Abstract
As current diagnostic markers for dry eye syndrome (DES) are lacking in both sensitivity and specificity, a pressing concern exists to develop activity markers that closely align with the principal axes of disease progression. In this study, a comprehensive lipidomic platform designated for analysis of the human tear lipidome was employed to characterize changes in tear lipid compositions from a cohort of 93 subjects of different clinical subgroups classified based on the presence of dry eye symptoms and signs. Positive correlations were observed between the tear levels of cholesteryl sulfates and glycosphingolipids with physiological secretion of tears, which indicated the possible lacrimal (instead of meibomian) origin of these lipids. Notably, we found wax esters of low molecular masses and those containing saturated fatty acyl moieties were specifically reduced with disease and significantly correlated with various DES clinical parameters such as ocular surface disease index, tear breakup time, and Schirmer's I test (i.e., both symptoms and signs). These structure-specific changes in tear components with DES could potentially serve as unifying indicators of disease symptoms and signs. In addition, the structurally-specific aberrations in tear lipids reported here were found in patients with or without aqueous deficiency, suggesting a common pathology for both DES subtypes.
Keywords: mass spectrometry, meibum, tear lipidome, wax esters
Dry eye syndrome (DES) represents one of the most frequently encountered ocular morbidities, which can affect up to approximately one-third of the population worldwide depending on the criteria and definition used in the various studies conducted across the continents (1). Recent studies in China and Japan have, however, revealed a much higher prevalence than the average value reported globally (2, 3), indicating that Asian populations might have a greater predisposition to the disease. Despite its prevalence, there is presently no universal consensus on the diagnostic guidelines for the disease (4). Current clinical tests lack reproducibility and are not sufficiently predictive of symptomatology to facilitate effective disease diagnosis and prognosis (5).
The importance of tear lipids in maintaining ocular surface homeostasis and visual acuity due to their critical roles in constituting the outermost layer of the tear film has been extensively reported and discussed (5–8). In particular, with the recent development in mass spectrometric technology, lipidomics has been transformed into a principal tool in biomedical research to decipher fine changes in lipid metabolism in various diseases including the DES (9, 10). Elucidating single tear components that are specifically altered with disease therefore marks the future of dry eye research by revealing novel molecular targets for improving current diagnostic and therapeutic platforms. Compared with tears, meibum-derived lipid markers indicative of DES are circumvented by the assumption that meibomian lipids are fully incorporated into the tear film lipid layer without proportional alterations of the various lipid classes. Moreover, manually expressed meibum might not provide accurate reflections of the physiological compositions normally secreted onto the lid margin and subsequently incorporated into tears. Tear lipid biomarkers can thus potentially offer a closer reflection of disease pathophysiology and display better correlation to clinical tests routinely used for dry eye diagnosis, such as the Schirmer's I test and the tear film breakup time. Besides, a comprehensive tear lipidome would also facilitate pharmaceutical development of artificial tears for alleviating DES and other ocular diseases.
In this study, we investigated the alterations in tear lipidomes from a clinical cohort of 93 subjects in order to elucidate lipid aberrations in tears that are pathologically relevant for DES. Subjects were classified into asymptomatic controls, individuals at-risk of developing DES (aqueous-deficient or nonaqueous-deficient) as well as symptomatic patients (aqueous-deficient or nonaqueous-deficient) based on a combination of dry eye symptoms (i.e., ocular surface disease index) and clinical signs [i.e., Schirmer's I test, tear breakup time (TBuT), and Baylor score for corneal staining]. Changes in tear lipid compositions in the respective clinical subgroups were compared against controls, and associations between tear lipid levels with DES clinical signs were evaluated. In addition, lipid correlates with DES-related physiological processes (i.e., aging, tear secretion, and tear drainage) were also reported.
On another note, the entrapment and thus prolonged stagnation of meibomian gland secretions within the partially obstructed glands in the case of meibomian gland dysfunction (MGD) could result in enhanced modifications by commensal bacteria residing within the glandular ducts (8), leading to reduced delivery of meibum (i.e., hyposecretion) with altered compositions to the lid reservoir. Indeed, it is still debatable whether the perturbations to tear film physiology associated with MGD result primarily from a diminished supply of meibum per se to the lid reservoir, or could mainly be attributed to an altered meibum composition that would adversely affect tear film integrity and its associated biophysical properties. To verify if tear lipid composition indeed varies considerably with MGD, we attempted to move away from the conventional scheme of classification by segregating subjects solely based on the absolute amounts of lipids detected in tears (i.e., low, medium, high). This classification is based on the assumption that under conditions of MGD, the compromised delivery of meibum to the lid reservoir would result in a diminished level of total lipids detected in the tear fluid. Compositional changes in tear lipids among the three subgroups were evaluated.
