Abstract
Acid sphingomyelinase (ASMase)-deficient Niemann-Pick disease (NPD) is caused by mutations in the sphingomyelin phosphodiesterase 1 (SMPD1) gene, resulting in accumulation of sphingomyelin in the lysosomes and secondary changes in cholesterol metabolism. We hypothesized that the oxidation product of cholesterol, 7-ketocholesterol (7-KC), might increase in the plasma of patients with ASMase-deficient NPD. In this study, a rapid and nonderivatized method of measurement of plasma 7-KC by liquid chromatography-tandem mass spectrometry (LC-MS/MS) was developed. Plasma samples from healthy subjects, patients with ASMase-deficient NPD, nonaffected ASMase-deficient NPD heterozygotes, Niemann-Pick type C (NPC) disease, glycogen storage disorder type II (GSDII), Gaucher disease (GD), mucopolysaccharidosis type II (MPSII), Krabbe disease (KD), and metachromatic leukodystrophy (MLD) were tested retrospectively. Markedly elevated 7-KC was found in patients with ASMase-deficient NPD and NPC disease that showed significant differences from ASMase-deficient NPD heterozygotes; patients with GSDII, GD, MPSII, KD, and MLD; and normal controls. The analysis of plasma 7-KC by LC-MS/MS offers the first simple, quantitative, and highly sensitive method for detection of ASMase-deficient NPD and could be useful in the diagnosis of both ASMase-deficient NPD and NPC disease.
Keywords: genetics, mass spectrometry, oxysterols, storage diseases
Acid sphingomyelinase (ASMase)-deficient Niemann-Pick disease (NPD) is an inherited lysosomal storage disorder (LSD) caused by mutations in the sphingomyelin phosphodiesterase 1 (SMPD1) gene (1, 2). The estimated birth prevalence reported worldwide is 0.5 up to 1 per 100,000 (3). However, the true prevalence may be higher due to a number of attenuated cases that were missed. ASMase-deficient NPD is classified into type A and type B according to the phenotype of the disease. Type A is an infantile-onset severe neurodegenerative disorder characterized by failure to thrive, hepatosplenomegaly, progressive retardation, and death in early childhood (2, 4). In contrast, type B can occur at different ages with variable degrees of hepatosplenomegaly, liver dysfunction, pulmonary disease, and retinal stigmata, but little or no neurologic involvement in most patients. Patients with type B NPD may survive into adolescence and adulthood (2, 5, 6). In recent years, an increasing number of patients intermediate between the A and B types have been described with presence of mental abnormality and onset at 2 to 7 years of age (7, 8). To date, diagnosis of ASMase-deficient NPD is mainly based on detecting the activity of ASMase in leukocytes or skin fibroblasts (2). In addition, testing of mutations of the SMPD1 gene is considered to be a confirmatory diagnosis for ASMase-deficient NPD. More than 100 mutations have been found in the SMPD1 gene and some important genotype-phenotype correlations have been established (9–12). At present, although bone marrow transplants have been done on a small number of patients with type B with encouraging results, no specific treatment is available for patients with ASMase-deficient NPD (13, 14). Enzyme replacement therapy has been largely investigated in recent years (1, 15–17). A phase I clinical trial of enzyme replacement therapy with recombinant SMase for ASMase-deficient NPD type B has been finished in Mt. Sinai School of Medicine in New York city.
An accurate diagnosis of ASMase deficiency in a timely manner is critical for treatment to provide a good chance of halting the course of the disease before major organ or tissue damage has occurred. The current diagnosis processes, including enzyme assay and gene analysis are time-consuming. A rapid, sensitive, and specific assay will benefit the diagnosis and treatment of this disease.
