, subsequently expediting the release of chlorhexidine. Due to its low molecular weight (130 daltons), unreacted HEMA also leaches more rapidly from the polymer matrix after crazing and cracking [30].
Antibacterial activity on Streptococcus mutans was only observed from the 1.0% and 2.0% CDA-incorporated resin disks after water storage. Moreover, no inhibition effect was further detected from resin disks derived from any group after 2 weeks of water storage. These results suggest that like the MPDB-containing resins [5–7], the experiment CDA-incorporated resins do not continuously release enough CDA to create a bacterial-free halo. For the 2.0% CDA-incorporated resin groups, the concentrations of chlorhexidine released was 0.48–0.78 mg/g or 0.0432–0.0702 mg/90 mg disk into 5 ml of water. It was not found that these consentrations exceeded the concentrations that are required to inhibit the activities of matrix metalloproteinases (MMPs). The minimal concentration of chlorhexidine that resulted in complete inhibition of MMP-9 activity was reported to be 0.002%, while MMP-2 activity was inhibited at a chlorhexidine concentration as low as 0.0001% [31]. Chlorhexidine concentrations between 0.01–0.02% are sufficient to completely inhibit the activities of MMP-8 [31]. Endogenous MMP-2, MMP-8 and MMP-9 have recently been shown to be present in human dentine [32–34]. Activated forms of these enzymes have been speculated to be responsible for auto-degradation of unprotected collagen fibrils within hybrid layers. Both in vitro and in vivo studies have shown that chlorhexidine applied directly to acid-etched matrices has the ability to prevent degradation of hybrid layers [14–16, 35].
Understandably, chlorhexidine released by the CDA-incorporated resins to an external environment (e.g. a gap along the resin-dentine interface) may be readily diluted so that after several weeks, the concentration is no longer sufficient to exert any appreciable antibacterial activity. However, chlorhexidine released by these resins to an internal environment (i.e. within hybrid layers) are likely to remain within the interfibrillar spaces due to the inherent substantivity of the chlorhexidine in human dentine [36, 37]. As there is concern that current dentine adhesives are too hydrophilic [38] and results in rapid deterioration of material properties via water sorption [39, 40], there is an ongoing trend to bond hydrophobic resins such as comonomer blend R2 to acid-etched dentine using the ethanol wet bonding technique [41, 42]. Although bonding of hydrophobic resins to acid-etched dentine reduces water sorption of the resin components, such a procedure does not prevent degradation of incompletely infiltrated collagen fibrils by endogenous MMPs. Thus, the results obtained from this study strongly point to the potential of incorporating CDA in ethanol-solvated hydrophobic resins to prevent collagen degradation within hybrid layers created by these hydrophobic adhesives. Further work should be performed to determine, with the use of gelatine zymography assays [31], the optimal amounts of chlorhexidine released into solution from CDA-incorporated hydrophobic resin blends that are required for inactivating MMPs.
5. CONCLUSION
Within the limits of this study, it may be concluded that:
Chlorhexidine release from chlorhexidine diacetate-incorporated resin disks is highly correlated with the degree of water uptake/sorption, which, in turn is dependent upon the hydrophilic characteristics of the resin compositions.
Solubility of the resin disks was probably affected by both the amount of chlorhexidine and unreacted residual monomers present in the polymer matrix.
ACKNOWLEDGMENTS
The resin blends used in this study were generous gifts from Bisco, Inc. This study was supported in part, by grant 10207401/14207/08004/323/01 and 10207821.14207.8004.324.01, Faculty of Dentistry, The University of Hong Kong, and by grants DE 014911 and DE 015306 from NIDCR to DHP (PI). The authors are grateful to Zinnia Pang and Michelle Barnes for secretarial assistance.