MATERIALS AND METHODS
Study group
A total of 93 subjects were recruited for the current study. Symptomatic patients were recruited from the dry eye clinic, while age-matched control subjects who did not exhibit dry eye symptoms were recruited from the glaucoma clinic. Detailed demographic information for the study group can be found in the supplementary section (supplementary Table I). Subjects were classified into different clinical subgroups based on a combination of dry eye symptoms and signs (supplementary Fig. III). Written informed consent was obtained from all participating subjects and the procedure for the project was specifically approved by the SingHealth Centralised Institutional Review Board (CIRB reference number: 2008/611/A). We adhered to the tenets of the Declaration of Helsinki for all human research conducted in this study. The detailed clinical procedures have been reported elsewhere (11).
Lipid extraction and mass spectrometric analyses
The detailed procedures for tear sample collection and lipid extraction have been reported elsewhere (11). A comprehensive lipidomic platform largely based upon principles of HPLC/MS/MS, which had been specifically developed for analysis of human tear lipids (12), was employed to measure changes in tear lipid components among the different clinical subgroups. In particular, development of a mass spectrometric method for quantitation of wax esters (WEs) in tears that has been reported in detail previously (13). In the current study, care was taken to minimize technical artifacts by randomizing samples from different clinical subgroups throughout the mass spectrometric runs. For all LC/MS analyses, individual peaks were manually examined and only peaks above the limit of quantitation and within the linearity range were used for quantitation and subsequent statistical analyses.
Statistical analysis
Correlation analyses between lipid levels in tears and meibum and common dry eye clinical indicators were investigated using Spearman's correlation. An eclipse demarcates the 95% confidence region of correlating parameters. The levels of various lipid classes and species in different clinical subgroups were compared against the control group using one-way ANOVA with a post hoc Dunnett's test. False discovery rate was controlled based on q-values calculated using R 3.0.1 (supplementary Table II). Compositional changes in lipid levels among subgroups with differing total lipid amounts were compared using one-way ANOVA with a post hoc TukeyHSD test. For all analyses, ***P < 0.001; **P < 0.01; *P < 0.05; #0.05 ≤ P < 0.10.
RESULTS
Associations of tear lipid levels with DES-related physiological processes
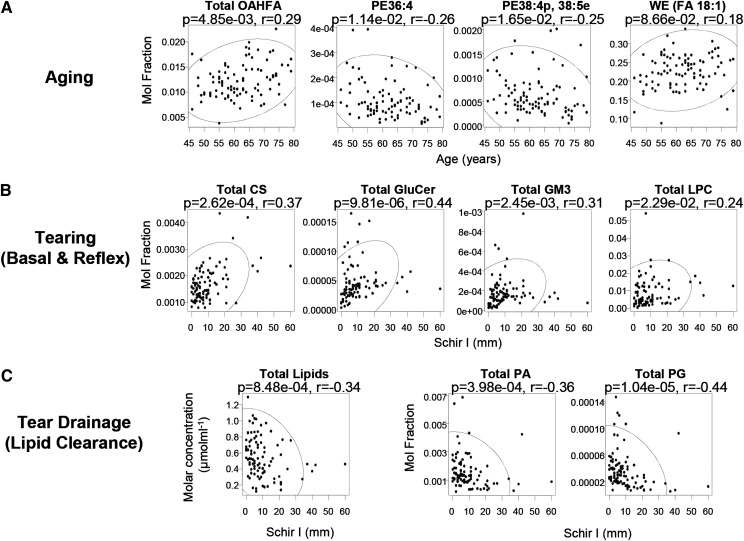
Aging is known to associate with physiological changes that predispose individuals to DES, such as lower tear film stability and compositional alterations in meibum (14). We found the entire class of O-acyl-ω-hydroxy-fatty acids (OAHFAs) to be positively correlated with increasing age (Fig. 1A). Several individual species of OAHFAs in tears were also positively correlated with age (see supplementary Fig. IA, B). On the other hand, several phospholipids containing PUFAs were decreased with increasing age, including both diacyl and ether/plasmalogen species (Fig. 1A, supplementary Fig. IC), which might be indicative of increased oxidative stress with advancing age, in corroboration with the previous observation of enhanced oxidation in meibum with aging, based on the ratio of aldehydes to hydroperoxides (15). Remarkably, the levels of oleic acid (FA 18:1)-based WEs were elevated with increasing age, with several individual oleic acid-containing species exhibiting similar upward trends (Fig. 1A, supplementary Fig. ID). Because oleic acid-based species represent the major WE species in tears (Fig. 2B), increased levels of such species are expected to alter the composition and nature of tear lipids considerably. This finding is in agreement with separate studies on human meibum using infrared spectroscopy and nuclear magnetic resonance spectroscopy, in which the authors reported an increase in double bonds in meibum with age (16), and a resultant decrease in hydrocarbon chain order (decreased viscosity) with advancing age (17). The increase in lipid disorder probably represents a natural phenomenon of aging, facilitating meibum outflow and its subsequent spreading across the ocular surface in forming an expanded film.
