Abstract
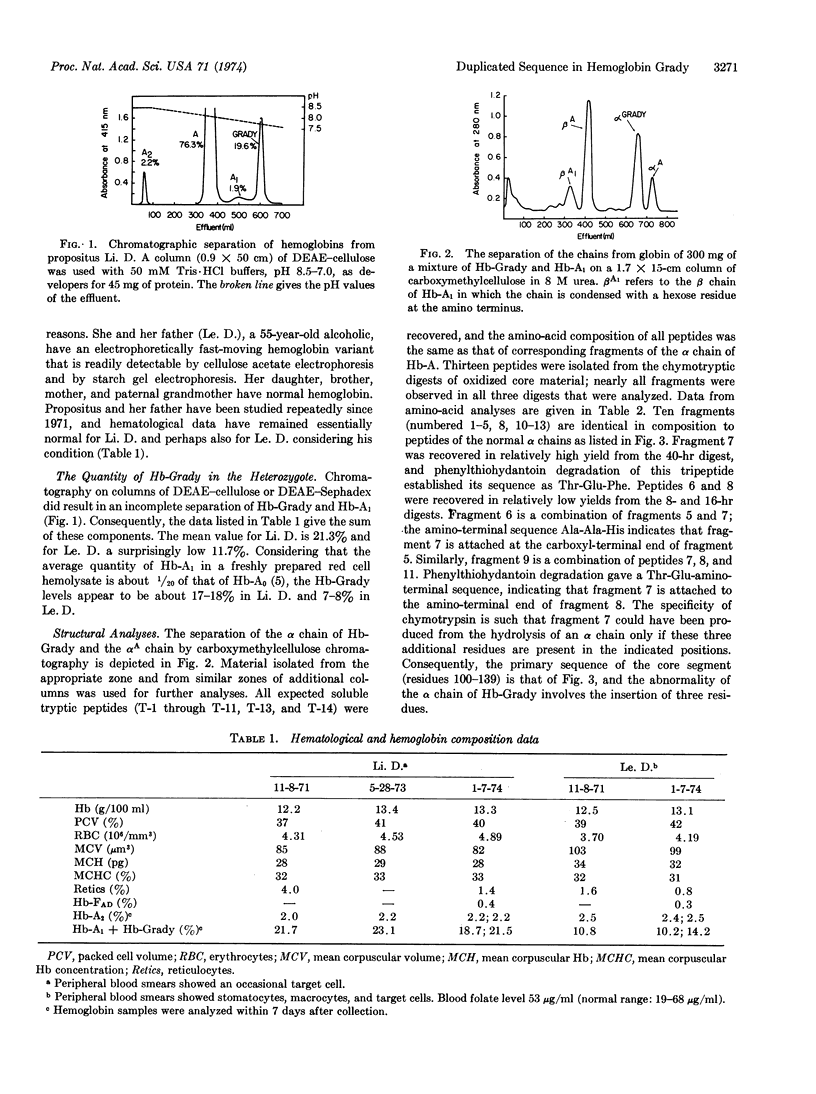
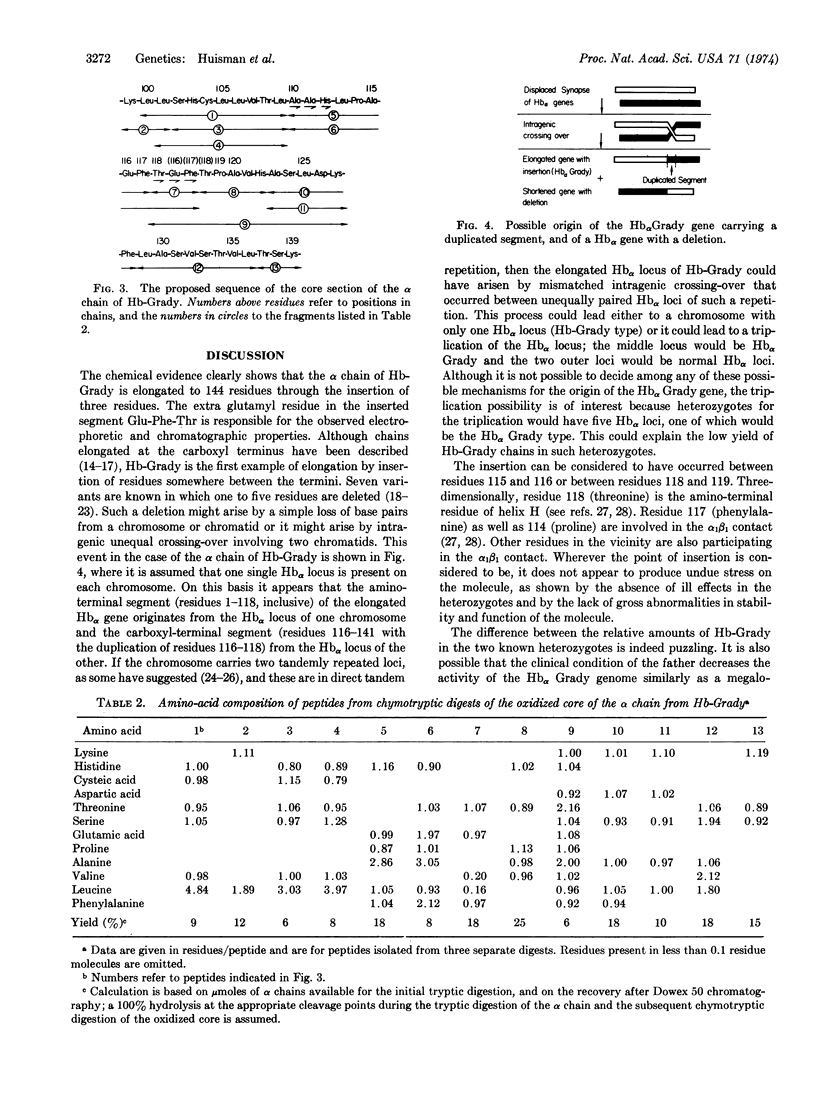
A black 25-year-old woman and her father have a fast-moving α chain variant in an amount of 8% (the father) and 18% (the daughter). Structural data indicate that this chain has been elongated by the addition of three amino-acid residues to give the sequence: -Pro(114)-Ala(115)-Glu(116)-Phe(117)-Thr(118)-Glu-Phe-Thr-Pro(119)-Ala(120)-.The underlying genetic alteration responsible for hemoglobin Grady appears, therefore, to be a tandem duplication of nine base pairs which may have arisen by a process of mismatched intragenic crossing over. Functional and physicochemical properties of the variant are not greatly altered, and hematological data are normal.
Keywords: alpha chain, gene duplication, amino-acid sequence
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BETKE K., MARTI H. R., SCHLICHT I. Estimation of small percentages of foetal haemoglobin. Nature. 1959 Dec 12;184(Suppl 24):1877–1878. doi: 10.1038/1841877a0. [DOI] [PubMed] [Google Scholar]
- Bradley T. B., Jr, Wohl R. C., Rieder R. F. Hemoglobin Gun Hill: deletion of five amino acid residues and impaired heme-globin binding. Science. 1967 Sep 29;157(3796):1581–1583. doi: 10.1126/science.157.3796.1581. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Milner P. F. Haemoglobin Constant Spring--a chain termination mutant? Nature. 1971 Dec 10;234(5328):337–340. doi: 10.1038/234337a0. [DOI] [PubMed] [Google Scholar]
- De Jong W. W., Went L. N., Bernini L. F. Haemoglobin Leiden: deletion of beta-6 or 7 glutamic acid. Nature. 1968 Nov 23;220(5169):788–790. doi: 10.1038/220788a0. [DOI] [PubMed] [Google Scholar]