It has been previously noted that tissue and plasma cholesterol and triglyceride levels are increased in patients with ASMase-deficient NPD (18). In patients with Niemann Pick type C (NPC) disease, another LSD caused by deficiency of gene NPC1 or NPC2, cholesterol oxidation products are found to be significantly elevated in the plasma (19, 20). Markedly increased 7-ketocholesterol (7-KC) and cholestan-3β,5α,6β-triol (3β,5α,6β-triol) are used as biomarkers to diagnose NPC disease. We therefore hypothesized that the excess cholesterol in the plasma of ASMase-deficient NPD patients may also be oxidized in some way that leads the increased oxidation products of cholesterol.
Here, to test whether the concentration of 7-KC might serve as a marker for ASMase-deficient NPD, we developed a liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based method without a derivatization step and analyzed plasma samples from patients with ASMase-deficient NPD, NPC disease, and patients that have similar clinical presentations or laboratory investigations to ASMase-deficient NPD, such as hepatomegaly, splenomegaly, hepatosplenomegaly, or abnormal lipid profile. Meanwhile, 7-KC was tested in ASMase-deficient NPD heterozygotes and healthy subjects. We found that 7-KC was markedly elevated in the plasma of patients with ASMase-deficient NPD and NPC disease, but not in other subjects. This rapid and highly sensitive LC-MS/MS method could be useful in the efficient diagnosis of ASMase-deficient NPD and NPC disease.
MATERIALS AND METHODS
Standards and reagents
7-KC, formic acid, acetonitrile, methanol, and activated charcoal were obtained from Sigma-Aldrich, Shanghai, China. The internal standard of 7-KC, d7-7-ketocholesterol (d7-7-KC), was from CND Isotopes (Quebec, Canada). Cholestan-3β,5α,6β-triol and d7-cholestan-3β,5α,6β -triol were obtained from Medical Isotopes, Inc. (Pelham, NH). Ultrapure water was generated using a Milli-Q system (Millipore, Shanghai, China).
Preparation of calibrators, quality controls, and internal standard
Whole blood from a healthy individuals was collected in EDTA vials and centrifuged at a speed of 12,000 rcf for 10 min to separate red blood cells and plasma. To remove the endogenous 7-KC, activated charcoal (1 g/10 ml) was added to the separated plasma and the mixture was stirred at room temperature overnight. Then, the charcoal was removed by centrifuging with a Beckman Optima MAX-E ultracentrifuge (Beckman Coulter, Inc., Brea, CA) at a speed of 80,000 rcf. The above procedure was repeated two additional times. The charcoal-stripped plasma was then used as blank plasma which was treated with a stock solution containing a 10 μg/ml concentration of 7-KC in methanol. The final concentrations of the calibration series were 1, 2, 5, 10, 25, 50, 200, and 400 ng/ml for 7-KC. The calibrators were stored at −80°C before analysis.
Quality controls (QCs) with three concentrations at 2.5, 25, and 250 ng/ml of 7-KC were made by spiking charcoal-stripped plasma and storing in aliquots at −80°C.
The internal standard solution (d7-7-KC) was prepared in methanol at a concentration of 50 ng/ml and stored at −80°C until use.
Clinical sample collection
Plasma samples were obtained from patients with a confirmed diagnosis of ASMase-deficient NPD, NPC disease, and other LSDs. ASMase-deficient NPD heterozygotes were obtained from the Department of Pediatric Endocrinology and Genetic Metabolism at Xinhua Hospital, Shanghai, China and plasma from healthy subjects was obtained from anonymous residual samples for blood lead level screening at Xinhua Hospital. All plasma samples were collected in ethylenediaminetetraacetic acid dipotassium salt-containing tubes. Ethical approval was obtained from the Ethics Committee of Xinhua Hospital.
Sample preparation
Plasma samples were thawed and vortex mixed, and 25 ul was transferred into a 1.5 ml tube to which 100 μl of internal standard solution was then added. The mixture was vigorously mixed and centrifuged at a speed of 13,300 rcf for 5 min at room temperature, and then the supernatant was transferred to a 96-well plate.