Fig. 1.
Association between tear lipid levels and dry eye-associated physiological processes. A: Correlations between selected classes/species of tear lipids with age. OAHFAs were positively correlated with age, while several highly unsaturated PE species were negatively correlated. Oleic acid-based WEs (FA 18:1) were positively correlated with age with marginal significance. B: Positive correlations were found between CSs, GluCers, GM3s, and LPCs with the Schirmer's I test (Schir I) that provides a surrogate measure of lacrimal reflex response. C: Absolute levels of total lipids and molar fractions of PA and PG were negatively correlated with Schir I, which might be associated with afflicted lipid clearance from the lid reservoirs under an overall AqD. The ellipse demarcates the 95% confidence region of the correlating parameters.
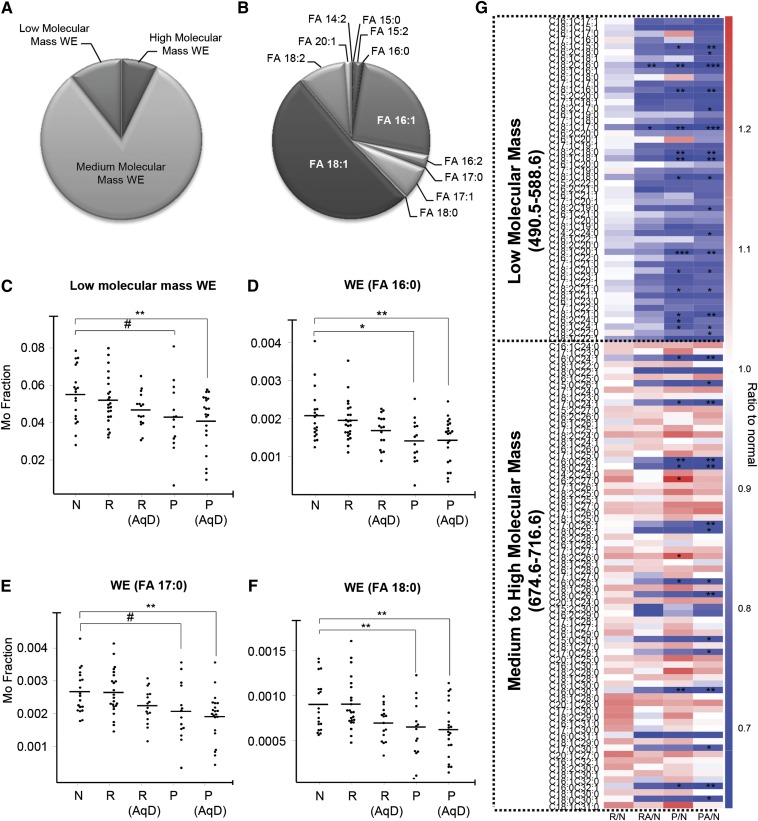
Fig. 2.
Structure-specific changes in WEs with DES. WEs were stratified into three categories based on molecular masses: low (490.5–588.6); medium (590.6–672.6); and high (674.6–716.6). The pie chart illustrates the relative abundances of WEs categorized based on molecular masses (i.e., low, medium, and high) (A) and fatty acyl moieties in their structures (B). Subclasses of WEs of low molecular masses (C) or those containing saturated acid moieties (D–F) were significantly reduced in symptomatic dry eye patients with or without AqD (cutoff: Schir I ≤ 5.5 mm). G: Heatmap illustrates changes in the levels of individual WE species in various clinical categories compared with normal controls. Values are plotted as ratios of levels of individual WE species in the respective clinical categories to that in normal individuals. Red indicates an increase compared with normal while blue indicates a decrease. R, individuals at risk; R (AqD), individuals at risk with AqD; P, Patients (nonaqueous-deficient); P (AqD), patients with AqD; N, normal. ***P < 0.001; **P < 0.01; *P < 0.05; #0.05 ≤ P < 0.10.
