Abstract
Isotope dilution is currently the most accurate technique in humans to determine vitamin A status and bioavailability/bioconversion of provitamin A carotenoids such as β-carotene. However, limits of MS detection, coupled with extensive isolation procedures, have hindered investigations of physiologically-relevant doses of stable isotopes in large intervention trials. Here, a sensitive liquid chromatography-tandem mass spectrometry (LC/MS/MS) analytical method was developed to study the plasma response from coadministered oral doses of 2 mg [13C10]β-carotene and 1 mg [13C10]retinyl acetate in human subjects over a 2 week period. A reverse phase C18 column and binary mobile phase solvent system separated β-carotene, retinol, retinyl acetate, retinyl linoleate, retinyl palmitate/retinyl oleate, and retinyl stearate within a 7 min run time. Selected reaction monitoring of analytes was performed under atmospheric pressure chemical ionization in positive mode at m/z 537→321 and m/z 269→93 for respective [12C]β-carotene and [12C] retinoids; m/z 547→330 and m/z 274→98 for [13C10]β-carotene and [13C5] cleavage products; and m/z 279→100 for metabolites of [13C10]retinyl acetate. A single one-phase solvent extraction, with no saponification or purification steps, left retinyl esters intact for determination of intestinally-derived retinol in chylomicrons versus retinol from the liver bound to retinol binding protein. Coadministration of [13C10]retinyl acetate with [13C10]β-carotene not only acts as a reference dose for inter-individual variations in absorption and chylomicron clearance rates, but also allows for simultaneous determination of an individual's vitamin A status.
Keywords: β-carotene 15,15′-monooxygenase; carotenoid metabolism; retinol metabolism; retinyl esters; tandem mass spectrometry
Vitamin A deficiency is a major public health issue in the developing world due to inadequate intake of both preformed vitamin A and provitamin A carotenoids in the diet (1). However, detection of subclinical deficiency is problematic because ∼85% of vitamin A is stored in the liver while the level of vitamin A circulating in the blood is under strict homeostatic control and not indicative of hepatic reserves (2). Increasing the intake of provitamin A carotenoids, primarily through β-carotene, is seen as a safe way of restoring the vitamin A reserves of an individual because high doses of preformed vitamin A have adverse health effects (3). Although the current vitamin A equivalency ratio for β-carotene is estimated at 12:1 (by weight) (4), large inter-individual variations in both absorption and conversion have been observed (5–8).
In the intestinal mucosa, a proportion of absorbed β-carotene undergoes centric cleavage by the β-carotene 15,15′-monooxygenase 1 (BCMO1) enzyme to produce two molecules of retinal which are further reduced to retinol (vitamin A) (9). For export into the circulation, retinol is esterified to a long chain fatty acid, typically palmitate, and incorporated, along with intact β-carotene, into chylomicrons (10). Subsequently, retinyl esters are either stored in hepatic stellate cells or hydrolyzed back to retinol by the liver for repartition to other tissue compartments bound to retinol binding protein (RBP).
Currently, stable isotope dilution offers the most accurate determination of β-carotene bioefficacy and vitamin A status irrespective of high endogenous circulating levels of these micronutrients (1, 2). However, the minimum dose to be administered has been dictated by the detection limit of the analytical method (2). Furthermore, isolation of carotenoids/retinoids from the plasma matrix for MS analysis often involves extensive and time-consuming extraction/purification procedures that have included: saponification, solid-phase extraction, preparative HPLC, and, in the case of GC-MS analysis, further conversion to tert-butyl-dimethylsilyl derivatives (11–18). The aim was to develop an analytical method that involved a simplified extraction procedure, sensitive MS/MS for detection of physiological doses of stable isotopes, and short LC runtimes so as to be suitable for high-throughput of samples from human intervention studies.
MATERIALS AND METHODS
Chemicals
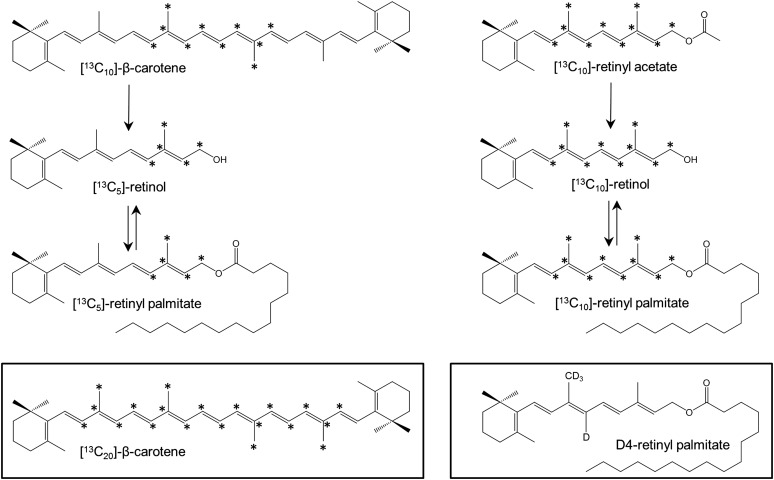
The following carotenoid and retinoid (>95% all-trans) standards were purchased from Sigma (St. Louis, MO): β-carotene, lycopene, retinol, retinyl acetate, and retinyl palmitate. The [12-, 12′-, 13-, 13′-, 14-, 14′-, 15-, 15′-, 20-, 20′-13C10]β-carotene and [8-, 9-, 10-, 11-, 12-, 13-, 14-, 15-, 19-, 20-13C10]retinyl acetate (Fig. 1) to be administered to human subjects were custom synthesized by Buchem BV (Apeldoorn, The Netherlands) and certified fit for human consumption. Similarly, the [8-, 8′-, 9-, 9′-, 10-, 10′-, 11-, 11′-, 12-, 12′-, 13-, 13′-, 14-, 14′-, 15-, 15′-, 19-, 19′-, 20-, 20′-13C20]β-carotene, [12-, 13-, 14-, 15-, 20-13C5]retinol, and [10-, 19-, 19-, 19-d4]retinyl palmitate stable isotopes (Fig. 1) were also purchased from Buchem BV. Methanol, propan-2-ol, chloroform, ethanol, ethyl acetate, toluene, and acetic acid were all of HPLC grade and purchased from Fisher Scientific (Loughborough, UK). Butylated hydroxytoluene (BHT), Novozyme 435, and ammonium acetate were obtained from Sigma-Aldrich (St. Louis, MO). Aberlyst A-21, linoleic acid, oleic acid, and stearic acid for synthesis of retinyl esters were obtained from Alfa Aesar (Heysham, Lancashire, UK).
