Abstract
The tear film covers the anterior eye and the precise balance of its various constituting components is critical for maintaining ocular health. The composition of the tear film amphiphilic lipid sublayer, in particular, has largely remained a matter of contention due to the limiting concentrations of these lipid amphiphiles in tears that render their detection and accurate quantitation tedious. Using systematic and sensitive lipidomic approaches, we validated different tear collection techniques and report the most comprehensive human tear lipidome to date; comprising more than 600 lipid species from 17 major lipid classes. Our study confers novel insights to the compositional details of the existent tear film model, in particular the disputable amphiphilic lipid sublayer constituents, by demonstrating the presence of cholesteryl sulfate, O-acyl-ω-hydroxyfatty acids, and various sphingolipids and phospholipids in tears. The discovery and quantitation of the relative abundance of various tear lipid amphiphiles reported herein are expected to have a profound impact on the current understanding of the existent human tear film model.
Keywords: lipidomics, mass spectrometry, dry eye syndrome, meibum, tear lipidome, cholesteryl sulfates, O-acyl-ω-hydroxy fatty acids
The tear fluid covers the anterior surface of the cornea and serves critical functions in maintaining the homeostasis of the ocular surface. Tears hydrate and lubricate the mucous membranes constituting the ocular surface, supply nourishment to the avascular corneal epithelium, and provide a smooth optical surface essential for visual acuity. The drainage of tears via the lacrimal puncta flushes contaminants and irritants out of the eye, thereby functioning as a first line of defense for the anterior eye against invading pathogens (1, 2). The typical volume of tears in normal eyes ranges from 3.4 to 10.7 μl per eye (3). Despite its small volume, tears represent a biological fluid of immense complexities with a wide array of proteins/peptides, electrolytes, lipids, and small molecule metabolites contributed by distinct sources (1). The precise balance of these various metabolites is crucial in ensuring proper physiological function and maintaining biophysical integrity of the precorneal tear film. Perturbations in this delicate equilibrium may be manifested in various ocular conditions such as dry eye syndrome (DES) and blepharitis (1, 4, 5).
Recent decades have witnessed tremendous progress in the systematic profiling of proteins as well as small molecule metabolites present in tears (1, 6–9). Furthermore, with technological advancements in MS and nuclear magnetic resonance spectroscopy, the lipid composition of the human meibomian gland secretions, the predominant source of lipids for the precorneal tear film, has been elucidated with great compositional and structural details (10–19). A comprehensive lipidomic analysis of the tear fluid per se, however, has lingered behind. The appreciably lower lipid concentration in human tears, which is largely aqueous compared with the meibum, as well as the limiting amount of unstimulated tears that could be obtained from individual subjects, present a new level of challenge for the field of analytical lipidomics. In spite of these analytical challenges, a comprehensive elucidation of the tear lipidome is imperative to unravel the biophysical properties of the tear film. As a proximal fluid to the anterior eye, the tear is a dynamic reflection of metabolites present at the ocular surface and therefore represents a rich source for the discovery of ocular disease-related biomarkers. A systematic comparison of tear collection techniques will therefore facilitate the use of human tears for biomarker discovery pertaining to both ocular and systemic diseases, which would be of immense importance to the field of ocular research and beyond.
A preliminary analysis of human tears using MS concluded that meibum represents the prominent source of lipids for the tear film (13). The presence of phosphatidylcholines (PCs), sphingomyelins (SMs), wax esters (WEs), and free cholesterols (Chos) in tears was demonstrated using infrared spectroscopy (20). Saville et al. (21) subsequently provided the relative abundance of individual species of PCs and SMs in human tears. The polar lipid composition was further expanded by two other groups, who reported a predominance of lysophosphatidylcholines (lyso-PCs) (2, 22). In addition to PCs and SMs, Rantamäki et al. (2) also reported the presence of triacylglycerides (TAGs), ceramides (Cers), and phosphatidylethanolamines (PEs) in tears. As most of these studies only focused on selected lipid groups in tears, a comprehensive lipidome with sufficient details to encompass the sheer complexities of the various lipid classes in tears is therefore still lacking. Apart from the analytical challenges posed by the limited abundance but yet great complexities of lipid species in tears, the biochemical analysis of tears has also been plagued by the problem of specimen collection. Thus, a systematic comparison of tear lipidomes obtained using different collection methods will therefore be of immense practical value for the ocular community in facilitating the standardization of collection procedures. This will also aid future clinical designs to use human tears as a proximal fluid for biomarker discovery.
Herein, we report HPLC/MS-based approaches for the comprehensive, qualitative, and comparative characterization of human tears and meibum lipidomes from individual subjects. Our analyses revealed that human tears comprise more than 600 individual lipid species from 17 distinct lipid classes in volumes less than 10 μl per sample. We also report, for the first time, the presence of a novel lipid amphiphile, cholesteryl sulfate (CS), in the human tears and meibum, which would have considerable impact on the existent tear film model. In addition, systematic comparisons of the tear lipidomes collected with different sampling techniques were evaluated.
MATERIALS AND METHODS
Study group
For preliminary analysis and method validation, 45 patients and 15 volunteers were recruited to contribute pooled or individual samples of tears and meibum. The patients were diagnosed with DES at the Singapore National Eye Center. Detailed demographic information for the study group can be found in the supplementary section (supplementary Tables I, II). Written informed consent was obtained from all participating subjects and the procedure for the project was specifically approved by the SingHealth Centralised Institutional Review Board (CIRB reference number 2008/611/A). We adhered to the tenets of the Declaration of Helsinki for all human research conducted in this study. The detailed clinical procedures have been reported elsewhere (10).
Sample collection
Capillary tears were collected using 5 μl glass microcapillary tubes (Blaubrand® IntraEND, Wertheim, Germany). The collection time was limited to a maximum of 5 min with collection volumes between 2 and 10 μl. The capillary tube rested in the lateral tear meniscus and care was exercised to minimize contact with the bulbar conjunctiva or the lid margin. Reflex tearing was observed in some subjects (supplementary Table II). Flush tears were collected by instilling 20 μl of nonpreserved unit dose saline (sodium chloride injection 0.9%, B Braun, Germany) into the inferior palpebral fold using an Eppendorf pipette. Care was taken not to contact the eye with the pipette tip. Participants were then instructed to gently close and rotate their eyes. Tears were then immediately collected by the capillary tubes as aforementioned. Tears were expelled from the capillary tubes into glass vials. Capillary tubes were washed two times with chloroform:methanol (2:1) to remove residual lipids and the washings were also pooled together with the tear samples and stored at −80°C until further analysis. Schirmer's tears were collected from both eyes of the subjects without anesthesia (Bausch and Lomb® Sno Strips, New York, NY) and the strips were stored at −80°C in glass vials until further analysis. Meibum samples were collected as previously described (10).