The Schirmer's I test (Schir I) provides a proximal measure of total tear secretion (basal and reflex) (18), a physiological process instrumental in determining DES onset and progression. The normalized levels of cholesteryl sulfates (CSs), glucosylceramides (GluCers), NeuAcα2-3Galβ1-4Glcβ-Cers (GM3s), and lyso-phosphatidylcholines (LPCs) were positively correlated with Schir I (Fig. 1B), indicating that the release of these lipids is elevated with increased tearing. In addition, contrary to other lipid classes found in tears, absolute concentrations of CSs, GluCers, GM3s, and LPCs were reduced as tear secretion decreased (supplementary Fig. IIB), implying the possible nonmeibomian origin of these lipids. These amphiphilic lipids possibly originate from the lacrimal glands and might represent suitable indicators of lacrimal function. Nagyová and Tiffany (19) have suggested that the lacrimal protein lipocalin might facilitate the lowering of surface tension by complexing with specific lipids and spreading out as a monolayer at the surface of the tear film. Using TLC, separate groups concluded that glycolipids might be involved in binding to tear lipocalins (18). It has been suggested that the lacrimal gland might be a probable source of such lipids and that the protein lipocalin might be secreted from the gland already fully charged with these lipids (20). Correspondingly, our results showed that GluCer is strongly positively correlated with increasing Schir I (Fig. 1B). Also, the propensity of GluCers to aggregate forming highly ordered gel domains has been shown to increase the order of fluid membranes (21). In addition, the trapping of ocular pathogens by soluble sialyated and/or glycated proteins has been shown to modulate the accessibility of such microbes to the epithelial glycocalyx, thereby protecting the cornea from infections (22). Similarly, soluble GluCer and GM3 could help to entrap invading microbes and therefore preventing microbial adherence to epithelial mucins. Thus, apart from possibly reducing surface tension of the tear film, the enhanced release of GluCer and GM3 with tearing might facilitate microbe clearance via drainage through the ocular punta.
In contrast to the CSs, GluCers, GM3s, and LPCs that exhibited positive trends with increasing tear secretion, the absolute concentrations (μmolml−1) of total lipids displayed a significantly decreasing trend with increasing Schir I (Fig. 1C). The higher lipid level with low Schir I is consistent with earlier work, which noted increased casual lipid levels at the lid margin with aqueous deficiency (AqD) (23). This might be attributed to an afflicted lipid clearance with reduced tear flow, as no known reflex control of meibomian gland secretion has been reported thus far (23). In particular, molar fractions of phosphatidic acids (PAs) and phosphatidylglycerols (PGs) were negatively correlated with Schir I (Fig. 1C), and found in significantly higher levels (P < 0.05) in aqueous-deficient patients (i.e., Schir I < 10 mm) than normal subjects. The enhanced levels of specific phospholipid classes might be associated with modifications by various ocular surface enzymes, such as phospholipases, due to the prolonged stagnation of tear lipids at the ocular surface and lid reservoir under an overall AqD.
Levels of WEs were altered in DES in a manner dependent on their molecular masses and fatty acyl chain saturation
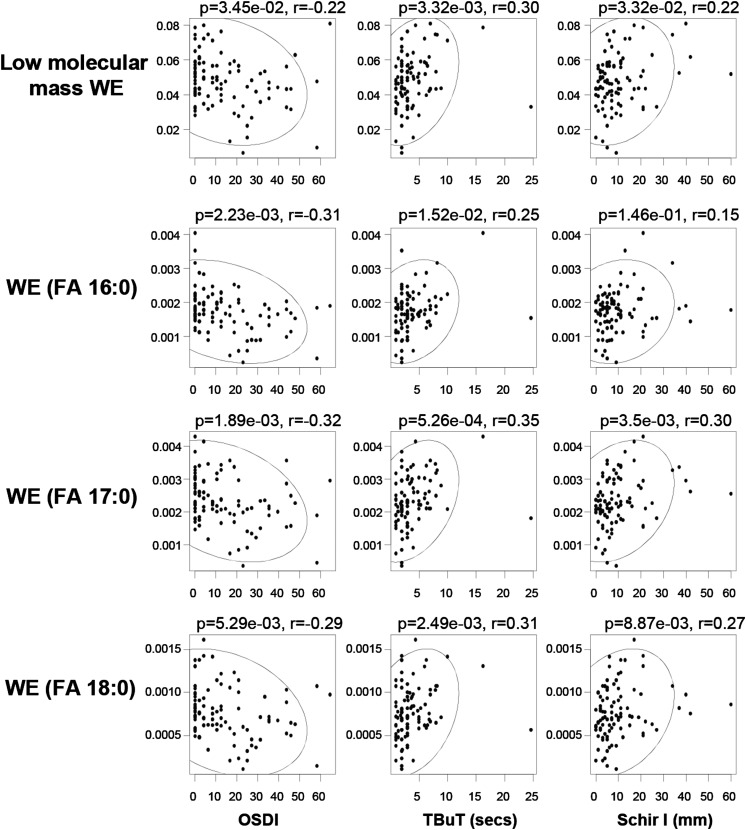
While the category of low molecular mass WEs constitutes only a minor fraction (approximately 7%) of the total waxes (Fig. 2A), their levels were found to steadily decrease with DES onset, reaching statistical significance in patients (AqD and nonAqD) (Fig. 2C). In agreement with their reduced levels in patients, low molecular mass WEs were negatively correlated with the ocular surface disease index, while positively correlated with TBuT and Schir I (Fig. 3). These observations cumulatively suggest that reductions in low molecular mass WEs were associated with DES pathogenesis. Individual species displayed similar trends to that observed for the entire category of low molecular mass WEs (Fig. 2G; supplementary Fig. VA). On the other hand, species of WEs containing