REFERENCES
- 1.Tay FR, Pashley DH. Dental adhesives of the future. J Adhes Dent. 2002;4:91–103. [PubMed] [Google Scholar]
- 2.Van Meerbeek B, De Munck J, Yoshida Y. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003;28:215–235. [PubMed] [Google Scholar]
- 3.Pashley DH. Dentin: a dynamic substrate--a review. Scanning Microsc. 1989;3:161–174. [PubMed] [Google Scholar]
- 4.Kidd EAM. Microleakage: a review. J Dent. 1976;4:199–205. doi: 10.1016/0300-5712(76)90048-8. [DOI] [PubMed] [Google Scholar]
- 5.Imazato S, Kinomoto Y, Tarumi H, Torii M, Russell RRB, McCabe JF. Incorporation of antibacterial monomer MDPB in dentin primer. J Dent Res. 1994;73:1437–1444. doi: 10.1177/00220345970760030901. [DOI] [PubMed] [Google Scholar]
- 6.Imazato S, Torii Y, Takatsuka T, Inoue K, Ebi N, Ebisu S. Bactericidal effect of dentin primer containing antibacterial monomer methacryloyloxydodecylpyridinium bromide (MDPB) against bacteria in human carious dentin. J Oral Rehabil. 2001;28:314–319. doi: 10.1046/j.1365-2842.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- 7.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dent Mater. 2003;19:313–319. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 8.Bergenholtz G, Cox CF, Loesche WJ. Bacterial leakage around dental restorations: its effect on the dental pulp. J Oral Pathol. 1982;11:439–450. doi: 10.1111/j.1600-0714.1982.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 9.Gwinnett AJ, Dickerson WD, Yu S. Dentin bond shear strength and microleakage for Syntac/Heliomolar: a comparison between the manufacturer’s and total etch technique. J Esthet Dent. 1992;4:164–168. doi: 10.1111/j.1708-8240.1992.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 10.Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995;20:18–25. [PubMed] [Google Scholar]
- 11.Sano H, Yoshiyama M, Ebisu S, Burrow MF, Takatsu T, Ciucchi B, Carvalho R, Pashley DH. Comparative SEM and TEM observations of nanoleakage within the hybrid layer. Oper Dent. 1995;20:160–170. [PubMed] [Google Scholar]
- 12.Li H, Burrow MF, Tyas MJ. Nanoleakage patterns of four dentin bonding systems. Dent Mater. 2000;16:48–56. doi: 10.1016/s0109-5641(99)00085-8. [DOI] [PubMed] [Google Scholar]
- 13.Eick JD, Gwinnett AJ, Pashley DH, Robinson SJ. Current concepts on adhesion to dentin. 1997;8:306–335. doi: 10.1177/10454411970080030501. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials. 2003;24:3795–3805. doi: 10.1016/s0142-9612(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 15.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 16.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–746. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 17.Riggs PD, Braden M, Patel M. Chlorhexidine release from room temperature polymerising methacrylate systems. Biomaterials. 2000;21:345–351. doi: 10.1016/s0142-9612(99)00187-8. [DOI] [PubMed] [Google Scholar]
- 18.Yiu CK, King NM, Carrilho MRO, Sauro S, Rueggeberg FR, Prati C, Carvalho RM, Pashley DH, Tay FR. Effect of resin hydrophilicity and temperature on water sorption of dental adhesive resins. Biomaterials. 2006;27:1695–1703. doi: 10.1016/j.biomaterials.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Malacarne J, Carvalho RM, de Goes MF, Svizero N, Pashley DH, Tay FR, Yiu CK, Carrilho MRO. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006;22:973–980. doi: 10.1016/j.dental.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Unemori M, Matsuya Y, Matsuya S, Akashi K, Akamine A. Water absorption of poly(methyl methacrylate) containing 4-methacryloxyethyl trimellitic anhydride. Biomaterials. 2003;24:1381–1387. doi: 10.1016/s0142-9612(02)00521-5. [DOI] [PubMed] [Google Scholar]
- 21.Anusavice KJ, Zhang NZ, Shen C. Controlled release of chlorhexidine from UDMA-TEGDMA resin. J Dent Res. 2006;85:950–954. doi: 10.1177/154405910608501016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel MP, Braden M, Downes S. Heterocylic methacrylate-based drug release polymer system. J Mater Sci Mater Med. 1994;5:793–797. [Google Scholar]