Lipid extraction
Capillary tears.
Lipids were extracted at 4°C for 1 h with 200 μl ice-cold chloroform:methanol (1:1) at 1,200 rpm in a thermomixer. Samples were then centrifuged at 3,000 rpm for 5min at 4°C and 200 μl of clear supernatant was transferred to fresh glass vials. The second extraction was carried out by repeating the procedures and the extracted organic fractions were pooled and dried using speed-valco (Thermo Savant, Milford, NY). Dried lipid extracts were stored at −80°C until mass spectrometric analysis.
Schirmer's tears.
The first three 5 mm segments of the strips were cut into fine pieces (of approximately 2 mm) using micro-scissors prewashed with methanol between samples. Lipids were extracted overnight at 4°C with 900 μl of ice-cold chloroform:methanol (1:2) at 1,200 rpm in a thermomixer. Deionized water (540 μl) and chloroform (300 μl) were added to separate phases. Samples were vortexed and incubated on ice for 3 min. Samples were then centrifuged at 3,000 rpm at 4°C for 5 min and the lower organic phase was extracted. The aqueous phase was reextracted with 500 μl of chloroform and 50 μl of 0.1M hydrochloric acid. The extracted organic fractions were pooled and dried using speed-valco (Thermo Savant). Dried lipid extracts were stored at −80°C until mass spectrometric analysis.
Meibum.
Meibum samples were extracted as previously described (10).
Mass spectrometric analysis
All lipid species were quantitated using LC-multiple reaction monitoring (MRM) in a combined workflow, but high-resolution MS was used for characterization and confirmation of lipid identities, or for illustrative purposes. Concentrations of individual samples were preadjusted for mass spectrometric analyses by taking out 5 μl of each sample for estimation of total cholesteryl ester (CE) and total WE content such that the final adjusted concentrations for mass spectrometric analyses would lie within the linear dynamic range of the various lipid classes analyzed.
Normal phase LC/MS.
Polar lipids were analyzed using an Agilent 1200 HPLC system coupled with a triple quadrupole/ion trap mass spectrometer (3200Qtrap; Applied Biosystems) as described previously (23). Separation of individual lipid classes of polar lipids by normal phase (NP)-HPLC was carried out using a Phenomenex Luna 3 μ-silica column (internal diameter 150 × 2.0 mm) with the following conditions: mobile phase A (chloroform:methanol:ammonium hydroxide, 89.5:10:0.5) and mobile phase B (chloroform:methanol:ammonium hydroxide:water, 55:39:0.5:5.5). MRM transitions were set up for comparative analysis of various polar lipids. Individual lipid species were quantified by referencing to spiked internal standards. PC-14:0/14:0, PC34:1-d31, LPC-C20, PE-14:0/14:0, PE34:1-d31, LPE-C17, phosphatidylserine (PS)-14:0/14:0, lysophosphatidylserine (LPS)-C17, phosphatidic acid (PA)-17:0/17:0, LPA-C14, phosphatidylglycerol (PG)-14:0/14:0, C14-lyso-bisphosphatidic acid (LBPA), C8-glucosylceramide (GluCer), C17-Cer, C17-sphingosine-1-phosphate (S1P), and C12-SM were obtained from Avanti Polar Lipids (Alabaster, AL) and LIPID MAPS. Dioctanoyl phosphatidylinositol (PI) (16:0-PI) was obtained from Echelon Biosciences, Inc. (Salt Lake City, UT) and used together with PI34:1-d31 (LIPID MAPS) for PI and lysophosphatidylinositol (lyso-PI) quantitation. Qualitative deuterated lipid standards from LIPID MAPS were precorrected using quantitative lipid standards prior to their use for quantitation. NeuAcα2-3Galβ1-4Glcβ-Cer (GM3) species were quantified using C17-GM3 as an internal standard. O-acyl-ω-hydroxy-fatty acid (OAHFA) species were quantitated using OAHFA 18:1/16:0, which was synthesized as previously described (17).
Reverse phase LC/MS.
Phospholipids, sphingolipids, CSs, glycerol lipids [diacylglycerides (DAGs) and TAGs], WEs, and CEs were analyzed using a modified version of reverse phase (RP)-HPLC/ESI/MS/MS described previously (24). Briefly, separation of the aforementioned lipids was carried out on a Phenomenex Kinetex 2.6 μ-C18 column (i.d. 4.6 × 100 mm) using an isocratic mobile phase chloroform:methanol:0.1M ammonium acetate (100:100:4) at a flow rate of 150 μl/min for 22 min. CSs and individual CE species were quantified using d7-CS and d6-C18 CE (CDN Isotopes) as internal standards, respectively. MRM analysis of CSs was validated using d7-CS as internal standard (supplementary Fig. I). Using neutral loss-based MS/MS techniques, the levels of TAGs were calculated as relative contents to the spiked d5-TAG 48:0 internal standard (CDN Isotopes), while DAG species were quantified using 4ME 16:0 diether DG as an internal standard (Avanti Polar Lipids). Levels of WEs were quantified using three standards; palmitoyl palmitate (C16:0C16:0) was used for WEs with saturated fatty acyl heads, while WEs containing unsaturated fatty acyl heads were quantitated using 13C18:1C26:0 and 13C18:1C28:0, which were synthesized in-house using oleic acid-1,2,3,7,8,9,10-13C7 following the procedure described previously (25). The detailed mass spectrometric procedures for analysis of WEs have been reported elsewhere (26).
High-resolution MS.
Single stage MS profiles of lipid extracts were also carried out using an Accela HPLC system coupled with an LTQ Orbitrap XL hybrid Fourier transform mass spectrometer (Thermo Fisher Scientific, Waltham, MA) using both a RP-HPLC/MS and a NP-HPLC/MS approach as aforementioned. In the case of RP-LC/MS, 5 μl of each extract was injected into a Phenomenex Kinetex 2.6 μ-C18 column (i.d. 4.6 × 100 mm) with the mobile phase as aforementioned at a flow rate of 120 μl/min for a total duration of 30 min. In NP-LC/MS, separation of individual lipid classes of polar lipids by NP-HPLC was carried out using a Phenomenex Luna 3 μ-silica column (i.d. 150 × 2.0 mm) with the following conditions: mobile phase A (chloroform:methanol:ammonium hydroxide, 89.5:10:0.5) and mobile phase B (chloroform:methanol:ammonium hydroxide:water, 55:39:0.5:5.5). MS profiles were recorded under both positive and negative modes in separate runs (resolution 60,000), and mass accuracy of less than 2 ppm was obtained throughout the analytical runs.