Experimental conditions
Analysis was performed on an Ultra Performance Liquid Chromatography system with a Xevo TQ MS detector (Waters, USA). The capillary voltage was set at +3,000 V and the desolvation temperature was 350°C with a gas flow rate of 1,000 l/h, and cone gas was set at 50 l/h. 7-KC and d7-7-KC were detected in positive electrospray ionization (ESI) through the multiple reaction monitoring (MRM) modes. The optimized ESI-MS/MS parameters are listed in Table 1. A Waters BEH column (1.7 μm, 2.1 mm × 50 mm) (Shanghai, China) was maintained at 30°C and eluted with a linear gradient at a flow rate of 0.5 ml/min. Mobile phase A consisted of water with 0.5% formic acid and mobile phase B contained HPLC-grade methanol with 0.5% formic acid. The gradient was increased from 80% mobile phase B to 95% of B over 3 min, stepped to 100% of B, and held for 1 min. The column was then reconditioned to the initial 80% of B for 1 min. The injection volume was 10 μl.
TABLE 1.
Optimal MRM functions and MS/MS parameters of 7-KC and internal standards
| Compound | Mass Transition | Cone Volt | CE |
| 7-KC | 401/81 | 38 | 32 |
| 401/95 | 38 | 40 | |
| d7-7-KC | 408/81 | 40 | 32 |
| 408/95 | 40 | 34 |
CE, collision energy.
Statistical analysis
Results are expressed as mean ± SD. For group comparisons, the statistical significance of differences in mean values was determined by a two-tailed single-factor ANOVA or Student's t-test. A P value of 0.05 or less was considered significant.
RESULTS
Method validation
The LC-MS/MS method for measuring 7-KC was fully validated. The linearity was excellent within ranges from 1 to 400 ng/ml. The coefficient of regression was ≥0.995 and the low limit of quantification was 1 ng/ml. The precision for QCs were 10.52–3.82% [intra-assay, coefficient of variation (CV) (%)] and 3.71–4.16% [inter-assay, CV (%)]. The accuracy of both intra- and inter-assays was within 85–110%. The recovery of 7-KC ranged from 90.8% to 113.2% (see Table 2 for details).
TABLE 2.
Precision, accuracy, recovery, linear range, and low limit of quantification of detection
| Compound | Level (ng/ml) | Precision CV (%) | Accuracy (%) | Recovery (%) | Linear Range (ng/ml) | Coefficient of Regression (r) | LOQ (ng/ml) | ||
| Intra-assay | Inter-assay | Intra-assay | Inter-assay | ||||||
| 7-KC | 2.5 | 10.52 | 3.71 | 86 | 85 | 90.8 | 1-400 | 0.996 | 1.0 |
| 25 | 3.49 | 3.86 | 105 | 101 | 113.2 | ||||
| 250 | 3.82 | 4.16 | 107 | 103 | 110.6 | ||||
Intra-assay, n = 8; inter-assay, n = 6. LOQ, limit of quantification.
Reference interval determination
To establish a reference interval, 314 healthy age-matched subjects were selected and their plasma was collected in purple top tubes (same collection conditions as patients). The concentration of 7-KC was measured and the data were found to be non-Gaussian. The 95th percentile data were used to establish a reference interval of <12.3 ng/ml.