Fig. 1.
β-carotene and retinyl acetate metabolism. Position of [13C] labels are shown for [13C10]β-carotene and [13C10]retinyl acetate, and derived metabolites. Inserts show the [13C20]β-carotene and d4-retinyl palmitate used for method validation. Asterisks (*) denote position of [13C] labels.
Synthesis of retinyl esters
Retinyl esters were synthesized via an enzyme-catalyzed transesterification (19) as follows. Into a dry Schlenk flask, retinyl acetate (33 mg, 0.10 mmol), Novozyme 435 (120 mg), and Aberlyst A-21 (50 mg) were suspended in dry toluene (5 ml). The reaction mixture was stirred under an atmosphere of N2, and five equivalents (0.50 mmol) of the appropriate acid (palmitic, stearic, linoleic, or oleic) were added. After 20 h at room temperature, the reaction mixture was filtered and the solvent was removed under reduced pressure to give a mixture (approximately 1:4) of the desired retinyl ester and unreacted acid. The resulting mixtures were used without further purification as LC/MS/MS standards for the corresponding retinyl esters.
Subjects and blood collection
Healthy male and female volunteers with an age range of 18–45 years were recruited into the “BetaSNP” dietary intervention study where written informed consent was obtained. Exclusion criteria were: pregnancy, smoking, high blood pressure, diabetes, BMI >30, liver/kidney/gastrointestinal disease, lipid metabolic disorders, and consumption of multivitamins (containing vitamins A, C, E) or β-carotene supplements 3 months prior to the study start. The study was conducted according to the guidelines set forth in the Declaration of Helsinki, and all procedures involving human subjects were approved by the National Research Ethics Service (NRES), North East - Sunderland Committee (REC 09/H0904/20) before registration with the UK Clinical Research Network (UKCRN: 7413). The [13C10]β-carotene and [13C10]retinyl acetate were prepared for oral administration in sunflower oil, at respective concentrations of 2 mg ml–1 and 1 mg ml–1, by sonication in amber bottles at room temperature for 30 min. Oil solutions were then stored in sterile 1 ml tip-cap amber oral syringes (Becton Dickinson, Oxford, UK) and used within 1 week of preparation. Fasted subjects were cannulated via the antecubital vein and blood was drawn into 10 ml EDTA Vacutainer tubes (Becton Dickinson). Subjects then received the dual isotopic oral dose of 2 mg [13C10]β-carotene and 1 mg [13C10]retinyl acetate along with a standardized breakfast meal consisting of a muffin and yogurt smoothie. The meal was designed to reflect the same nutrient content as described by Borel et al. (5) containing 46.3 g of fat (55.5% of total energy intake). Blood was subsequently collected at 2, 4, 6, 8, 10, and 12 h postdose via cannulation, and at 24, 48, 168, and 336 h by simple venipuncture. Each blood sample was immediately centrifuged at 4°C upon collection and the plasma stored at −80°C until analysis.
Plasma extraction and analyte recovery
An ethanol/ethyl acetate (1:1) solvent extraction was applied to plasma samples to ensure adequate recovery of all analytes without coextraction of lipids known to interfere with LC/MS analyses. All extraction procedures were performed under yellow lighting. To 1 ml of plasma, 10 μl (50 pmol) each of the [13C10]retinyl acetate and [13C20]β-carotene internal standards were added before denaturing with 5 ml of ethanol and 5 ml of ethyl acetate. The sample was then shaken on an orbital shaker for 10 min and centrifuged at 10,000 rpm for 30 min at 4°C. The supernatant was transferred to a clean glass tube and the solvent evaporated to dryness under a stream of nitrogen. The residue was resuspended in 100 μl of ethyl acetate, by vortexing briefly, and transferred to amber glass vials ready for LC/MS/MS injection.
Due to endogenous levels of [12C]β-carotene, retinol, and retinyl palmitate always being present in “control” plasma, recovery of target analytes from the plasma matrix was assessed using the following stable isotopes: [13C10]β-carotene, [13C5]retinol, and d4-retinyl palmitate. Blank plasma was generously provided by the Blood Transfusion Service, Newcastle upon Tyne Hospitals (UK). For extraction efficiency experiments, 10 μl of [13C10]β-carotene, [13C5]retinol, and d4-retinyl palmitate in ethanol were spiked into 1 ml of control plasma at a final concentration of 5 μM. Plasma was then extracted as described above.
LC/MS/MS analysis
Chromatographic separation of β-carotene and retinoids was achieved using a Perkin Elmer Series 200 LC (Beckonsfield, UK) equipped with a Gemini C18 column (3 μm; 50 mm × 2 mm i.d.) and SecurityGuard C18 column (4 × 3 mm) both from Phenomenex (Cheshire, UK) maintained at 30°C. Reverse phase elution of analytes was performed with mobile phases of 0.1M aqueous ammonium acetate pH 5 (A) and 50:50 (w/w) methanol/isopropanol (B). The mobile phase system consisted of a 1 min linear gradient from 80% to 99% B, held at 99% B for 3 min, then immediately returned to 80% B for 3 min to re-equilibrate. Flow rate was 1.0 ml min−1 with an injection volume of 10 μl.
An API4000 triple quadrupole LC/MS/MS (Applied Biosystems, Carlsbad, CA) was used for analysis with atmospheric pressure chemical ionization (APCI) performed in positive ion mode using nitrogen gas with the following optimum settings: collision gas, 7; curtain gas, 10; ion source gas 1, 60; ion source gas 2, 15. Temperature of the heated nebulizer was 400°C with an ionspray voltage of 5,500. Optimization of MS/MS parameters for all analytes was performed by selecting precursor ions of [M+H]+ for β-carotene, [M+H-18]+ for retinol, [M+H-256]+ for retinyl palmitate, and [M+H-60]+ for retinyl acetate to obtain product ion spectra. Quantitation of analytes was performed in selected reaction monitoring (SRM) mode; mass transitions and optimized MS/MS parameters are given in Table 1. Analyst® software v1.4.1 (AB SCIEX, Framingham, MA) was used for SRM, peak integration, and analyte quantitation. Peak areas were adjusted according to internal standard recovery ([13C10]retinyl acetate for retinoids and [13C20]β-carotene for carotenes) and quantified against external calibration curves of [12C]β-carotene, [12C]retinol, and [12C]retinyl palmitate (Table 2).