saturated fatty acyl moieties in tears were found in considerably lower proportion compared with their unsaturated counterparts (Fig. 2B). Intriguingly, among medium to high molecular mass WEs, only species with saturated FA chains were specifically and significantly decreased in DES patients (both AqD and nonAqD), while the levels of species with unsaturated FAs were increased, albeit not reaching statistical significance (Fig. 2D–G). In addition, only species with saturated FA chains were specifically and significantly correlated with Schir I and TBuT (positive correlations); as well as OSDI (negative correlations) (Fig. 3). These observations indicate that reductions in WEs containing saturated FA chains are associated with disease pathology. On the other hand, several high molecular mass WEs with unsaturated FA chains were positively correlated with the Baylor score for corneal staining (supplementary Fig. VB), implying that the saturation status of WE fatty acyl moieties might exert critical roles in the manifestations of dry eye disease signs and symptoms. The subclasses of WEs containing the respective saturated FA moieties (i.e., FA 16:0, FA 17:0, and FA 18:0) displayed trends consistent with those of individual species (Fig. 2D–F).
Fig. 3.
Correlations between structural-specific aberrations in WEs and clinical signs of DES. The levels of low molecular mass WEs and those containing saturated acid moieties were negatively correlated with OSDI but positively correlated with both TBuT and Schir I. The ellipse demarcates the 95% confidence region of the correlating parameters.
Compositional changes in tear lipids with varying total lipid levels in tears
Rather surprisingly, we did not observe significant reductions in total tear lipids (μmolml−1) in both DES subtypes (AqD and nonAqD) according to the classification scheme based on clinical parameters (supplementary Fig. III part I). This is contrary to the common perception that evaporative dry eye (i.e., nonAqD) results from compromised tear film stability due to insufficient delivery of meibomian lipids to the lid margin (4). As no current clinical tests for DES are sufficiently predictive of symptomatology to render them as leading disease indicators, it is possible that these clinical signs do not truly reflect physiological processes aligned with the principal axes of DES pathogenesis (10). Meibomian gland secretions could undergo enhanced modifications by host- and/or bacterial-derived enzymatic activities in the gland ducts if secretions become entrapped and stagnate behind partially obstructed glands (24), leading to reduced delivery of meibum (i.e., hyposecretion) with altered compositions into tears. Thus, nonAqD dry eye might have resulted from an altered composition of tear lipids accompanying hyposecretion, instead of an overall insufficiency in lipids per se. To test this hypothesis, we attempted to move away from the conventional scheme of classification by segregating subjects solely based on the absolute amounts of lipids detected in tears (see supplementary Figs. VI–VIII).
Interestingly, we found significant compositional changes in a number of lipid classes as total lipid amount in tears varied from low to medium to high. For instance, the elevated levels of diacylglycerides in the low-lipid category (supplementary Fig. VIIIA) could be attributed to enhanced activities of esterases and lipases from lid commensal bacteria in MGD subjects, leading to irritation or other DES-associated symptoms (25). The molar fraction of cholesteryl esters (CEs) was significantly lower in the low-lipid group (supplementary Fig. VIIIB), with a corresponding marginal increase in the level of free cholesterol (Cho) (data not shown), suggestive of an enhanced breakdown of CEs to free Cho. The hydrolysis of CEs releasing free Cho was previously reported to promote the growth of ocular flora in chronic blepharitis (26). Molar fractions of phosphatidylcholines (PCs) (supplementary Fig. VIIIC), which are major membrane lipid constituents, were also significantly elevated in the low-lipid category. Elevated levels of these lipids might have resulted from increased cellular debris in entrapped meibum associated with MGD.