Measurement of 7-KC in ASMase-deficient NPD patients, ASMase-deficient NPD heterozygotes, NPC disease, and other LSD patients
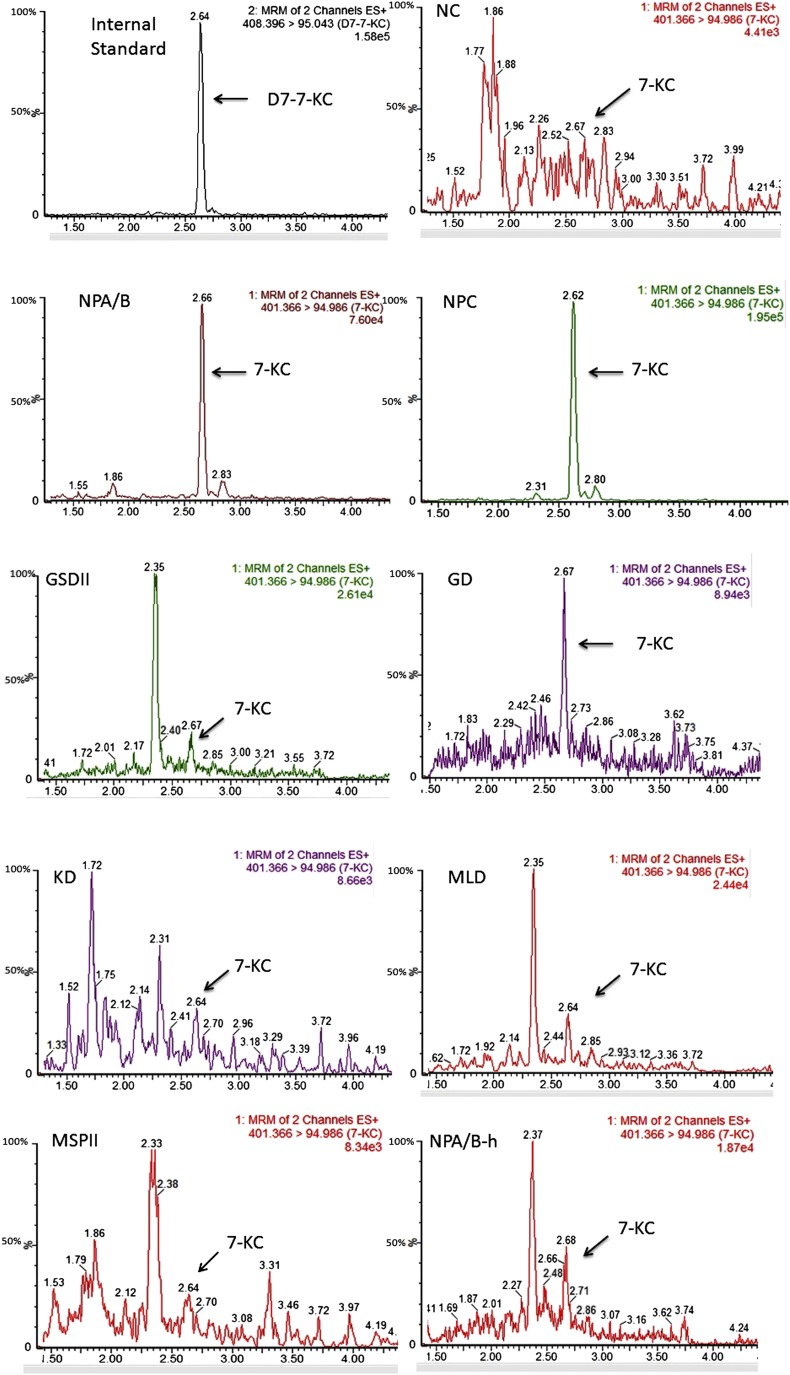
Specimens from ASMase-deficient NPD (n = 38), ASMase-deficient NPD heterozygotes (n = 8), NPC disease (n = 7), glycogen storage disorder type II (GSDII) (n = 19), Gaucher disease (GD) (n = 34), mucopolysaccharidosis type II (MPSII) (n = 38), Krabbe disease (KD) (n = 12), and metachromatic leukodystrophy (MLD) (n = 9) were tested retrospectively. The typical chromatograms for each group are shown in Fig. 1. The significant peaks of 7-KC were found in both ASMase-deficient NPD and NPC disease. The mean values were 245 and 279 ng/ml in patients with ASMase-deficient NPD and NPC disease, respectively. For ASMase-deficient NPD heterozygotes and patients with GD, MPSII, GSDII, KD, and MLD, the mean values were 3.7, 6.7, 6.4, 6.3, and 3.7 ng/ml (KD and MLD together), respectively. The difference was unambiguous in ASMase-deficient NPD and NPC disease results and these two kinds of diseases were easily distinguished from other disorders presented with similar symptoms (all P < 0.001) (see Table 3 for details).