Atmospheric pressure chemical ionization.
Free Chos and CEs were further analyzed using HPLC/atmospheric pressure chemical ionization (APCI)/MS/MS as previously described with corresponding d6-Cho and d6-C18 CE (CDN Isotopes) as internal standards (27). As we had previously shown that HPLC/APCI/MS allows for a wide dynamic linearity range in measuring the levels of CEs in plasma samples due to less ion suppression compared with the ESI mode (27), the absolute amount of total CEs obtained in APCI/MS/MS was used to correct results obtained in the ESI/MS/MS analysis of individual CE species.
Statistical analysis
The absolute levels of lipids in tears obtained using different collection techniques were compared using one-way ANOVA with a post hoc TukeyHSD test. For all analyses, ***P < 0.001; **P < 0.01; *P < 0.05; #0.05 ≤ P < 0.10.
RESULTS
Validation of the Schirmer's strip method of tear collection
In order to ensure that tears collected using Schirmer's strips represented an accurate reflection of the dynamic lipid microenvironment at the ocular surface, we considered a few key issues: 1) What is the lipid background in the paper strip per se? 2) To what extent does the strip act as a chromatographic system? 3) Does the portion of tears captured by the strip represent a true reflection of the concentrations of lipid metabolites in the tear reservoir?
We found that lipids were concentrated in the first two segments of the strip, with the −5 to 0 mm segment, which was in direct contact with the conjunctiva during the collection process, having the highest abundance of all lipid classes (supplementary Fig. II). The blank Schirmer's strips gave rise to considerable background noise in the mass spectrometric analysis of the individual lipid classes, but did not produce appreciable peak shapes in the mass spectra (data not shown). This indicated that the strip materials did not contain the endogenous tear lipids and that the Schirmer's strips were suitable for collecting tears intended for lipid analysis. In order to maximize the accuracy of our analytical results, we chose to extract and analyze lipids only from the first three segments (i.e., −5 to 10 mm, Schirmer's length = 10 mm) of the strips regardless of the actual length of the entire wetted portion, as it was apparent from our preliminary analyses that lipids, especially the nonpolar lipids such as CEs and WEs, do not travel along the strips as efficiently as the aqueous portion of the tears. This approach minimized the level of background noise presented by the strip materials without significantly compromising the extraction efficiency of the endogenous tear lipids, as the first three strip segments contained at least 80% of all lipids along the entire wetted area (supplementary Table III). Furthermore, lipid extraction from a fixed length of the strip standardized the background noise in individual samples for unbiased comparison of endogenous lipid levels between samples. Also, the region of Schirmer's strips farthest away from the notch of the papers might unavoidably contact with facial skin during the collection process, especially for subjects with longer Schirmer's lengths, and therefore consideration of only the first three 5 mm segments of the strips minimized contamination with skin lipids.
We next investigated whether the Schirmer's strips captured lipids in a manner that reflected the actual concentrations of lipids from the respective classes in the tear reservoir using a lipid cocktail consisting of different lipid standards with concentrations that approximated the relative abundance of various lipid classes in human tears. The lipid cocktail was first dried under pure nitrogen gas and the dried samples were then reconstituted in phosphate-buffered saline and diluted to create different concentrations of “artificial tear lipids.” Schirmer's strips were placed in glass vials containing varying concentrations of the artificial tear lipids for 5 min and lipid extraction and mass spectrometric analyses were subsequently carried out on the first three 5 mm segments of the strips. An approximately linear relationship was observed between the raw intensities of individual lipid standards and their relative concentrations in the reconstituted lipid mixture (supplementary Fig. III), implying that the Schirmer's strip-collected portions were reflective of the actual concentration of lipids in the original aqueous lipid mixture.
Collectively, the data obtained demonstrated that the first three 5 mm segments of the wetted strip area (regardless of total wetted length) were sufficient to provide a tear lipidome that was reflective of the actual lipid compositions in the tear reservoir with negligible background interference (Fig. 1; supplementary Figs. I–III; supplementary Table III). The Schirmer's strip method of tear collection is thus suitable for use in lipid analysis.
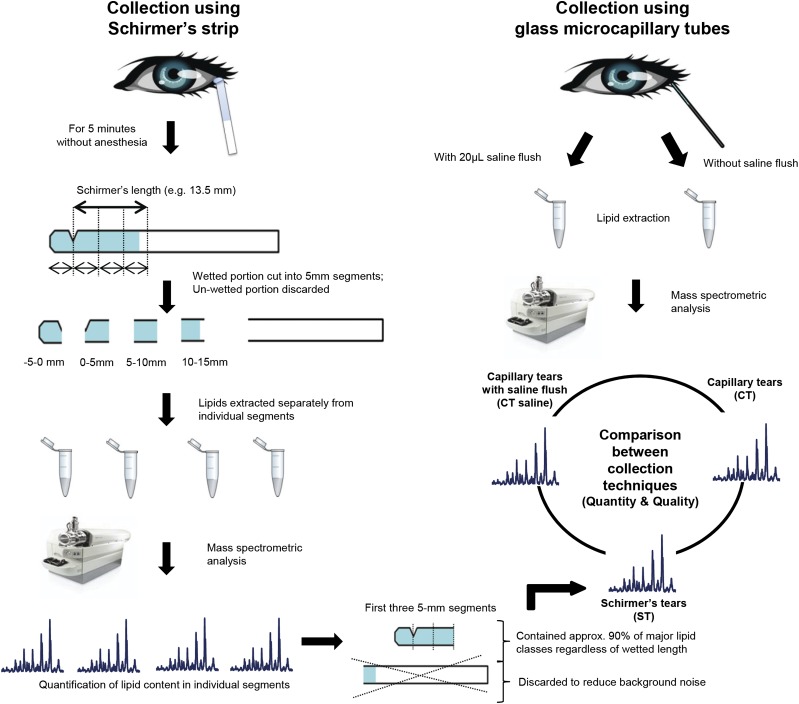
Fig. 1.