TABLE 1.
LC retention times, SRM mass ion transitions (Q1/Q3), and MS parameters of analytes
| Analyte | Retention Time (min) | SRM Transitions (m/z) | Declustering Potential (V) | Entrance Potential (V) | Collision Energy (eV) | Collision Exit Potential (V) |
| [12C]retinol | 0.63 | 269→93 | 51 | 10 | 27 | 6 |
| [13C5]retinol | 0.62 | 274→98 | 51 | 10 | 27 | 6 |
| [13C10]retinol | 0.62 | 279→100 | 41 | 10 | 27 | 6 |
| [13C10]retinyl acetate | 0.91 | 279→100 | 41 | 10 | 27 | 6 |
| [12C]retinyl linoleate | 2.20 | 269→93 | 51 | 10 | 27 | 6 |
| [13C5]retinyl linoleate | 2.20 | 274→98 | 51 | 10 | 27 | 6 |
| [13C10]retinyl linoleate | 2.20 | 279→100 | 41 | 10 | 27 | 6 |
| [12C]retinyl palmitate/oleate | 2.36 | 269→93 | 51 | 10 | 27 | 6 |
| [13C5]retinyl palmitate/oleate | 2.36 | 274→98 | 51 | 10 | 27 | 6 |
| [13C10]retinyl palmitate/oleate | 2.35 | 279→100 | 41 | 10 | 27 | 6 |
| d4-Retinyl palmitate | 2.34 | 273→94 | 41 | 10 | 31 | 2 |
| [12C]retinyl stearate | 2.63 | 269→93 | 51 | 10 | 27 | 6 |
| [13C5]retinyl stearate | 2.63 | 274→98 | 51 | 10 | 27 | 6 |
| [13C10]retinyl stearate | 2.63 | 279→100 | 41 | 10 | 27 | 6 |
| [12C]β-carotene | 2.96 | 537→321 | 46 | 10 | 33 | 32 |
| [13C10]β-carotene | 3.00 | 547→330 | 86 | 10 | 33 | 18 |
| [13C20]β-carotene | 2.99 | 557→335 | 66 | 10 | 29 | 24 |
TABLE 2.
Limits of detection, limits of quantitation, linear dynamic ranges, calibration curves, correlation coefficients, and intra-/inter-day variations of [12C] standards used for quantitation of analytes
| Analyte | LODa (pmol) | LOQb (pmol) | Linear Range (pmol) | Slopec (a × 105) | Interceptc (b × 104) | Correlation Coefficient (r2) | Intra-dayd (%RSD) | Inter-daye (%RSD) |
| [12C]retinol | 0.01 | 0.03 | 0.03–110 | 7.937 | 4.219 | 0.999 | 3.8 | 6.5 |
| [12C]retinyl palmitate | 0.03 | 0.10 | 0.10–100 | 4.388 | 1.689 | 0.999 | 3.7 | 7.1 |
| [12C]β-carotene | 0.05 | 0.17 | 0.17–90 | 1.701 | 0.455 | 1.000 | 3.7 | 7.8 |
Limit of detection (S/N = 3; n = 5)
Limit of quantitation (S/N = 10; n = 5)
Calibration curves (y = ax + b).
Intra-day, n = 50.
Inter-day, n = 8.
LC/MS/MS validation
The [12C] species of β-carotene, retinol, and retinyl palmitate were used to assess linear dynamic ranges, limits of detection, limits of quantitation, intra-/inter-day assay precision, and to construct external calibration curves. Stock solutions of β-carotene and retinyl palmitate were prepared in chloroform containing 0.1% BHT at respective concentrations of 0.2 mg ml−1 and 1.0 mg ml−1. Retinol was dissolved in ethanol containing 0.1% BHT at 1.0 mg ml−1. Stock solutions were diluted in ethanol for spectrophotometric determination of absolute concentration at λmax 450 nm for β-carotene and λmax 325 nm for retinol and retinyl palmitate. Concentrations were calculated from published extinction coefficients (E1%1cm) for these compounds in ethanol (20, 21). A standard mix of analytes was prepared in ethanol to study linear dynamic range via serial dilution (11 μM–5 nM), and for determination of intra- and inter-day assay precision (1 μM) through multiple injections.