Fig. 1.
Chromatograms of 7-KC in normal subjects (NC), ASMase-deficient NPD (NPA/B), NPC disease, GSDII, GD, KD, MLD, MPSII, and ASMase-deficient NPD heterozygote (NPA/B-h).
TABLE 3.
Plasma 7-KC mean values, range, and P value in healthy subjects, ASMase-deficient NPD, ASMase-deficient NPD heterozygotes, and other LSD patients
| NC | NPA/B | NPA/B-h | NPC | GD | MPSII | GSDII | MLD/KD | |
| n | 314 | 38 | 8 | 7 | 34 | 38 | 19 | 21 |
| Mean and range (ng/ml) | 5.2 (ND–23) | 245 (101–976) | 3.7 (ND–6.1) | 279 (32–603) | 6.7 (1.5–19) | 6.4 (ND–20) | 6.3 (ND–20) | 3.7 (1–7.9) |
| P | <0.001a | NA | <0.001a | 0.849a | <0.001a | <0.001a | <0.001a | <0.001a |
NC, healthy subjects; NPA/B, ASMase-deficient NPD; NPA/B-h, ASMase-deficient NPD heterozygote; NA, not applicable; ND, not detectable.
Comparison versus NPA/B.
DISCUSSION
The current diagnosis of ASMase-deficient NPD is mainly based on enzyme assay and then confirmed by gene analysis. But these time-consuming processes may delay the start of proper treatment of the disease. In order to develop a simple and robust diagnosis method, we selected 7-KC and 3β,5α,6β-triol, the oxidation products of cholesterol previously demonstrated elevated in patients with NPC disease, as the potential markers (19, 20). Although derivatization is one of the common approaches for determining oxidation products of cholesterol (21–25), it is liable to be a source of assay error due to unwanted reaction (26). Therefore, we first detected 7-KC, 3β,5α,6β-triol, and their deuterated internal standards without derivatization using LC/atmospheric-pressure chemical ionization (APCI)-MS. However, we found the most abundant ions by APCI-MS/MS were those resulting from nonspecific fragmentation (e.g., loss of water), which make the unequivocal identification impossible. We then used ESI-MS, an ionization mode in general more sensitive than APCI-MS for compounds that carry polar groups, and recently used for analyses of anabolic steroids (27). Unfortunately, 3β,5α,6β-triol and its deuterated internal standard were not readily detectable by ESI-MS due to their relatively low ionization efficiency, but 7-KC and its deuterated internal standard were efficiently ionized under this circumstance. Accordingly, 7-KC was selected as the potential diagnosis biomarker.
We found plasma 7-KC was markedly elevated in patients of both ASMase-deficient NPD and NPC disease and showed significant differences from ASMase-deficient NPD heterozygotes, patients with GSDII, GD, MPSII, KD, and MLD, and normal controls. Though we have only included seven patients with confirmed diagnosis of NPC disease in our study, the mean value of 7-KC was very consistent with the cholesterol oxidation signature found in the previous study of Jiang et al. (20) (279 vs. 229 ng/ml). The increased plasma 7-KC in ASMase-deficient NPD indicated that the excess cholesterol was oxidized nonenzymatically, similar to the finding in NPC subjects, and this may reflect the presence of oxidative damage in patients of ASMase-deficient NPD (18, 28, 29). We wondered whether 7-KC might also elevate in ASMase-deficient NPD heterozygotes as in NPC heterozygotes (20), so we included eight ASMase-deficient NPD heterozygotes in this study and measured their plasma 7-KC. However, the results showed the mean value of 7-KC is similar to healthy controls (3.7 vs. 5.2 ng/ml). This may be a true reflection due to the small number of samples (n = 8). Given the variation of phenotype between NPD type A and type B, we compared the plasma 7-KC level in these two types of patients. No difference was found between the two groups (data not shown).