Schematic diagram illustrating the procedures for validating and comparing different tear collection techniques. Schirmer's tears collected from four volunteers on two separate occasions were used to validate the gradient of lipid metabolites along the wetted length of the strips. Briefly, the wetted portion of each strip was cut into 5 mm segments and lipid extractions were carried out on individual segments. The segment before the notch of the Schirmer's strip, which was not taken into account during the measurement of the Schirmer's length, was denoted the −5 to 0 mm segment. The representative lipidome of Schirmer's strip-collected tears from normal volunteers was compared with that of capillary-collected tears with and without saline flush.
Comparison of tear lipidomes obtained using different collection techniques
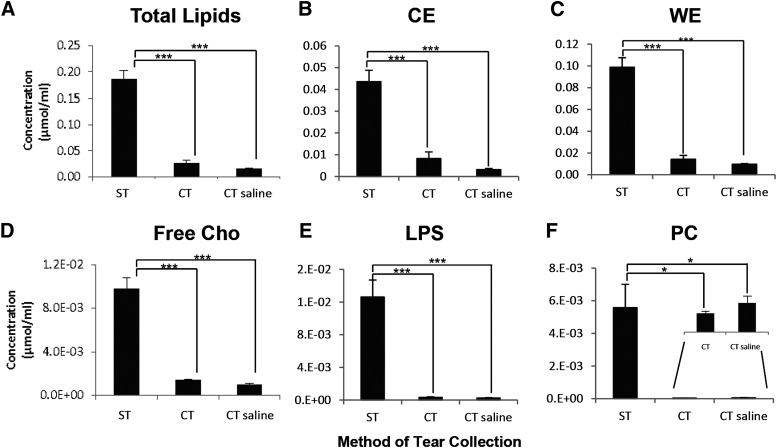
It has been documented that conflicting reports on the clinical biochemistry of tears could be attributed to differences in specimen collection (e.g., Schirmer's strips or capillary tubes) (28), so we next investigated the quantitative and qualitative differences in tear lipids collected using different techniques. Thus far, only preliminary statements had been made on the generally similar lipid profiles between tears collected using Schirmer's strips and capillary tubes, but no detailed comparison was conducted (13). We systematically compared the different clinical techniques for tear collection based on the quantity and composition of lipids captured (Fig. 1, Fig. 2). The Schirmer's method of tear collection generally captured the highest absolute amounts of lipids (Fig. 2). CEs and WEs were found to be the predominant nonpolar lipid classes and PC was the major polar lipid fraction in all three collection procedures. Higher levels of all lipid classes, except GM3, in tears collected using Schirmer's strips could be attributed to the overall greater amount of tears collected with this method. The enhanced detection of specific lipid classes under an overall limiting absolute concentration of total lipids in capillary-collected tears could be attributed to the different limits of detection using MRM for the various lipid classes investigated (data not shown). We found no appreciable differences in the lipid composition of tears collected using a capillary without a saline flush from that with a saline flush (apart from the lower absolute amounts of lipids in the latter), which is in good agreement with a previous study that reported an essentially similar tear proteome using the two methods (29).
Fig. 2.
Comparison of the absolute amounts of lipids captured using the Schirmer's strips (n = 10) and capillary tubes with (n = 5) or without (n = 5) saline flush. Mean values were plotted. Error bars indicate standard errors of the means. ST, Schirmer's tears; CT, capillary tears; CT saline, capillary tears with saline flush. *** P < 0.001;* P < 0.05.
On the basis of the similar tear lipid profiles collected using the three methods, we adopted the Schirmer's method for subsequent tear analysis in our clinical cohort primarily due to the overall greater absolute amounts of tear lipids that this method could capture. Furthermore, the Schirmer's method is a routine clinical procedure in dry eye clinics and could be performed with relative ease. Aside from providing tear samples for analysis, the Schirmer's method of tear collection also confers additional clinical information pertaining to the ocular health status of subjects compared with the capillary method (i.e., a lower Schirmer's I implies impaired tear secretion).
Analytical workflow facilitated characterization of polar lipid species based on retention times, accurate masses, and specific MRM transitions
In the current study, all individual lipid species in human tears were measured in a simple analytical workflow, using either NP- or RP-HPLC/MS (Figs. 3, 4; supplementary Fig. IV). Pooled tear samples from DES patients were first analyzed to determine representative baseline values (supplementary Table IV). The lipid composition of tear fluid derived from the pooled patient sample had also been validated in individual Schirmer's samples from a cohort of 28 DES patients with a reasonable range in individual DES clinical indicators, including the tear breakup time, Schirmer's type I length, modified ocular surface disease index, and Baylor score for corneal staining (see supplementary Fig. V and supplementary Table V). The lipidomic composition of tear fluid displayed a striking overall consistency even for samples with very low wetted lengths (i.e., 0–3 mm), demonstrating the sensitivity of our analytical methods and the feasibility of our protocol to be translated for use in dry eye clinics for large-scale analysis of tear samples from severe DES patients with significantly impaired lacrimal function.
Fig. 3.
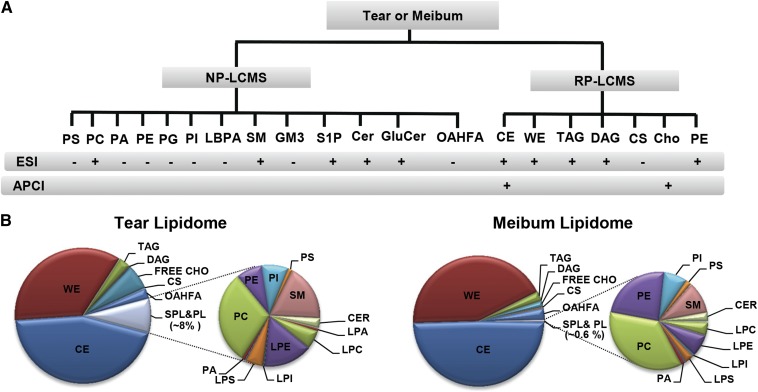
Establishment of a comprehensive lipidomic platform for qualitative and comparative elucidation of human tear and meibum lipidomes. A: Schematic summary of the analytical workflow employed. Human tear and meibum lipidomes were elucidated using HPLC/MS in different ionization modes (ESI+/− APCI+). Different lipid classes were analyzed and quantitated chiefly using RP- or NP-HPLC-MRM. B: Pie charts illustrate the lipidomes of the pooled sample of Schirmer's strip-collected tears (n = 8) and meibum (n = 7) from DES patients.