RESULTS
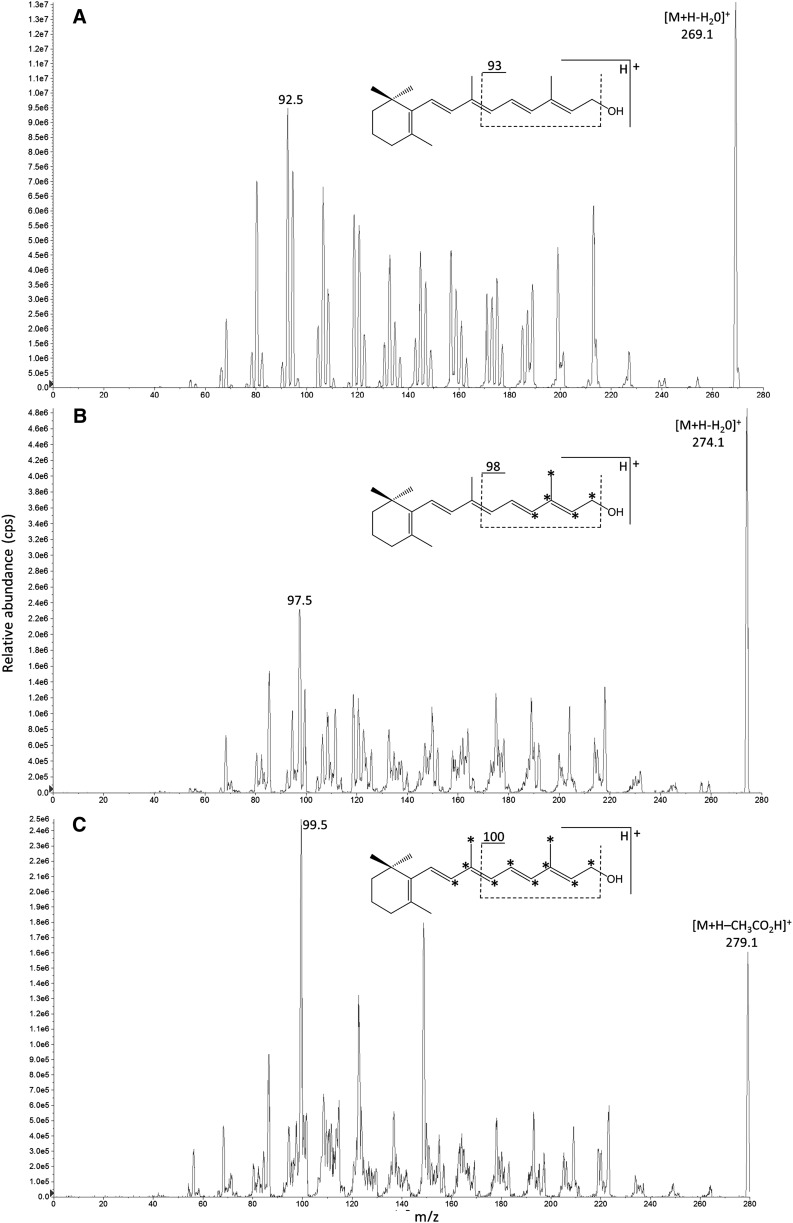
APCI in positive mode offered greater linear dynamic range for both β-carotene and retinoids compared with electrospray ionization (ESI). APCI of retinoids resulted in the elimination of terminal functional groups to produce identical Q1 precursor ions of [M+H–H2O]+ for retinol, [M+H–CH3CO2H]+ for retinyl acetate, and [M+H–CH3(CH2)14CO2H]+ for retinyl palmitate. Consequently, it was necessary to adequately separate retinoids by LC before selected reaction monitoring (SRM) at m/z 269→93, m/z 274→98, and m/z 279→100 for respective [12C], [13C5], and [13C10] isotopologues (Table 1). The abundant Q3 product ion for retinoids was due to cleavage at the C9-C10 double bond where the selected polyene chain fragment contained all [13C] labels from m/z 274 and seven of the [13C] labels from m/z 279 (Fig. 2).
Fig. 2.
Flow-injection APCI-MS/MS product ion mass spectra of m/z 269 [12C]retinol (A), m/z 274 [13C5]retinol (B), and m/z 279 [13C10]retinol (C) in positive mode. Asterisks (*) denote position of [13C] labels.
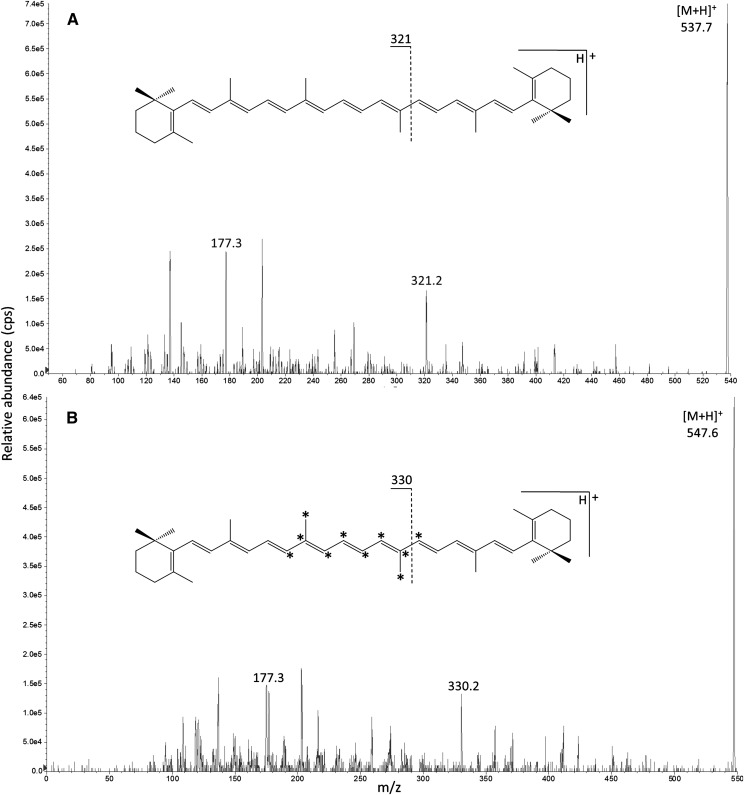
APCI of β-carotene resulted in protonation of the molecule [M+H]+ with an abundant Q3 product ion at m/z 177 irrespective of isotopic composition (m/z 537→177 [12C] and m/z 547→177 [13C]; Fig. 3). The geometric isomer of β-carotene, lycopene, also produced a fragment Q3 ion at m/z 537→177 and possessed an identical LC retention time to β-carotene. Furthermore, an unidentified compound was observed in “blank” plasma at m/z 547→177 which could not be separated from β-carotene by LC. Therefore, an alternative less abundant fragment of higher m/z was selected for [13C]β-carotene at m/z 330 (Fig. 3). This product ion was the result of cleavage at C12-C13 and contained the majority of the [13C] labeling from m/z 547 and also from m/z 557 as internal standard. The corresponding fragment for [12C]β-carotene at m/z 321 was not present for lycopene. Both trans- and cis-β-carotene isomers produced the same Q3 product ions (supplementary Fig. I). Optimized MS/MS parameters and SRM transitions for all analytes are given in Table 1.
Fig. 3.
Flow-injection APCI-MS/MS product ion mass spectra of m/z 537 [12C]β-carotene (A) and m/z 547 [13C10]β-carotene (B) in positive mode. Asterisks (*) denote position of [13C] labels.