Compared with recently published reports (19, 20), our LC-MS/MS method for detection of 7-KC is advantageous because it needs a smaller amount of plasma (25 μl) and less laborious sample preparation. In this method, we omitted the derivatization step and detected 7-KC by ESI positive mode directly. Protein precipitation is the only applied step in sample processing. The application of UPLC interfaced with MS for plasma 7-KC analysis is very simple and highly reproducible, as well as being robust and able to accommodate high sample throughput. The total run time of testing needs only 5 min. It is suitable to be routinely used in clinical laboratories.
Though we only included seven patients with NPC disease in this study, we noticed that the range of plasma 7-KC in NPC disease is very broad (32–603 ng/ml), which is similar to the previous result obtained from a large group of patients (20). As for ASMase-deficient NPD, 38 patients were recruited and the range of 7-KC was relatively narrower (101–976 ng/ml). We think the range of 7-KC in this group may change if we can obtain more samples. Because of the scarcity of NPD, the sample size was relatively small. We believe quite a few patients were misdiagnosed or missed due to insufficient understanding of the disease and different levels of diagnosis among hospitals in different area of China. More patients will be diagnosed quickly and easily if this LC-MS/MS assay with simple sample processing can be routinely used in hospitals.
In summary, our method for plasma 7-KC allows ASMase-deficient NPD and NPC disease to be distinguished from other LSDs with lipid metabolism defects and healthy subjects. Measurement of plasma 7-KC by LC-MS/MS can be applied as the first diagnostic step to infants presenting with neurological deterioration and any children, adolescents, or adults with hepatomegaly or splenomegaly. This step can greatly facilitate further biochemical and genetic confirmations in cases of ASMase-deficient NPD and NPC disease.
Acknowledgments
The authors are grateful to Xiaoyan Li for her assistance in obtaining plasma samples from the patients. We also thank Xiaogang Yu for providing plasma samples from healthy subjects.
Footnotes
Abbreviations:
- APCI
- atmospheric-pressure chemical ionization
- ASMase
- acid sphingomyelinase
- CV
- coefficient of variation
- d7-7-KC
- internal standard for 7-ketocholesterol
- GD
- Gaucher disease
- GSDII
- glycogen storage disorder type II
- 7-KC
- 7-ketocholesterol
- KD
- Krabbe disease
- LSD
- lysosomal storage disorder
- MLD
- metachromatic leukodystrophy
- MPSII
- mucopolysaccharidosis type II
- MRM
- multiple reaction monitoring
- NPC
- Niemann-Pick type C
- NPD
- Niemann-Pick disease
- QC
- quality control
- SMPD1
- sphingomyelin phosphodiesterase 1
- 3β,5α,6β-triol
- cholestan-3β,5α,6β-triol
This study was supported by a grant from Shanghai Municipal Health Bureau (20124128), a grant from the Science and Technology Commission of the Shanghai Municipality Key Program (11dz1950300), and a grant from the National Key Technology R&D Program (2012BAI09B04).