Fig. 4.
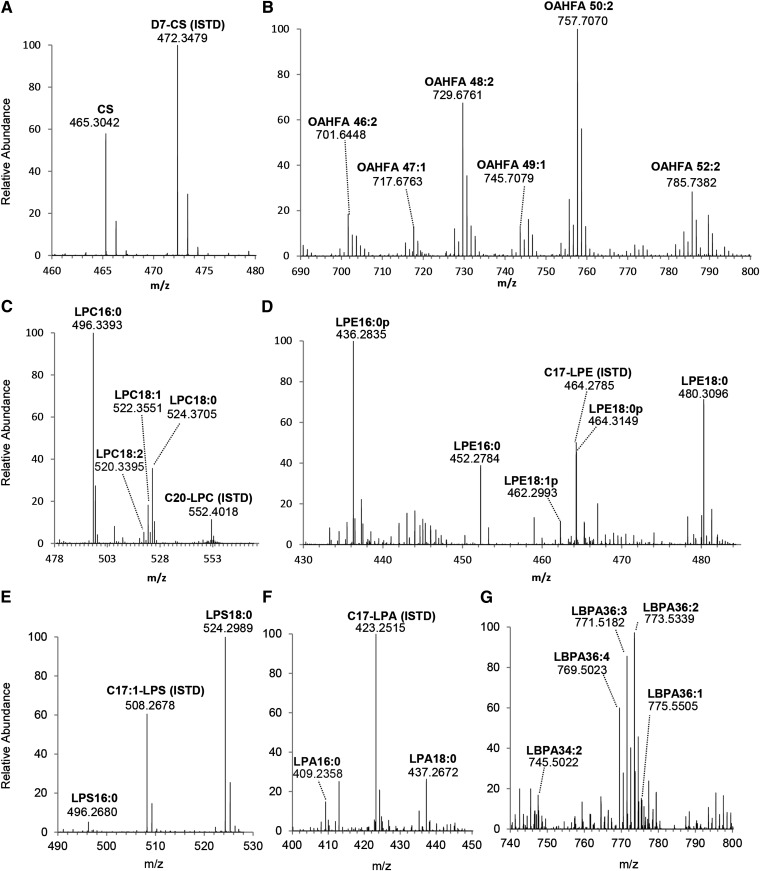
High resolution mass spectra for selected classes of polar lipids in tears. Accurate masses of major species from lipid classes of CS (A), OAHFA (B), LPC (C), LPE (D), LPS (E), LPA (F), and LBPA (G) are presented. Mass spectra were obtained using the LTQ Orbitrap XL with NP-LC-separation.
Our analyses revealed the astonishing complexities of the human tear fluid lipidome, consisting of the nonpolar lipid classes of CEs, WEs, TAGs, DAGs, and free Chos; the glycerophospholipid classes such as PCs, PEs, PIs, PGs, PSs, PAs, and LBPAs; the sphingolipid classes including SMs, Cers, GluCers, GM3, and S1P; as well as CSs and OAHFAs, a unique class of amphiphilic compounds first reported in meibum by Butovich, Wojtowicz, and Foulks (17). The tear (and meibum) lipidomes presented in this study are a significant expansion from a previous report by our group (10) and those of others (2, 21, 22). First, CSs, LBPAs, S1P, and GM3 have not been previously reported in tears or meibum (Table 1). Second, we expanded the list of phospholipid classes found in tears to include PIs, PGs, and PAs from previously reported PCs, PEs, and PSs (Table 1) (2, 22, 30). In addition, we report the relative abundance of individual OAHFA species in the tear fluid using the MRM method we had previously developed based on meibum samples (10).
TABLE 1.
An overview of major lipid classes in human tears
| Ham et al., 2004–2006 (30, 31) | Butovich, 2008 (13) | Saville et al., 2010 (21) | Rantamäki et al., 2011 (2) | Dean and Glasgow, 2012 (22) | Current, 2013 | |
| Neutral lipids | ||||||
| CE | +a | + | − | − | − | 44.82 |
| WE | +a | + | − | − | − | 35.21 |
| TAG | + | + | − | 5.1 | − | 2.84 |
| DAG | + | + | − | − | − | 0.30 |
| Cho | − | + | − | +b | − | 5.94 |
| Sphingolipids | ||||||
| SM | + | − | +c | 3.0 | + | 1.50 |
| Cer | − | − | − | 3.0 | − | 0.26 |
| GluCer | − | − | − | − | − | 0.003 |
| GM3 | − | − | − | − | − | 0.01 |
| S1P | − | − | − | − | − | 0.0001 |
| Phospholipidd | ||||||
| PC | + | − | +c | 70.6 | + | 2.96 |
| PE | − | − | − | 17.6 | −e | 1.99 |
| PS | − | − | − | 0.3 | −e | 0.57 |
| PA | − | − | − | − | − | 0.14 |
| PI | − | − | − | − | − | 0.76 |
| PG | − | − | − | − | − | 0.008 |
| LBPA | − | − | − | − | − | 0.03 |
| Others | ||||||
| OAHFA | − | − | − | − | − | 2.52 |
| CS | − | − | − | − | − | 0.14 |
Values are presented as molar percentages of total lipids measured. Comparisons were made only with selected recent studies on human tear lipidomes derived using MS. While the presence of PA in tears had been earlier suggested based on TLC results (22, 41), no prior mass spectrometric analysis of tears has confirmed the presence of PA in tears. In contrast to earlier works, we found appreciable amounts of serine-containing phospholipids in tears. This apparent disparity might possibly be due to the usage of only ESI positive mode in the analysis of tears by Rantamäki et al. (2), as PS species ionize preferentially in the negative mode. On the other hand, while Dean and Glasgow (22) employed neutral loss scan in the negative mode for analysis of PS, they did not detect an appreciable amount of PS in tears, which was possibly due to insensitive methodology (limit of detection, 12 ng/μl). The lack of column separation in the method used by Dean and Glasgow (22), coupled with the complex pool of phospholipids inherent in tear samples might have also significantly suppressed the ionization of PS species. Contrary to Saville et al. (21) and Dean and Glasgow (22) who reported PE to be lower than the limit of detection in their analyses, we noted the unambiguous presence of PE in our tear samples. +, detected but relative abundance was not reported; −, not detected.