- Dozy A. M., Kleihauer E. F., Huisman T. H. Studies on the heterogeneity of hemoglobin. 13. Chromatography of various human and animal hemoglobin types on DEAE-Sephadex. J Chromatogr. 1968 Feb 20;32(4):723–727. doi: 10.1016/s0021-9673(01)80551-3. [DOI] [PubMed] [Google Scholar]
- Efremov G. D., Huisman T. H., Smith L. L., Wilson J. B., Kitchens J. L., Wrightstone R. N., Adams H. R. Hemoglobin Richmond, a human hemoglobin which forms asymmetric hybrids with other hemoglobins. J Biol Chem. 1969 Nov 25;244(22):6105–6116. [PubMed] [Google Scholar]
- Efremov G. D., Wrightstone R. N., Huisman T. H., Schroeder W. A., Hyman C., Ortega J., Williams K. An unusual hemoglobin anomaly and its relation to alpha-thalassemia and hemoglobin-H disease. J Clin Invest. 1971 Aug;50(8):1628–1636. doi: 10.1172/JCI106651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatz G., Kinderlerer J. L., Kilmartin J. V., Lehmann H. Haemoglobin Tak: a variant with additional residues at the end of the beta-chains. Lancet. 1971 Apr 10;1(7702):732–733. doi: 10.1016/s0140-6736(71)91994-5. [DOI] [PubMed] [Google Scholar]
- HELLER P., YAKULIS V. J., EPSTEIN R. B., FRIEDLAND S. Variation in the amount of hemoglobin S in a patient with sickle cell trait and megaloblastic anemia. Blood. 1963 Apr;21:479–483. [PubMed] [Google Scholar]
- Hollán S. R., Szelenyi J. G., Brimhall G., Duerst M., Jones R. T., Koler R. D., Stocklen Z. Multiple alpha chain loci for human haemoglobins: Hb J-Buda and Hb G-Pest. Nature. 1972 Jan 7;235(5332):47–50. doi: 10.1038/235047a0. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Huisman T. H. Normal and abnormal human hemoglobins. Adv Clin Chem. 1972;15:149–253. doi: 10.1016/s0065-2423(08)60160-2. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Schroeder W. A. IV. The chemical heterogeneity of the chain from human fetal hemoglobin. CRC Crit Rev Clin Lab Sci. 1970 Jul;1(3):514–526. doi: 10.3109/10408367009027956. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Wrightstone R. N., Wilson J. B., Schroeder W. A., Kendall A. G. Hemoglobin Kenya, the product of fusion of amd polypeptide chains. Arch Biochem Biophys. 1972 Dec;153(2):850–853. doi: 10.1016/0003-9861(72)90408-0. [DOI] [PubMed] [Google Scholar]
- Jones R. T., Brimhall B., Huisman T. H., Kleihauer E., Betke K. Hemoglobin Freiburg: abnormal hemoglobin due to deletion of a single amino acid residue. Science. 1966 Nov 25;154(3752):1024–1027. doi: 10.1126/science.154.3752.1024. [DOI] [PubMed] [Google Scholar]
- Kattamis C., Lehmann H. Duplication of alpha-thalassaemia gene in three Greek families with haemoglobin H disease. Lancet. 1970 Sep 26;2(7674):635–637. doi: 10.1016/s0140-6736(70)91401-7. [DOI] [PubMed] [Google Scholar]
- LEVERE R. D., LICHTMAN H. C., LEVINE J. EFFECT OF IRON-DEFICIENCY ANAEMIA ON THE METABOLISM OF THE HETEROGENIC HAEMOGLOBINS IN SICKLE CELL TRAIT. Nature. 1964 May 2;202:499–501. doi: 10.1038/202499a0. [DOI] [PubMed] [Google Scholar]
- Wajcman H., Labie D., Schapira G. Two new hemoglobin variants with deletion. Hemoglobin tours: Thr 8 7 (F 3 ) deleted and hemoglobin St Antoine: Gly-Leu 74-75 (E 18-19 deleted. Consequences for oxygen affinity and protein stability. Biochim Biophys Acta. 1973 Feb 21;295(2):495–504. [PubMed] [Google Scholar]
- Wasi P. Is the human globin -chain locus duplicated? Br J Haematol. 1973 Mar;24(3):267–273. doi: 10.1111/j.1365-2141.1973.tb01651.x. [DOI] [PubMed] [Google Scholar]