REFERENCES
- 1.Schuchman E. H. 2007. The pathogenesis and treatment of acid sphingomyelinase-deficient Niemann-Pick disease. J. Inherit. Metab. Dis. 30: 654–663 [DOI] [PubMed] [Google Scholar]
- 2.McGovern M. M., Schuchman E. H. 2006. Acid sphingomyelinase deficiency. In GeneReviews™ [Internet]. R. A. Pagon, M.P. Adam, T. D. Bird, et al., editors. University of Washington, Seattle, WA. Accessed November 6, 2012, at http://www.ncbi.nlm.nih.gov/books/NBK1370/ [PubMed]
- 3.Meikle P. J., Hopwood J. J., Clague A. E., Carey W. F. 1999. Prevalence of lysosomal storage disorders. JAMA. 281: 249–254 [DOI] [PubMed] [Google Scholar]
- 4.McGovern M. M., Aron A., Brodie S. E., Desnick R. J., Wasserstein M. P. 2006. Natural history of type A Niemann-Pick disease, possible endpoints for therapeutic trials. Neurology. 66: 228–232 [DOI] [PubMed] [Google Scholar]
- 5.McGovern M. M., Wasserstein M. P., Giugliani R., Bembi B., Vanier M., Mengel E., Brodie S. E., Mendelson D., Skloot G., Desnick R. J., et al. 2008. A prospective, cross-sectional survey study of the natural history of Niemann-Pick disease type B. Pediatrics. 122: e341–e349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGovern M. M., Lippa N., Bagiella E., Schuchman E. H., Desnick R. J., Wasserstein M. P. 2013. Morbidity and mortality in type B Niemann-Pick disease. Genet. Med. 15: 618–623 [DOI] [PubMed] [Google Scholar]
- 7.Pavlů-Pereira H., Asfaw B., Poupctová H., Ledvinová J., Sikora J., Vanier M. T., Sandhoff K., Zeman J., Novotná Z., Chudoba D., et al. 2005. Acid sphingomyelinase deficiency. Phenotype variability with prevalence of intermediate phenotype in a series of twenty-five Czech and Slovak patients. A multi-approach study. J. Inherit. Metab. Dis. 28: 203–227 [DOI] [PubMed] [Google Scholar]
- 8.Wasserstein M. P., Aron A., Brodie S. E., Simonaro C., Desnick R. J., McGovern M. M. 2006. Acid sphingomyelinase deficiency: prevalence and characterization of an intermediate phenotype of Niemann-Pick disease. J. Pediatr. 149: 554–559 [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Pascau L., Gort L., Schuchman E. H., Vilageliu L., Grinberg D., Chabás A. 2009. Identification and characterization of SMPD1 mutations causing Niemann-Pick types A and B in Spanish patients. Hum. Mutat. 30: 1117–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desnick J. P., Kim J., He X., Wasserstein M. P., Simonaro C. M., Schuchman E. H. 2010. Identification and characterization of eight novel SMPD1 mutations causing types A and B Niemann-Pick disease. Mol. Med. 16: 316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittis M. G., Ricci V., Guerci V. I., Marçais C., Ciana G., Dardis A., Gerin F., Stroppiano M., Vanier M. T., Filocamo M., et al. 2004. Acid sphingomyelinase: identification of nine novel mutations among Italian Niemann Pick type B patients and characterization of in vivo functional in-frame start codon. Hum. Mutat. 24: 186–187 [DOI] [PubMed] [Google Scholar]
- 12.Zhang H., Wang Y., Gong Z., Li X., Qiu W., Han L., Ye J., Gu X. 2013. Identification of a distinct mutation spectrum in the SMPD1 gene of Chinese patients with acid sphingomyelinase-deficient Niemann-Pick disease. Orphanet J. Rare Dis. 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneiderman J., Thormann K., Charrow J., Kletzel M. 2007. Correction of enzyme levels with allogeneic hematopoeitic progenitor cell transplantation in Niemann-Pick type B. Pediatr. Blood Cancer. 49: 987–989 [DOI] [PubMed] [Google Scholar]
- 14.Shah A. J., Kapoor N., Crooks G. M., Parkman R., Weinberg K. I., Wilson K. 2005. Successful hematopoietic stem cell transplantation for Niemann-Pick disease type B. Pediatrics. 116: 1022–1025 [DOI] [PubMed] [Google Scholar]