Undetectable using MS but presence indicated via TLC.
Detected using TLC; estimated to be approximately twice of TAG in abundance.
Amount of total SM approximately equal to total PC detected in tears.
Values presented were sum of diacyl- and lysophospholipids.
Below the limit of detection in their study.
Of particular relevance to tear film structural integrity, a diverse range of amphiphilic lipid compounds was detected. CSs (Fig. 4A) and total OAHFAs (Fig. 4B) were found to constitute approximately 0.14 and 2.52% of the total tear lipidomes, respectively (Table 1). We also detected the unambiguous presence of LBPAs in human tears, with LBPA 36:2 as the most abundant species (Fig. 4G). LBPA represents an isobaric class of lipids to PGs, for which the predominant species in tears was found to be PG34:2. Individual species of LBPA and their corresponding isobaric PG species were distinguishable via their different retention times using HPLC separation. In addition, various classes of phospholipids and their lyso-forms were also detected. In accordance with previous reports (2, 22), lysophospholipids formed a major proportion of the phospholipid pool (Fig. 3B; supplementary Table IV). In agreement with Rantamäki et al. (2), we detected appreciable quantities of PEs (supplementary Fig. IIIC) and lyso-PEs (Fig. 4D) in human tears collected using the Schirmer's strips in both pooled and individual samples. An appreciable amount of LPS species were found in human tears that consisted predominantly of three distinct species, namely LPS16:0, LPS18:0, and LPS18:1 (Fig. 4E), which were different from the unusual species (LPS19:3; LPS20:2) earlier reported by Rantamäki et al. (2).
Quantitative corrections of major neutral lipid classes in tears and meibum
Consistent with previous reports (13, 20), CEs and WEs were unambiguously detected in human tears as the predominant nonpolar lipid classes in our study. A notable analytical advancement in our current study is the quantitative corrections of the proportions of WEs and CEs in the human tears and meibum (see Materials and Methods). Using our improved approach incorporating in-house synthesized 13C-labeled WEs as internal standards (26), we found that WEs constitute approximately 43% of the total lipids in the human meibum (Fig. 3B; supplementary Table IV), which is in excellent agreement with a recent value [40 ± 10% (wt/wt)] determined by gas chromatography (GC)-MS (19). Furthermore, we detected and characterized the comprehensive profile of DAG species in human tears (supplementary Fig. VI), which were at appreciably higher levels than those in human meibum (supplementary Table IV). This class of compounds corresponded to an earlier preliminary observation stating that human tears contain a new range of compounds with higher polarity than the nonpolar components of meibum that coelute with authentic DAGs (13). On the other hand, Ham et al. (31) had also previously reported the presence of a few DAG species, such as DAG16:0/16:0 and DAG 16:0/18:0, in the human tears.
DISCUSSION
The foregoing results evaluated the suitability of the Schirmer's strip for collecting tears designated for lipid analyses; and systematically compared the different tear collection methods currently available in eye clinics in terms of both quantity and quality of lipids captured. A comprehensive lipidome of the human tear fluid was presented; and the relative abundance of various novel lipid amphiphiles in tears including CSs and OAHFAs were reported. The compositional details of the human tear lipidome reported herein have major implications on ocular surface biochemistry as well as tear film biophysics.
Physiological significance of the tear lysophospholipidome
In considering the possible physiological functions of the novel lipids detected in human tears, it is important to reckon the unique biochemistry at the ocular surface and the critical importance of ocular homeostasis in maintaining precise vision. Valuable insights can be drawn from the tear fluid proteome, of which bactericidal proteins such as lysozyme, lactoferrin, and lipocalin form the bulk constituents (1). In line with this, lyso-PC has been reported to increase the bactericidal activity of neutrophils (36); while lyso-PEs were shown to possess antifungal and antibacterial activity in the housefly (37). Therefore, the physiological significance of the tear lysophospholipidome seems to coincide with that of the tear proteome, which comprises a predominance of metabolites with microbicidal properties (38).
Implications of lipid compositional details on the existent tear film model
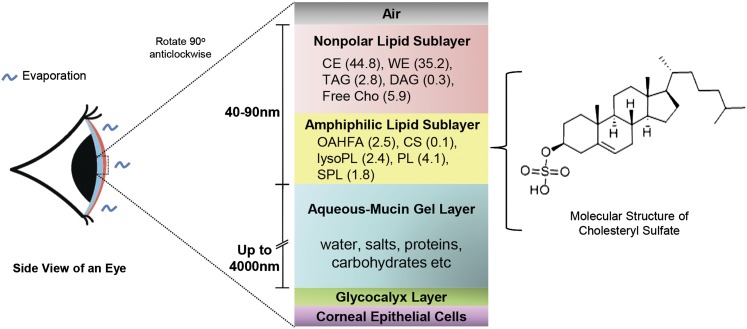
The longstanding perception that phospholipids serve as an amphiphilic layer of the tear film lipid layer has been recently challenged due to the ambiguous occurrence of phospholipids in human meibum, the predominant source of lipids for tears (10, 32). Our current analysis of the tear lipidome revealed that phospholipids comprise a substantial amount (approximately 6%) of the total tear lipids. The significant enrichment of phospholipids and sphingolipids in the human tear fluid compared with the meibum therefore has important implications for our understanding of tear film physiology. On the other hand, the detection of a considerable amount of CSs in the tear fluid (approximately 0.14%) has introduced a novel, hitherto unknown amphiphile to the tear film (Fig. 5). CS has been shown to interact with phospholipids in a manner akin to Chos, inducing hydrocarbon ordering in lipid bilayers and stabilizing model membranes (33). Moreover, under physiological conditions, the sulfate moiety of CS is ionized, converting CS from a relatively hydrophobic molecule into a highly amphiphilic compound comprising a highly charged headgroup (34). While the physiological significance of CS in tears is presently obscure, CS might be a suitable candidate for the amphiphilic sublayer of the tear film based on its biophysical property as a stabilizing agent of biological membranes (34). Furthermore, CS (P < 0.001) and OAHFA (P < 0.05) represent the only lipid classes that were positively correlated with tear breakup time, which is a proximal measure of tear film stability after blinking (35).
Fig. 5.