- 23.Nerurkar MJ, Zentner GM, Rytting JH. Effect of chloride on the release of chlorhexidine salts from methyl methacrylate-2-hydroxyethyl methacrylate copolymer reservoir devices. J Control Release. 1995;33:357–363. [Google Scholar]
- 24.Leung D, Spratt DA, Pratten J, Gulabivala K, Mordan NJ, Young LA. Chlorhexidine-releasing methacrylate dental composite materials. Biomaterials. 2005;26:7145–7153. doi: 10.1016/j.biomaterials.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Haung X, Christopher SB. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73:121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 26.Wadgaonkar B, Ito S, Svizero N, Elrod D, Foulger S, Rodgers R, Oshida Y, Kirkland K, Sword J, Rueggeberg F, Tay F, Pashley D. Evaluation of the effect of water-uptake on the impedance of dental resins. Biomaterials. 2006;27:3287–3294. doi: 10.1016/j.biomaterials.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 27.Riggs PD, Braden M, Tilbrook DA, Swai H, Clarke RL, Patel MP. Water uptake of poly(tetrahydrofurfuryl methacrylate) Biomaterials. 1999;20:435–441. doi: 10.1016/s0142-9612(98)00188-4. [DOI] [PubMed] [Google Scholar]
- 28.Sideridou I, Tserki V, Papanastasiou G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials. 2003;24:655–665. doi: 10.1016/s0142-9612(02)00380-0. [DOI] [PubMed] [Google Scholar]
- 29.Sideridou I, Tserki V, Papanastasiou G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials. 2002;23:1819–1829. doi: 10.1016/s0142-9612(01)00308-8. [DOI] [PubMed] [Google Scholar]
- 30.Michelsen VB, Lygre H, Skalevik R, Tveit AB, Solheim E. Identification of organic eluates from four polymer-based dental filling materials. Eur J Oral Sci. 2003;111:263–271. doi: 10.1034/j.1600-0722.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 31.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol. 1999;6:437–439. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinases gelatinase A in human dentine. Arch Oral Biol. 2000;45:757–765. doi: 10.1016/s0003-9969(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 33.Sulkala M, Tervahartiala T, Sorsa T, Lamas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–127. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Mazzoni A, Mannello F, Tay FR, Tonti GAM, Papa S, Mazzotti G, DiLenarda R, Pashley DH, Breschi L. Zymographic analyses and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res. doi: 10.1177/154405910708600509. (in press). [DOI] [PubMed] [Google Scholar]
- 35.Carrilho MRO, Geraldeli S, Tay FR, de Goes MF, Carvalho RM, Tjaderhane L, Reis A, Hebling J, Mazzoni A, Breschi L. In vivo preservation of hybrid layer by chlorhexidine. J Dent Res. doi: 10.1177/154405910708600608. (in press). [DOI] [PubMed] [Google Scholar]
- 36.Basrani B, Santos JM, Tjäderhane L, Grad H, Gorduysus O, Huang J, Lawrence HP, Friedman S. Substantive antimicrobial activity in chlorhexidine-treated human root dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:240–245. doi: 10.1067/moe.2002.124002. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal S, Spångberg L, Safavi K. Chlorhexidine substantivity in root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:488–492. doi: 10.1016/j.tripleo.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? J Cad Dent Assoc. 2003;69:726–731. [PubMed] [Google Scholar]
- 39.Yiu CKY, King NM, Pashley DH, Suh BI, Carvalho RM, Carrilho MRO, Tay FR. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials. 2004;25:5789–5796. doi: 10.1016/j.biomaterials.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 40.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, Rueggeberg FA, Foulger S, Saito T, Nishitani Y, Yoshiyama M, Tay FR, Pashley DH. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–6459. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 41.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, Garcia-Godoy F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007;20:7–20. [PubMed] [Google Scholar]
- 42.Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, Pashley DH. Effects of resin hydrophilicity on dentin bond strength. J Dent Res. 2006;85:1016–1021. doi: 10.1177/154405910608501108. [DOI] [PMC free article] [PubMed] [Google Scholar]