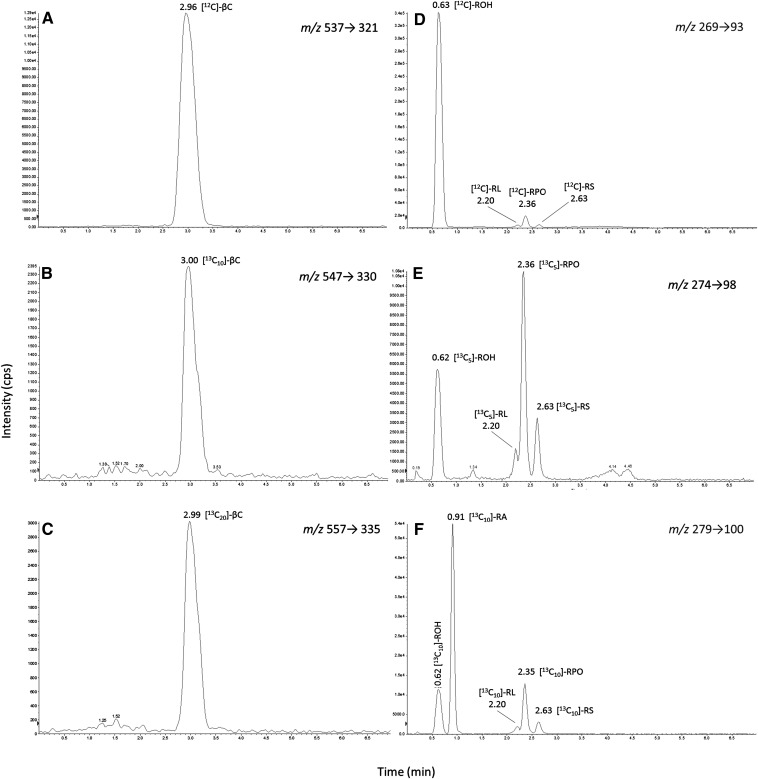
Retinol and retinyl acetate were separated to baseline on a C18 reversed-phase column with a 1 min linear gradient of 80–99% methanol/isopropanol (50:50, w/w); their respective retention times were 0.63 and 0.91 min (Fig. 4). Retinyl palmitate and β-carotene eluted at 2.36 min and 2.96 min respectively under isocratic conditions of 99% methanol/isopropanol. From extracted control plasma, two additional peaks were observed at m/z 269→93 that flanked the retinyl palmitate peak. As these peaks were suspected to be alternative fatty acid esters of retinol, it was necessary to synthesize noncommercially available retinyl esters. The presence of the postulated retinyl esters was confirmed through the use of natural abundance 13C NMR measured in CDCl3 using a Jeol ECS-400 MHz. 13C NMR analysis of the reaction between palmitic acid and retinyl acetate revealed a signal at 174.0 ppm which correlates to the carbonyl carbon of retinyl palmitate (in comparison to commercial standards) and was clearly distinct from retinyl acetate (171.2 ppm) and palmitic acid (180.4 ppm). Similar 13C NMR signals were observed for retinyl stearate (174.0 ppm), retinyl oleate (174.0 ppm), and retinyl linoleate (173.9 ppm), confirming the production of each of the retinyl esters. Synthetic retinyl palmitate was compared against commercially-available retinyl palmitate by LC/MS/MS providing the same retention time and mass spectra, further confirming the formation of the desired retinyl esters. Consequently, LC/MS/MS peaks at 2.20 and 2.63 min were confirmed as retinyl linoleate and retinyl stearate while retinyl oleate coeluted with retinyl palmitate at 2.36 min. Total LC run-time was 7 min, which included a column re-equilibration period of 3 min.
Fig. 4.
APCI (positive mode) LC/MS/MS chromatograms from a human subject plasma sample 6 h postdose showing [12C], [13C10], and [13C5] isotopologues of β-carotene (βC), retinol (ROH), retinyl linoleate (RL), retinyl palmitate/oleate (RPO), and retinyl stearate (RS). [13C10]retinyl acetate (RA) and [13C20]β-carotene were used as internal standards. SRM transitions are given for each chromatogram.
From extraction efficiency experiments (n = 6), the recoveries of [13C5]retinol, d4-retinyl palmitate, and [13C20]β-carotene were 39% (±1.9% SD), 36% (±2.3% SD), and 30% (±1.6% SD) respectively. Although recovery of analytes was relatively low, the mild extraction procedure employed negated the detrimental effects associated with co-extracted lipids during MS analysis. Furthermore, total analyte concentrations were calculated using the internal standards [13C10]retinyl acetate and [13C20]β-carotene, thus correcting for the low recovery. On-column validation of linear dynamic range, limit of detection, and intra- and inter-day precision for [12C] analytes are given in Table 2. Limits of detection ranged from 10 fmol for retinol to 50 fmol for β-carotene. Linear dynamic ranges were over two to three orders of magnitude with r2 values of >0.999 (supplementary Fig. II). Intra- and inter-day precision ranged from 3.7 to 3.8% relative standard deviation (RSD) and from 6.5 to 7.8% RSD, respectively.
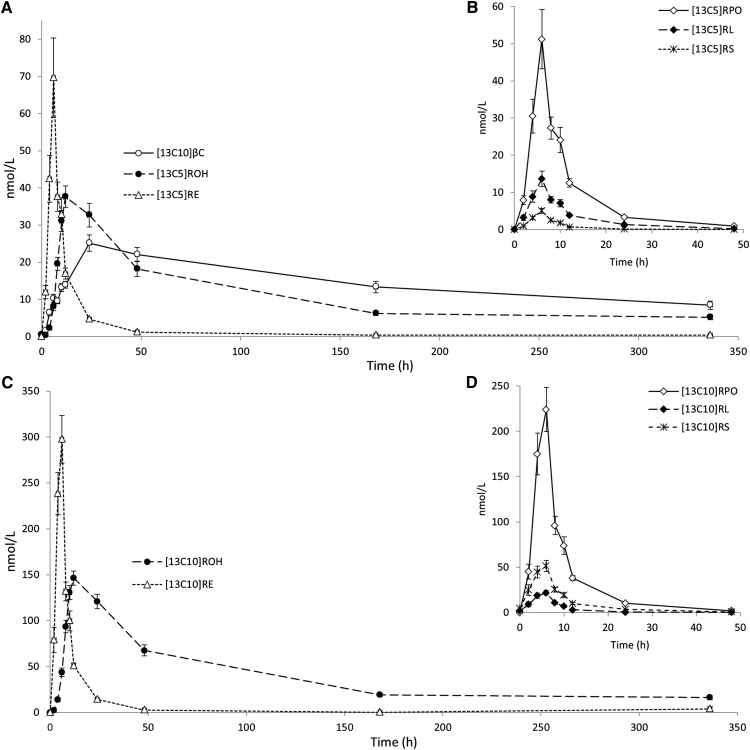
Administered 2 mg [13C10]β-carotene could be detected in plasma from 2 h to 2 weeks postdose (Fig. 5). The [13C10]β-carotene plasma response exhibited an initial increase to 10 nmol/l at 6 h, followed by a brief plateau to 8 h, then a steady rise to a maximum of 25 nmol/l at 24 h. The [13C10]β-carotene cleavage product, [13C5]retinyl palmitate, rapidly attained a maximum concentration of 50 nmol/l at 4 h postdose, while [13C5]retinol started to appear at 3–4 h in plasma and peaked at 10 h. Metabolites of the 1 mg [13C10]retinyl acetate dose reached plasma concentrations 4- to 6-fold higher than [13C10]β-carotene and derived cleavage products. Plasma kinetics of [13C10]retinol and [13C10]retinyl palmitate mirrored those observed for [13C5]retinol and [13C5]retinyl palmitate. Retinol secreted from the intestine was predominantly esterified to palmitate and oleate. However, retinyl linoleate levels were higher than retinyl stearate for [13C5] cleavage products while retinyl stearate was higher than retinyl linoleate for [13C10]retinol.