- 15.Miranda S. R., He X., Simonaro C. M., Gatt S., Dagan A., Desnick R. J., Schuchman E. H. 2000. Infusion of recombinant human acid sphingomyelinase into Niemann-Pick disease mice leads to visceral, but not neurological, correction of the pathophysiology. FASEB J. 14: 1988–1995 [DOI] [PubMed] [Google Scholar]
- 16.Ziegler R. J., Brown C., Barbon C. M., D'Angona A. M., Schuchman E. H., Andrews L., Thurberg B. L., McPherson J. M., Karey K. P., Cheng S. H. 2009. Pulmonary delivery of recombinant acid sphingomyelinase improves clearance of lysosomal sphingomyelin from the lungs of a murine model of Niemann-Pick disease. Mol. Genet. Metab. 97: 35–42 [DOI] [PubMed] [Google Scholar]
- 17.Desnick R. J., Schuchman E. H. 2012. Enzyme replacement therapy for lysosomal diseases: lessons from 20 years of experience and remaining challenges. Annu. Rev. Genomics Hum. Genet. 13: 307–335 [DOI] [PubMed] [Google Scholar]
- 18.McGovern M. M., Pohl-Worgall T., Deckelbaum R. J., Simpson W., Mendelson D., Desnick R. J., Schuchman E. H., Wasserstein M. P. 2004. Lipid abnormalities in children with types A and B Niemann Pick disease. J. Pediatr. 145: 77–81 [DOI] [PubMed] [Google Scholar]
- 19.Porter F. D., Scherrer D. E., Lanier M. H., Langmade S. J., Molugu V., Gale S. E., Olzeski D., Sidhu R., Dietzen D. J., Fu R., et al. 2010. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci. Transl. Med. 2: 56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang X., Sidhu R., Porter F. D., Yanjanin N. M., Speak A. O., te Vruchte D. T., Platt F. M., Fujiwara H., Scherrer D. E., Zhang J., et al. 2011. A sensitive and specific LC-MS/MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J. Lipid Res. 52: 1435–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sevanian A., Seraglia R., Traldi P., Rossato P., Ursini F., Hodis H. 1994. Analysis of plasma cholesterol oxidation products using gas- and high-performance liquid chromatography/mass spectrometry. Free Radic. Biol. Med. 17: 397–409 [DOI] [PubMed] [Google Scholar]
- 22.Dzeletovic S., Breuer O., Lund E., Diczfalusy U. 1995. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 225: 73–80 [DOI] [PubMed] [Google Scholar]
- 23.Jiang X., Ory D. S., Han X. 2007. Characterization of oxysterols by electrospray ionization tandem mass spectrometry after one-step derivatization with dimethylglycine. Rapid Commun. Mass Spectrom. 21: 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honda A., Yamashita K., Numazawa M., Ikegami T., Doy M., Matsuzaki Y. 2007. Highly sensitive quantification of 7a-hydroxy-4-cholesten-3-one in human serum by LC-ESI-MS/MS. J. Lipid Res. 48: 458–464 [DOI] [PubMed] [Google Scholar]
- 25.Honda A., Yamashita K., Hara T., Ikegami T., Miyazaki T., Shirai M., Xu G., Numazawa M., Matsuzaki Y. 2009. Highly sensitive quantification of key regulatory oxysterols in biological samples by LC-ESI-MS/MS. J. Lipid Res. 50: 350–357 [DOI] [PubMed] [Google Scholar]
- 26.Santa T., Al-Dirbashi O. Y., Fukushima T. 2007. Derivatization reagents in liquid chromatography/electrospray ionization tandem mass spectrometry for biomedical analysis. Drug Discov. Ther. 1: 108–118 [PubMed] [Google Scholar]
- 27.Deshmukh N., Hussain I., Barker J., Petroczi A., Naughton D. P. 2010. Analysis of anabolic steroids in human hair using LC-MS/MS. Steroids. 75: 710–714 [DOI] [PubMed] [Google Scholar]
- 28.Tint G. S., Pentchev P., Xu G., Batta A. K., Shefer S., Salen G., Honda A. 1998. Cholesterol and oxygenated cholesterol concentrations are markedly elevated in peripheral tissue but not in brain from mice with the Niemann-Pick type C phenotype. J. Inherit. Metab. Dis. 21: 853–863 [DOI] [PubMed] [Google Scholar]
- 29.Zhang J. R., Coleman T., Langmade S. J., Scherrer D. E., Lane L., Lanier M. H., Feng C., Sands M. S., Schaffer J. E., Semenkovich C. F., et al. 2008. Niemann-Pick C1 protects against atherosclerosis in mice via regulation of macrophage intracellular cholesterol trafficking. J. Clin. Invest. 118: 2281–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]