DISCUSSION
As an evolutionary adaptation to a nonaquatic environment, humans developed a structurally and biochemically intricate preocular tear film stabilized by compositional and hydrodynamic factors (26). Thus, even minor aberrations in tear components could have a detrimental impact on the overall structural integrity and stability of the tear film. A notable finding in our study is the structurally-specific reductions in low molecular mass WEs, as well as species with saturated fatty acyl moieties in DES (Fig. 4). Consistent with our finding, Dougherty, Osgood, and McCulley (10) reported lower levels of saturated FAs in meibum WEs from chronic blepharitis patients, with corresponding increases in species containing monounsaturated FAs. The saturation of the fatty acyl component of WEs, instead of the alcohol moiety, is shown to be critical in determining the stiffness of WE films (27). The presence of a saturated FA component generally makes the film stiffer, eliciting greater resistance to spreading out again after being compressed (27). On the other hand, having a saturated FA component translates to a smaller surface area taken up by the WE molecule as a whole, indicating a greater fluidly in sliding over each other to lift from the surface (27). WEs containing saturated FAs might thus facilitate the formation of a stable multilayered lipid film after a blink. By virtue of their saturated FA components that confer fluidity in sliding across different planes, such species might intercalate between different layers of an expanded multilayered film, thereby providing a specific degree of stiffness and resistance to collapsing after an intact film is formed. On the other hand, low molecular mass WEs would have comparatively higher polarity, thus serving as transitional lipids in bridging the interaction between the amphiphilic lipid sublayer with the bulk of the other nonpolar lipids. Hence, a reduction in WEs of low molecular mass or those containing saturated FAs could possibly exert an adverse effect on overall tear film stability.
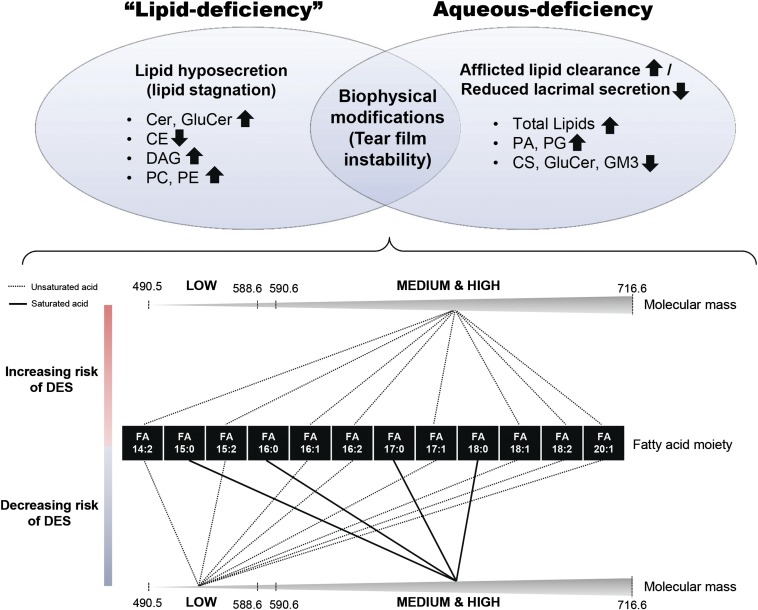
Fig. 4.
Schematic diagram summarizing lipid alterations in DES subtypes. Lipids at the ocular surface can exert biochemical and/or biophysical functions to maintain ocular health and homeostasis. Both “lipid-deficient” (our analyses showed that patients did not have an actual physiological deficiency in absolute lipid levels compared with normal controls) and aqueous-deficient DES exhibited distinct lipid perturbations that might be specific to the respective biochemical aberrations associated with each disease subtype. On the other hand, lipids eliciting biophysical (i.e., structural) roles in maintaining overall tear film integrity and stability might be similarly modified in both disease subtypes. Low molecular mass WEs in tears containing unsaturated acid moieties were associated with a decreased risk of DES. Medium to high molecular mass WEs containing unsaturated fatty acyl components were associated with increased risk of DES, while the opposite trend was observed for species containing saturated acid moieties in the same molecular mass range.