Human tear film model. The tear film lipid layer comprises the superficial sublayer consisting predominantly of nonpolar lipids and an inner amphiphilic sublayer, which facilitates the interaction between the polar and nonpolar components of tears. The composition of the amphiphilic lipid sublayer has remained a matter of contention (32, 40). Our global lipidomic analysis introduced CS as a novel candidate for the amphiphilic sublayer. Other possible amphiphilic lipid candidates are also listed. Percent abundances of respective lipid classes are presented in parentheses. The figure is not drawn to scale.
CONCLUSIONS
Our in-depth analysis of the human tear lipidome has provided new insights pertaining to ocular surface biochemistry and tear film biophysics, especially with regard to composition of the amphiphilic lipid sublayer of the tear film. We have shown that the tear fluid is appreciably enriched in phospholipids and sphingolipids relative to the meibum, and demonstrated that a surplus of phospholipids and sphingolipids is available in tears to constitute the amphiphilic lipid sublayer. We have also put forth CS as a novel candidate for this interfacial lipid sublayer. The detailed evaluations of various sampling techniques could help standardize collection protocols, thereby facilitating future biomarker studies on tears. Furthermore, we demonstrated the translational feasibility of using Schirmer's strip for tear collection designated for lipid analyses, which confers high reproducibility in terms of lipid profiles across samples with a wide range of wetted lengths; and is thus applicable even to patients with severe DES.
The ocular tissues and fluids are potential reporters of integrated metabolic stress over prolonged periods, otherwise obscured by complex homeostatic mechanisms on a systemic scale (39). The tear fluid, being the most accessible of all ocular fluids, therefore also represents a novel source of biomarkers for systemic diseases marked by long prodromal periods, extending the translational significance of the comprehensive human tear lipidome reported herein from the ocular field to other realms of clinical medicine.
Supplementary Material
Acknowledgments
The authors wish to thank Lam Wan Yee for technical help with illustrations in this manuscript.
Footnotes
Abbreviations:
- APCI
- atmospheric pressure chemical ionization
- CE
- cholesteryl ester
- Cer
- ceramide
- Cho
- cholesterol
- CS
- cholesteryl sulfate
- DAG
- diacylglyceride
- DES
- dry eye syndrome
- GluCer
- glucosylceramide
- GM3
- NeuAcα2-3Galβ1-4Glcβ-Cer
- LBPA
- lyso-bisphosphatidic acid
- LPS
- lysophosphatidylserine
- lyso-PC
- lysophosphatidylcholine
- lyso-PI
- lysophosphatidylinositol
- MRM
- multiple reaction monitoring
- NP
- normal phase
- OAHFA
- O-acyl-ω-hydroxy fatty acid
- PA
- phosphatidic acid
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- PI
- phosphatidylinositol
- PS
- phosphatidylserine
- RP
- reverse phase
- S1P
- sphingosine-1-phosphate
- TAG
- triacylglyceride
- WE
- wax ester
This research was supported by grants from the Chinese Academy of Sciences (KYQY-162 and Y265091891) and the Singapore National Research Foundation under its clinician scientist award NMRC/CSA/013/2009, under its individual research grant NMRC/1206/2009, and under its CRP award number 2007-4. S.M.L. was a recipient of the President's Graduate Fellowship from the National University of Singapore.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of six figures and six tables.
REFERENCES
- 1.Zhou L., Beuerman R. W. 2012. Tear analysis in ocular surface diseases. Prog. Retin. Eye Res. 31: 527–550 [DOI] [PubMed] [Google Scholar]
- 2.Rantamäki A. H., Seppänen-Laakso T., Oresic M., Jauhiainen M., Holopainen J. M. 2011. Human tear fluid lipidome: from composition to function. PLoS ONE. 6: e19553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherz W., Doane M. G., Dohlman C. H. 1974. Tear volume in normal eyes and keratoconjunctivitis sicca. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 192: 141–150 [DOI] [PubMed] [Google Scholar]
- 4.Zhou L., Beuerman R. W., Chan C. M., Zhao S. Z., Li X. R., Yang H., Tong L., Liu S., Stern M. E., Tan D. 2009. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J. Proteome Res. 8: 4889–4905 [DOI] [PubMed] [Google Scholar]
- 5.Tong L., Zhou L., Beuerman R. W., Zhao S. Z., Li X. R. 2011. Association of tear proteins with Meibomian gland disease and dry eye symptoms. Br. J. Ophthalmol. 95: 848–852 [DOI] [PubMed] [Google Scholar]
- 6.Zhou L., Zhao S. Z., Koh S. K., Chen L., Vaz C., Tanavde V., Li X. R., Beuerman R. W. 2012. In-depth analysis of the human tear proteome. J. Proteomics. 75: 3877–3885 [DOI] [PubMed] [Google Scholar]
- 7.Green-Church K. B., Nichols K. K., Kleinholz N. M., Zhang L., Nichols J. J. 2008. Investigation of the human tear film proteome using multiple proteomic approaches. Mol. Vis. 14: 456–470 [PMC free article] [PubMed] [Google Scholar]
- 8.de Souza G. A., Godoy L. M. F., Mann M. 2006. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 7: R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L., Zhou L., Chan E. C., Neo J., Beuerman R. W. 2011. Characterization of the human tear metabolome by LC-MS/MS. J. Proteome Res. 10: 4876–4882 [DOI] [PubMed] [Google Scholar]
- 10.Lam S. M., Tong L., Yong S. S., Li B., Chaurasia S. S., Shui G., Wenk M. R. 2011. Meibum lipid composition in Asians with dry eye disease. PLoS ONE. 6: e24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borchman D., Foulks G. N., Yappert M. C., Milliner S. E. 2012. Differences in human meibum lipid composition with meibomian gland dysfunction using NMR and principal component analysis. Invest. Ophthalmol. Vis. Sci. 53: 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saville J. T., Zhao Z., Willcox M. D. P., Ariyavidana M. A., Blanksby S. J., Mitchell T. W. 2011. Identification of phospholipids in human meibum by nano-electrospray ionisation tandem mass spectrometry. Exp. Eye Res. 92: 238–240 [DOI] [PubMed] [Google Scholar]
- 13.Butovich I. A. 2008. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest. Ophthalmol. Vis. Sci. 49: 3779–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butovich I. A. 2009. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J. Lipid Res. 50: 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butovich I. A., Uchiyama E., McCulley J. P. 2007. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J. Lipid Res. 48: 2220–2235 [DOI] [PubMed] [Google Scholar]