Fig. 5.
Quantitative LC/MS/MS analysis of mean plasma responses from 45 human subjects (± SEM) over the whole 14 day study period (A, C) and during the first 48 h (B, D). Administered [13C10]β-carotene (βC) and resulting [13C5] cleavage products (ROH, retinol; RE, total retinyl esters; RL, retinyl linoleate; RPO, retinyl palmitate/retinyl oleate; RS, retinyl stearate) are shown in (A) and (B). [13C10] metabolites of administered [13C10]retinyl acetate are shown in (C) and (D).
DISCUSSION
In human intervention studies, the size of stable isotope dose given is largely determined by the limit of detection of the analytical method (1, 2). Although carotene absorption and metabolism may be tracked by the very sensitive method of accelerator MS (22, 23), this method involves the administration of radiolabeled material, albeit at micro-doses, and requires laborious sample fractionation to distinguish metabolites, followed by very expensive analysis using highly specialized equipment that is not widely available. Even if other MS methods such as gas chromatography/combustion/isotope-ratio MS and electron capture negative chemical ionization MS allow effective use of physiological doses of retinol (24, 25) and β-carotene (26) tracers, these methods have the disadvantage of requiring extensive sample preparation, including HPLC purification and derivatization, before injection into the MS. In contrast, the application of liquid chromatography mass spectrometry (LC/MS) to the analysis of retinoid and carotenoid tracers offers the advantages of high sensitivity and selectivity without the need for hydrolysis and derivatization (17, 27–30). However, isolation of carotenoids and retinoids from the plasma matrix is frequently carried out individually leading to separate injections, use of different LC systems, MS ionization methods (APCI/ESI) and modes (positive/negative) (11–18). The current method allows for the first time the analysis of both [13C] retinoid and β-carotene tracers simultaneously using chemical ionization (APCI) in positive mode. Furthermore, the new method is more sensitive than comparable LC/MS methods, with detection limits of 10 fmol for retinol and 50 fmol for β-carotene compared with 233 (27) and 672 fmol (29) for retinol and 250 (17), 559 (28), and 57 fmol (27) for β-carotene in previous methods.
The single solvent extraction procedure developed here for both carotenoids and retinoids negated the effect of interfering plasma lipids (31), without saponification, leaving retinyl esters intact. Consequently, it was not necessary to prepare triglyceride-rich lipoprotein (TRL) fractions to discriminate newly-absorbed intestinally-derived retinyl esters from retinol secreted by the liver bound to RBP. However, it is recognized that small amounts (∼3%) of unesterified retinol, derived from administered retinyl acetate and β-carotene, may be present in lymph chylomicrons (32, 33). Although TRL fractions, obtained by ultracentrifugation at a solution density of <1.006 g ml−1, contain >83% of retinyl esters in the first 6 h postprandial period, a large percentage of plasma retinyl esters is progressively and irreversibly transferred to the denser LDL fraction resulting in 32% of the plasma retinyl esters localized to the LDL fraction 12 h after fat load (34). This transfer of retinyl esters is even more substantial in subjects with familial hypercholesterolemia (35). Furthermore, inter-individual variation in chylomicron clearance kinetics, such as delayed chylomicron remnant clearance in subjects with endogenous hypertriglyceridemia (36) or variation in chylomicron recovery during TRL preparation and analysis, reduces the accuracy of this approach to directly measure the mass of retinyl esters or β-carotene absorbed (37). Thus, the current method can detect intestinally-derived retinyl esters with more accuracy compared with methods employing TRL separations (27, 37, 38).
The current method also allows β-carotene bioefficacy and vitamin A dilution to be studied concurrently due to differential extrinsic [13C] labeling of administered compounds. [13C] isotopes were selected because deuterated compounds are subject to hydrogen-deuterium exchange and possess different physicochemical characteristics resulting in altered LC retention times and solvent extraction efficiencies (2, 11, 28). Position of [13C10] labels around the centric 15,15′ double bond on the β-carotene molecule allowed BCMO1 [13C5] cleavage products to be distinguished from [13C10] metabolites of [13C10]retinyl acetate. Although both [13C10] and [13C5] metabolites displayed similar plasma kinetic profiles, concentrations of [13C5] retinol and retinyl esters were 3- to 4-fold lower even though twice the dose of [13C10]β-carotene was administered. It is known that intestinal absorption of synthetic β-carotene is limited although bioavailability is distinctly enhanced when dissolved in oil (39). Regarding retinyl esters, both [13C10] and [13C5]retinol were preferentially esterified to palmitate and oleate. However, subsequent specificities of [13C5]retinol for linoleate and [13C10]retinol for stearate were observed, which suggests differences in subcellular compartmentalization between preformed retinol and retinol from provitamin A sources in the enterocyte before incorporation in chylomicrons.
Retinyl acetate was coadministered with β-carotene as a reference dose to correct for inter- and intra-individual variations in intestinal absorption and chylomicron clearance rates (37). The [13C10]retinyl acetate dose can also be used to determine total body vitamin A reserves after a sufficient period (circa 3 days) of isotope dilution with endogenous pools (1). In some previous studies, the reference dose was not administered concomitantly with β-carotene to avoid competition during intestinal absorption (12, 14). Single doses of β-carotene have ranged from 5 to 126 mg due to analytical detection limits dictating the minimum dose that can be administered to human subjects. However, β-carotene bioefficacy is dose-dependent when >4 mg is ingested (40), while doses >6 mg perturb the steady-state equilibrium in the blood (41). The 2 mg utilized in the current study represents a true physiological dose according to the estimated daily intake of β-carotene in UK and US populations (39). Although lower doses have been administered daily over a prolonged period to reach a plateau of isotopic enrichment in the blood (15, 16), multiple dosing cannot establish uptake kinetics.