Surprisingly, we did not observe a significant reduction in total lipid levels, even in nonAqD DES patients. It has been reported that in MGD patients with some, but not all, of the glands blocked, there were adequate lipids (17–53 times the amounts required) on the lid margin to form a multilayered tear film lipid layer. The quality, instead of quantity, of meibum could thus potentially contribute to MGD in such patients (28). In agreement with this hypothesis, we found appreciable compositional changes in tear lipids as the absolute amounts of lipids in tears varied (Fig. 4). In particular, proportional increases in major phospholipid classes [i.e., PC and phosphatidylethanolamine (PE)] were observed in the low-lipid category. In addition, elevated levels of PA and PG were also detected in aqueous-deficient individuals. Due to their polar nature, phospholipids can easily transverse the tear film aqueous layer and adhere to the ocular epithelial and mucin components, thereby creating “unwettable” regions that hinder the supply of nutrients to the avascular cornea, which might lead to reduced TBuT and corneal epithelial damage. Indeed, elevated molar fractions of PA and PG were significantly correlated with increasing Baylor score (supplementary Fig. IIA), indicative of corneal epithelial damages with enhanced levels of these lipids. It has been shown that both lipocalin (29) and phospholipid transfer protein (30) in human tears serve to scavenge lipids from corneal surfaces to ensure a “wettable” cornea. Specifically, it was stated that phospholipid transfer protein knockout mice developed appreciable DES and elevated corneal epithelial permeability (30), which is also in accordance with the accumulation of phospholipids in DES observed in our study.
CONCLUSION
This study has underscored the pathological relevance of structural-specific alterations (in terms of molecular mass and degree of unsaturation) in tear lipid components, particularly the WEs, to the pathogenesis of DES. Remarkably, our comprehensive analysis of tear lipid aberrations in DES did not reveal an actual “lipid deficiency” on the lid margins in both DES subtypes (i.e., with or without AqD), but suggested that compositional alterations possibly leading to impeded spreading and/or compromised stability of the tear film might instead represent the key to disease pathogenesis.
Supplementary Material
Footnotes
Abbreviations:
- AqD
- aqueous deficiency
- CE
- cholesteryl ester
- Cho
- cholesterol
- CS
- cholesteryl sulfate
- DES
- dry eye syndrome
- GluCer
- glucosylceramide
- GM3
- NeuAcα2-3Galβ1-4Glcβ-Cer
- LPC
- lyso-phosphatidylcholine
- MGD
- meibomian gland dysfunction
- OAHFA
- O-acyl-ω-hydroxy-fatty acid
- PA
- phosphatidic acid
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- Schir I
- Schirmer's I test
- TBuT
- tear breakup time
- WE
- wax ester
This research was supported by grants from the Chinese Academy of Sciences (KYQY-162 and Y265091891) and the Singapore National Research Foundation under its clinician scientist award NMRC/CSA/013/2009, under its individual research grant NMRC/1206/2009, and under its CRP award number 2007-4. S.M.L. was a recipient of the President's Graduate Fellowship from the National University of Singapore.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of eight figures and two tables.
REFERENCES
- 1.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 5: 93–107 [DOI] [PubMed] [Google Scholar]
- 2.Uchino M., Dogru M., Yagi Y., Goto E., Tomita M., Kon T., Saiki M., Matsumoto Y., Uchino Y., Yokoi N., et al. 2006. The features of dry eye disease in a Japanese elderly population. Optom. Vis. Sci. 83: 797–802 [DOI] [PubMed] [Google Scholar]
- 3.Jie Y., Xu L., Wu Y. Y., Jonas J. B. 2009. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye (Lond.). 23: 688–693 [DOI] [PubMed] [Google Scholar]
- 4.Sullivan B. D., Crews L. A., Sönmez B., de la Paz M. F., Comert E., Charoenrook V., de Araujo A. L., Pepose J. S., Berg M. S., Kosheleff V. P., et al. 2012. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 31: 1000–1008 [DOI] [PubMed] [Google Scholar]
- 5.Foulks G. N. 2007. The correlation between the tear film lipid layer and dry eye disease. Surv. Ophthalmol. 52: 369–374 [DOI] [PubMed] [Google Scholar]
- 6.McCulley J. P., Shine W. 1997. A compositional based model for the tear film lipid layer. Trans. Am. Ophthalmol. Soc. 95: 79–88; discussion 88–93 [PMC free article] [PubMed] [Google Scholar]
- 7.Yokoi N., Yamada H., Mizukusa Y., Bron A. J., Tiffany J. M., Kato T., Kinoshita S. 2008. Rheology of tear film lipid layer spread in normal and aqueous tear-deficient dry eyes. Invest. Ophthalmol. Vis. Sci. 49: 5319–5324 [DOI] [PubMed] [Google Scholar]