- 16.Borchman D., Yappert M. C., Foulks G. N. 2010. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp. Eye Res. 91: 246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butovich I. A., Wojtowicz J. C., Molai M. 2009. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J. Lipid Res. 50: 2471–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J., Green-Church K. B., Nichols K. K. 2010. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Invest. Ophthalmol. Vis. Sci. 51: 6220–6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butovich I. A., Arciniega J. C., Lu H., Molai M. 2012. Evaluation and quantitation of intact wax esters of human meibum by gas-liquid chromatography-ion trap mass spectrometry. Invest. Ophthalmol. Vis. Sci. 53: 3766–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borchman D., Foulks G. N., Yappert M. C., Tang D., Ho D. V. 2007. Spectroscopic evaluation of human tear lipids. Chem. Phys. Lipids. 147: 87–102 [DOI] [PubMed] [Google Scholar]
- 21.Saville J. T., Zhao Z., Willcox M. D. P., Blanksby S. J., Mitchell T. W. 2010. Detection and quantification of tear phospholipids and cholesterol in contact lens deposits: the effect of contact lens material and lens care solution. Invest. Ophthalmol. Vis. Sci. 51: 2843–2851 [DOI] [PubMed] [Google Scholar]
- 22.Dean A. W., Glasgow B. J. 2012. Mass spectrometric identification of phospholipids in human tears and tear lipocalin. Invest. Ophthalmol. Vis. Sci. 53: 1773–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shui G., Stebbins J. W., Lam B. D., Cheong W. F., Lam S. M., Gregoire F., Kusonoki J., Wenk M. R. 2011. Comparative plasma lipidome between human and cynomolgus monkey: are plasma polar lipids good biomarkers for diabetic monkeys? PLoS ONE. 6: e19731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shui G., Guan X. L., Low C. P., Chua G. H., Goh J. S. Y., Yang H., Wenk M. R. 2010. Toward one step analysis of cellular lipidomes using liquid chromatography coupled with mass spectrometry: application to Saccharomyces cerevisiae and Schizosaccharomyces pombe lipidomics. Mol. Biosyst. 6: 1008–1017 [DOI] [PubMed] [Google Scholar]
- 25.Vrkoslav V., Urbanová K., Cvačka J. 2010. Analysis of wax ester molecular species by high performance liquid chromatography/atmospheric pressure chemical ionisation mass spectrometry. J. Chromatogr. A. 1217: 4184–4194 [DOI] [PubMed] [Google Scholar]
- 26.Lam S. M., Tong L., Reux B., Lear M. J., Wenk M. R., Shui G. 2013. Rapid and sensitive profiling of tear wax ester species using high performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A. 1308: 166–171 [DOI] [PubMed] [Google Scholar]
- 27.Shui G., Cheong W. F., Jappar I. A., Hoi A., Xue Y., Fernandis A. Z., Tan B. K. H., Wenk M. R. 2011. Derivatization-independent cholesterol analysis in crude lipid extracts by liquid chromatography/mass spectrometry: applications to a rabbit model for atherosclerosis. J. Chromatogr. A. 1218: 4357–4365 [DOI] [PubMed] [Google Scholar]
- 28.Van Haeringen N. J. 1981. Clinical biochemistry of tears. Surv. Ophthalmol. 26: 84–96 [DOI] [PubMed] [Google Scholar]
- 29.Markoulli M., Papas E., Petznick A., Holden B. 2011. Validation of the flush method as an alternative to basal or reflex tear collection. Curr. Eye Res. 36: 198–207 [DOI] [PubMed] [Google Scholar]
- 30.Ham B. M., Cole R. B., Jacob J. T. 2006. Identification and comparison of the polar phospholipids in normal and dry eye rabbit tears by MALDI-TOF mass spectrometry. Invest. Ophthalmol. Vis. Sci. 47: 3330–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ham B. M., Jacob J. T., Keese M. M., Cole R. B. 2004. Identification, quantification and comparison of major non-polar lipids in normal and dry eye tear lipidomes by electrospray tandem mass spectrometry. J. Mass Spectrom. 39: 1321–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butovich I. A. 2009. The Meibomian puzzle: combining pieces together. Prog. Retin. Eye Res. 28: 483–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitson N., Monck M., Wong K., Thewalt J., Cullis P. 1992. The influence of cholesterol 3-sulphate on phase behaviour and hydrocarbon order in model membrane systems. Biochim. Biophys. Acta. 1111: 127–133 [DOI] [PubMed] [Google Scholar]
- 34.Bleau G., Bodley F. H., Longpré J., Chapdelaine A., Roberts K. D. 1974. Cholesterol sulfate. I. Occurrence and possible biological function as an amphipathic lipid in the membrane of the human erythrocyte. Biochim. Biophys. Acta. 352: 1–9 [DOI] [PubMed] [Google Scholar]
- 35.Lam S. M., Tong L., Reux B., Duan X., Petznick A., Yong S. S., Khee C. B. S., Lear M. J., Wenk M. R., Shui G. 2014. Lipidomic analysis of human tear fluid reveals structure-specific lipid alterations in dry eye syndrome. J. Lipid Res. 55: 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan J. J., Jung J. S., Lee J. E., Lee J., Huh S. O., Kim H. S., Jung K. C., Cho J. Y., Nam J. S., Suh H. W., et al. 2004. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat. Med. 10: 161–167 [DOI] [PubMed] [Google Scholar]
- 37.Meylaers K., Clynen E., Daloze D., DeLoof A., Schoofs L. 2004. Identification of 1-lysophosphatidylethanolamine (C(16:1)) as an antimicrobial compound in the housefly, Musca domestica. Insect Biochem. Mol. Biol. 34: 43–49 [DOI] [PubMed] [Google Scholar]
- 38.Fleiszig S. M. J., Kwong M. S. F., Evans D. J. 2003. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect. Immun. 71: 3866–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sapieha P., Stahl A., Chen J., Seaward M. R., Willett K. L., Krah N. M., Dennison R. J., Connor K. M., Aderman C. M., Liclican E., et al. 2011. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acids. Sci. Transl. Med. 3: 69ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dilly P. N. 1994. Structure and function of the tear film. Adv. Exp. Med. Biol. 350: 239–247 [DOI] [PubMed] [Google Scholar]
- 41.Glasgow B. J., Abduragimov A. R., Farahbakhsh Z. T., Faull K. F., Hubbell W. L. 1995. Tear lipocalins bind a broad array of lipid ligands. Curr. Eye Res. 14: 363–372 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.