In summary, this new sensitive analytical method allows for the simultaneous study of β-carotene bioefficacy and vitamin A status in human subjects at physiological doses for at least 2 weeks. The simple extraction procedure and single 7 min LC/MS run-time for all analytes makes the method applicable to the high throughput of samples generated in large human intervention studies.
Supplementary Material
Acknowledgments
The authors are grateful for the comments of Dr. Achim Treumann (Proteomics and Biological Mass Spectrometry Facility, Newcastle University) during manuscript preparation.
Footnotes
Abbreviations:
- APCI
- atmospheric pressure chemical ionization
- BCMO1
- β-carotene 15,15′-monooxygenase 1
- BHT
- butylated hydroxytoluene
- LOD
- limit of detection
- LOQ
- limit of quantitation
- RBP
- retinol binding protein
- RSD
- relative standard deviation
- SRM
- selected reaction monitoring
- TRL
- triglyceride-rich lipoprotein
This research was funded by BBSRC (grant reference BB/G004056/1) and supported in part by Cancer Research UK.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures.
REFERENCES
- 1.Furr H. C., Green M. H., Haskell M., Mokhtar N., Nestel P., Newton S., Ribaya-Mercado J. D., Tang G. W., Tanumihardjo S., Wasantwisut E. 2005. Stable isotope dilution techniques for assessing vitamin A status and bioefficacy of provitamin A carotenoids in humans. Public Health Nutr. 8: 596–607 [DOI] [PubMed] [Google Scholar]
- 2.van Lieshout M., West C. E., van Breemen R. B. 2003. Isotopic tracer techniques for studying the bioavailability and bioefficacy of dietary carotenoids, particularly beta-carotene, in humans: a review. Am. J. Clin. Nutr. 77: 12–28 [DOI] [PubMed] [Google Scholar]
- 3.Haskell M. J. 2012. The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion–evidence in humans. Am. J. Clin. Nutr. 96: 1193S–1203S [DOI] [PubMed] [Google Scholar]
- 4.Tang G. 2010. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am. J. Clin. Nutr. 91: 1468S–1473S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borel P., Grolier P., Mekki N., Boirie Y., Rochette Y., Le Roy B., Alexandre-Gouabau M. C., Lairon D., Azais-Braesco V. 1998. Low and high responders to pharmacological doses of beta-carotene: proportion in the population, mechanisms involved and consequences on beta-carotene metabolism. J. Lipid Res. 39: 2250–2260 [PubMed] [Google Scholar]
- 6.Hickenbottom S. J., Follett J. R., Lin Y. M., Dueker S. R., Burri B. J., Neidlinger T. R., Clifford A. J. 2002. Variability in conversion of beta-carotene to vitamin A in men as measured by using a double-tracer study design. Am. J. Clin. Nutr. 75: 900–907 [DOI] [PubMed] [Google Scholar]
- 7.Leung W. C., Hessel S., Meplan C., Flint J., Oberhauser V., Tourniaire F., Hesketh J. E., von Lintig J., Lietz G. 2009. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15′-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J. 23: 1041–1053 [DOI] [PubMed] [Google Scholar]
- 8.Lietz G., Oxley A., Leung W., Hesketh J. 2012. Single nucleotide polymorphisms upstream from the β-carotene 15,15′-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J. Nutr. 142: 161S–165S [DOI] [PubMed] [Google Scholar]
- 9.Lietz G., Oxley A., Boesch-Saadatmandi C., Kobayashi D. 2012. Importance of β,β-carotene 15,15′-monooxygenase 1 (BCMO1) and β,β-carotene 9′,10′-dioxygenase 2 (BCDO2) in nutrition and health. Mol. Nutr. Food Res. 56: 241–250 [DOI] [PubMed] [Google Scholar]
- 10.Harrison E. H. 2012. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta. 1821: 70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dueker S. R., Jones A. D., Smith G. M., Clifford A. J. 1994. Stable isotope methods for the study of beta-carotene-d8 metabolism in humans utilizing tandem mass spectrometry and high-performance liquid chromatography. Anal. Chem. 66: 4177–4185 [DOI] [PubMed] [Google Scholar]
- 12.Lin Y., Dueker S. R., Burri B. J., Neidlinger T. R., Clifford A. J. 2000. Variability of the conversion of beta-carotene to vitamin A in women measured by using a double-tracer study design. Am. J. Clin. Nutr. 71: 1545–1554 [DOI] [PubMed] [Google Scholar]
- 13.Novotny J. A., Dueker S. R., Zech L. A., Clifford A. J. 1995. Compartmental analysis of the dynamics of beta-carotene metabolism in an adult volunteer. J. Lipid Res. 36: 1825–1838 [PubMed] [Google Scholar]
- 14.Tang G., Qin J., Dolnikowski G. G., Russell R. M. 2000. Vitamin A equivalence of beta-carotene in a woman as determined by a stable isotope reference method. Eur. J. Nutr. 39: 7–11 [DOI] [PubMed] [Google Scholar]
- 15.van Lieshout M., West C. E., Muhilal S., Permaesih D., Wang Y., Xu X., van Breemen R. B., Creemers A. F., Verhoeven M. A., Lugtenburg J. 2001. Bioefficacy of beta-carotene dissolved in oil studied in children in Indonesia. Am. J. Clin. Nutr. 73: 949–958 [DOI] [PubMed] [Google Scholar]
- 16.Van Loo-Bouwman C. A., Naber T. H. J., van Breemen R. B., Zhu D., Dicke H., Siebelink E., Hulshof P. J. M., Russel F. G. M., Schaafsma G., West C. E. 2010. Vitamin A equivalency and apparent absorption of beta-carotene in ileostomy subjects using a dual-isotope dilution technique. Br. J. Nutr. 103: 1836–1843 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Xu X. Y., van Lieshout M., West C. E., Lugtenburg J., Verhoeven M. A., Creemers A. F. L., van Breemen R. B. 2000. A liquid chromatography-mass spectrometry method for the quantification of bioavailability and bioconversion of beta-carotene to retinol in humans. Anal. Chem. 72: 4999–5003 [DOI] [PubMed] [Google Scholar]
- 18.Zhu D., Wang Y., Pang Y., Liu A., Guo J., Bouwman C. A., West C. E., van Breemen R. B. 2006. Quantitative analyses of beta-carotene and retinol in serum and feces in support of clinical bioavailability studies. Rapid Commun. Mass Spectrom. 20: 2427–2432 [DOI] [PubMed] [Google Scholar]
- 19.Boaz N. W., Clendennen S. K.2006. Preparation of retinyl esters. United States patent application 20080085534. 2006 June 10.