- 8.Lam S. M., Tong L., Yong S. S., Li B., Chaurasia S. S., Shui G., Wenk M. R. 2011. Meibum lipid composition in Asians with dry eye disease. PLoS ONE. 6: e24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam S. M., Shui G. 2013. Lipidomics as a principal tool for advancing biomedical research. J. Genet. Genomics. 40: 375–390 [DOI] [PubMed] [Google Scholar]
- 10.Dougherty J. M., Osgood J. K., McCulley J. P. 1991. The role of wax and sterol ester fatty acids in chronic blepharitis. Invest. Ophthalmol. Vis. Sci. 32: 1932–1937 [PubMed] [Google Scholar]
- 11.Lam S. M., Tong L., Duan X., Petznick A., Wenk M. R., Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J. Lipid Res. 55: 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam S. M., Tong L., Reux B., Lear M. J., Wenk M. R., Shui G. 2013. Rapid and sensitive profiling of tear wax ester species using high performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A. 1308: 166–171 [DOI] [PubMed] [Google Scholar]
- 13.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf . 5: 75–92 [DOI] [PubMed] [Google Scholar]
- 14.Borchman D., Foulks G. N., Yappert M. C., Milliner S. E. 2012. Changes in human meibum lipid composition with age using nuclear magnetic resonance spectroscopy. Invest. Ophthalmol. Vis. Sci. 53: 475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borchman D., Yappert M. C., Foulks G. N. 2010. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp. Eye Res. 91: 246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borchman D., Foulks G. N., Yappert M. C. 2010. Confirmation of changes in human meibum lipid infrared spectra with age using principal component analysis. Curr. Eye Res. 35: 778–786 [DOI] [PubMed] [Google Scholar]
- 17.Savini G., Prabhawasat P., Kojima T., Grueterich M., Espana E., Goto E. 2008. The challenge of dry eye diagnosis. Clin. Ophthalmol. 2: 31–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagyová B., Tiffany J. M. 1999. Components responsible for the surface tension of human tears. Curr. Eye Res. 19: 4–11 [DOI] [PubMed] [Google Scholar]
- 19.Glasgow B. J., Abduragimov A. R., Farahbakhsh Z. T., Faull K. F., Hubbell W. L. 1995. Tear lipocalins bind a broad array of lipid ligands. Curr. Eye Res. 14: 363–372 [DOI] [PubMed] [Google Scholar]
- 20.Varela A. R. P., Gonçalves da Silva A. M. P. S., Fedorov A., Futerman A. H., Prieto M., Silva L. C. 2013. Effect of glucosylceramide on the biophysical properties of fluid membranes. Biochim. Biophys. Acta. 1828: 1122–1130 [DOI] [PubMed] [Google Scholar]
- 21.Fleiszig S. M. J., Kwong M. S. F., Evans D. J. 2003. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect. Immun. 71: 3866–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokoi N., Mossa F., Tiffany J. M., Bron A. J. 1999. Assessment of meibomian gland function in dry eye using meibometry. Arch. Ophthalmol. 117: 723–729 [DOI] [PubMed] [Google Scholar]
- 23.Bron A. J., Tiffany J. M. 2004. The contribution of meibomian disease to dry eye. Ocul. Surf. 2: 149–165 [DOI] [PubMed] [Google Scholar]
- 24.Shimazaki J., Sakata M., Tsubota K. 1995. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch. Ophthalmol. 113: 1266–1270 [DOI] [PubMed] [Google Scholar]
- 25.Shine W. E., Silvany R., McCulley J. P. 1993. Relation of cholesterol-stimulated Staphylococcus aureus growth to chronic blepharitis. Invest. Ophthalmol. Vis. Sci. 34: 2291–2296 [PubMed] [Google Scholar]
- 26.Pflugfelder S. C., Tseng S. C., Sanabria O., Kell H., Garcia C. G., Felix C., Feuer W., Reis B. L. 1998. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 17: 38–56 [DOI] [PubMed] [Google Scholar]
- 27.Schuett B. S., Millar T. J. 2012. Lipid component contributions to the surface activity of meibomian lipids. Invest. Ophthalmol. Vis. Sci. 53: 7208–7219 [DOI] [PubMed] [Google Scholar]
- 28.Faheem S., Kim S. H., Nguyen J., Neravetla S., Ball M., Foulks G. N., Yappert M. C., Borchman D. 2012. Wax-tear and meibum protein, wax-β-carotene interactions in vitro using infrared spectroscopy. Exp. Eye Res. 100: 32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glasgow B. J., Gasymov O. K., Abduragimov A. R., Engle J. J., Casey R. C. 2010. Tear lipocalin captures exogenous lipid from abnormal corneal surfaces. Invest. Ophthalmol. Vis. Sci. 51: 1981–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setälä N. L., Holopainen J. M., Metso J., Yohannes G., Hiidenhovi J., Andersson L. C., Eriksson O., Robciuc A., Jauhiainen M. 2010. Interaction of phospholipid transfer protein with human tear fluid mucins. J. Lipid Res. 51: 3126–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.