- 20.Barua A. B., Furr H. C. 1998. Properties of retinoids. Structure, handling, and preparation. Mol. Biotechnol. 10: 167–182 [DOI] [PubMed] [Google Scholar]
- 21.Craft N. E., Soares J. H. 1992. Relative solubility, stability, and absorptivity of lutein and β-carotene in organic-solvents. J. Agric. Food Chem. 40: 431–434 [Google Scholar]
- 22.Dueker S. R., Lin Y. M., Buchholz B. A., Schneider P. D., Lame M. W., Segall H. J., Vogel J. S., Clifford A. J. 2000. Long-term kinetic study of beta-carotene, using accelerator mass spectrometry in an adult volunteer. J. Lipid Res. 41: 1790–1800 [PubMed] [Google Scholar]
- 23.Ho C. C., de Moura F. F., Kim S-H., Burri B. J., Clifford A. J. 2009. A minute dose of C-14-beta-carotene is absorbed and converted to retinoids in humans. J. Nutr. 139: 1480–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang G., Qin J., Dolnikowski G. 1998. Deuterium enrichment of retinol in humans determined by gas chromatography electron capture negative chemical ionization mass spectrometry. J. Nutr. Biochem. 9: 408–414 [Google Scholar]
- 25.Tanumihardjo S. A. 2000. Vitamin A status assessment in rats with C-13(4)-retinyl acetate and gas chromatography/combustion/isotope ratio mass spectrometry. J. Nutr. 130: 2844–2849 [DOI] [PubMed] [Google Scholar]
- 26.Parker R. S., Swanson J. E., Marmor B., Goodman K. J., Spielman A. B., Brenna J. T., Viereck S. M., Canfield W. K. 1993. Study of beta-carotene metabolism in humans using C-13-beta-carotene and high-precision isotope ratio mass-spectrometry. Ann. N. Y. Acad. Sci. 691: 86–95 [DOI] [PubMed] [Google Scholar]
- 27.Fleshman M. K., Riedl K. M., Novotny J. A., Schwartz S. J., Harrison E. H. 2012. An LC/MS method for d8-β-carotene and d4-retinyl esters: β-carotene absorption and its conversion to vitamin A in humans. J. Lipid Res. 53: 820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawlosky R. J., Flanagan V. P., Novotny J. A. 2000. A sensitive procedure for the study of beta-carotene-d8 metabolism in humans using high performance liquid chromatography-mass spectrometry. J. Lipid Res. 41: 1027–1031 [PubMed] [Google Scholar]
- 29.van Breemen R. B., Nikolic D., Xu X. Y., Xiong Y. S., van Lieshout M., West C. E., Schilling A. B. 1998. Development of a method for quantitation of retinol and retinyl palmitate in human serum using high-performance liquid chromatography atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A. 794: 245–251 [DOI] [PubMed] [Google Scholar]
- 30.van Breemen R. B., Dong L., Pajkovic N. D. 2012. Atmospheric pressure chemical ionization tandem mass spectrometry of carotenoids. Int. J. Mass Spectrom. 312: 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagiwara T., Yasuno T., Funayama K., Suzuki S. 1998. Determination of lycopene, alpha-carotene and beta-carotene in serum by liquid chromatography atmospheric pressure chemical ionization mass spectrometry with selected-ion monitoring. J. Chromatogr. B Biomed. Sci. Appl. 708: 67–73 [DOI] [PubMed] [Google Scholar]
- 32.Huang H. S., Goodman D. S. 1965. Vitamin A and carotenoids. I. Intestinal absorption and metabolism of 14C-labelled vitamin A alcohol and beta-carotene in the rat. J. Biol. Chem. 240: 2839–2844 [PubMed] [Google Scholar]
- 33.Goodman D. S., Blomstrand R., Werner B., Huang H. S., Shiratori T. 1966. The intestinal absorption and metabolism of vitamin A and beta-carotene in man. J. Clin. Invest. 45: 1615–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krasinski S. D., Cohn J. S., Russell R. M., Schaefer E. J. 1990. Postprandial plasma vitamin A metabolism in humans: a reassessment of the use of plasma retinyl esters as markers for intestinally derived chylomicrons and their remnants. Metabolism. 39: 357–365 [DOI] [PubMed] [Google Scholar]
- 35.Rubinsztein D. C., Cohen J. C., Berger G. M., Vanderwesthuyzen D. R., Coetzee G. A., Gevers W. 1990. Chylomicron remnant clearance from the plasma is normal in familial hypercholesterolemic homozygotes with defined receptor defects. J. Clin. Invest. 86: 1306–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortner J. A., Coates P. M., Le N. A., Cryer D. R., Ragni M. C., Faulkner A., Langer T. 1987. Kinetics of chylomicron remnant clearance in normal and in hyperlipoproteinemic subjects. J. Lipid Res. 28: 195–206 [PubMed] [Google Scholar]
- 37.Edwards A. J., You C. S., Swanson J. E., Parker R. S. 2001. A novel extrinsic reference method for assessing the vitamin A value of plant foods. Am. J. Clin. Nutr. 74: 348–355 [DOI] [PubMed] [Google Scholar]
- 38.van Vliet T., Schreurs W. H. P., Vandenberg H. 1995. Intestinal beta-carotene absorption and cleavage in men: response of beta-carotene and retinyl esters in the triglyceride-rich lipoprotein fraction after a single oral dose of beta-carotene. Am. J. Clin. Nutr. 62: 110–116 [DOI] [PubMed] [Google Scholar]
- 39.Grune T., Lietz G., Palou A., Ross A. C., Stahl W., Tang G., Thurnham D., Yin S-a., Biesalski H. K. 2010. Beta-carotene is an important vitamin A source for human. J. Nutr. 140: 2268S–2285S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brubacher G. B., Weiser H. 1985. The vitamin A activity of beta-carotene. Int. J. Vitam. Nutr. Res. 55: 5–15 [PubMed] [Google Scholar]
- 41.Tang G. 2012. Techniques for measuring vitamin A activity from β-carotene. Am. J. Clin. Nutr. 96: 1185S–1188S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.