Abstract
Background
Our purpose was to examine the incidence and impact on survival of other primary malignancies (OPM) outside of the breast in breast cancer patients and to identify risk factors associated with OPM.
Methods
Patients with stage 0–III breast cancer treated with breast conserving therapy at our center from 1979 to 2007 were included. Risk factors were compared between patients with/without OPM. Logistic regression was used to identify factors that were associated with OPM. Standardized incidence ratios (SIRs) were calculated.
Results
Among 4,198 patients in this study, 276 (6.6 %) developed an OPM after breast cancer treatment. Patients with OPM were older and had a higher proportion of stage 0/I disease and contralateral breast cancer compared with those without OPM. In a multivariate analysis, older patients, those with contralateral breast cancer, and those who did not receive chemotherapy or hormone therapy were more likely to develop OPM after breast cancer. Patients without OPM had better overall survival. The SIR for all OPM sites combined after a first primary breast cancer was 2.91 (95 % confidence interval: 2.57–3.24). Significantly elevated risks were seen for numerous cancer sites, with SIRs ranging from 1.84 for lung cancer to 5.69 for ovarian cancer.
Conclusions
Our study shows that breast cancer patients have an increased risk of developing OPM over the general population. The use of systemic therapy was not associated with increased risk of OPM. In addition to screening for a contralateral breast cancer and recurrences, breast cancer survivors should undergo screening for other malignancies.
Cancer statistics continue to demonstrate that breast cancer is the most frequently diagnosed malignancy in the United States and the second leading cause of cancer-related deaths in American women.1 Many studies and awareness programs have promoted early detection of breast cancer, and there have been significant advances in treatment modalities. Breast conserving therapy (BCT) is considered standard of care for the local–regional management of early-stage breast cancer, offering equivalent survival benefit and acceptable local control rates when compared with mastectomy.2–4 Radiation therapy is an essential part of BCT for local–regional control of the disease. Chemotherapy and hormonal therapy have proven benefits for reducing local–regional recurrence as well as distant metastasis in certain patient subsets. Owing to improvements in cancer treatment, women with newly diagnosed breast cancer now have a 90 % 5-year relative survival, and there are approximately 2.6 million breast cancer survivors in the United States.5 It has been proposed that women with breast cancer may be at higher risk for developing other primary malignancies (OPM), and this may be influenced by treatments used at their initial diagnosis.
Several studies have reported the risks of OPM in women with breast cancer and hypothesized that these malignancies were due to shared environmental, lifestyle, and genetic factors or treatment effects.6, 7 The possibility of non-breast malignancies secondary to adjuvant therapies has been hypothesized and examined in population-based studies.8–20 Several investigators have reported a low rate of development of OPM among patients treated with radiation therapy and chemotherapy.9,11–18,20,21 Treatment with adjuvant tamoxifen has been shown to be associated with an excess risk of endometrial cancers.8,10,19 Obedian et al.22 reported that for early-stage breast cancer patients there was no increased risk of OPM.
Whether or not OPM have an impact on survival in breast cancer patients after BCT has not been established. The focus of our study was to examine the incidence of OPM after breast cancer treatment and to identify risk factors associated with post breast cancer OPM. In addition, we investigated the survival impact of these OPM on our breast cancer survivors.
PATIENTS AND METHODS
Patient Selection and Data Collection
Patients with stage 0–III breast cancer who underwent BCT from 1979 through 2007 were identified. The MD Anderson Institutional Review Board approved this study. Patients were excluded if the patients did not receive radiation therapy or if they were lost to follow-up within 2 years (157 patients). Also excluded were 23 patients who developed OPM both prior and post breast cancer treatment and 209 patients who had an OPM prior to the diagnosis of breast cancer. Records of 17 patients presenting with stage IV breast cancer were excluded. A total of 4,198 patients were included in this study after exclusions. Because basal cell carcinoma and squamous cell carcinoma of the skin rarely affect survival, these non-melanoma skin cancers were not included as OPM.
Surgical Treatment
Patients underwent resection of the primary breast tumor plus either sentinel lymph node biopsy or axillary lymph node dissection (ALND) for lymph node staging. Macroscopic complete excision of the primary tumor was performed for all patients, and margin status was reviewed intraoperatively by the pathologists. Clips were used to mark the breast cavity to aid in the delivery of radiation therapy.
Radiation Therapy
The ipsilateral breast was treated with 45–50 Gy, delivered in 25 fractions over a 5-week period. Medial and lateral tangential fields of cobalt-60, 6-MV or 18-MV photons were used for radiation. In addition, some patients received a boost of 10–20 Gy to the tumor bed with electrons or an interstitial implant.23
Systemic Therapy
Patients with histologically proven lymph node metastasis were offered systemic chemotherapy. After 1990, systemic chemotherapy was also offered to node-negative patients depending on primary tumor factors that were used for risk stratification. For postmenopausal patients with estrogen receptor positive tumors, tamoxifen was offered with or without chemotherapy.24 In the later years of the study, endocrine therapy was also offered to premenopausal women with estrogen receptor positive tumors.
Statistical Analysis
Patients were divided into two groups: patients without OPM and patients with OPM. Patient, tumor, and treatment characteristics were compared among those groups. Differences in characteristics were analyzed by the Chi-square test or Fisher exact test, t test/rank sum test or Kruskal– Wallis H test. We used cancer incidence rates of first primary cancer (based on NAACCR publication Cancer Incidence in North America, 2002–2006, Cancer Incidence rate for females in the United States) for the calculation of standardized incidence ratios (SIRs) of post breast cancer OPM.25 SIR confidence intervals (CI) were derived using exact Poisson probabilities under the usual assumption that the numbers of observed cancers followed a Poisson distribution with means and variances equal to the numbers expected. Backward stepwise logistic regression analysis was used to identify factors that were associated with post breast cancer OPM. Overall survival was defined as time from the diagnosis of breast cancer to death of any cause. A multivariate Cox proportional hazards model was used to identify significant factors associated with overall survival stratifying by clinical TNM stage and age. Stata statistical software (SE 10, StataCorp LP, College Station, TX, USA) was used for statistical analyses. All P values were two tailed, and P ≤.05 was considered significant.
RESULTS
Demographics and Clinical and Pathologic Characteristics
There were 4,198 patients included in the cohort. A total of 276 (6.6 %) developed an OPM after breast cancer treatment. For those 276 patients with OPM (6.6 %), median time to diagnosis of OPM was 1,835 days (mean 2,391 days, range 3–10,026 days). The 276 patients with OPM after the initial breast cancer treatment were followed for 37,188 person-years. The median follow-up time was 7.2 years (range 2–30.5 years). The majority of the patients were white (76.6 %), and the median age at the time of breast cancer diagnosis was 55 years (range 22–89 years). The most common histologic type of breast cancer was invasive ductal carcinoma (74.6 %).
Patient, tumor, and treatment characteristics for the two groups are summarized in Table 1. Patients with OPM were older and had a higher proportion of stage 0/I disease and contralateral breast cancer compared with those without OPM. No significant differences were noted between the two groups with respect to race, histologic subtype, histologic grade, final margin status, body mass index (BMI), estrogen receptor (ER), progesterone receptor (PR), and family history of cancer.
TABLE 1.
Comparisons of clinical, pathologic, and treatment characteristics between patients with OPM and without OPM
| Characteristics | Breast cancer without OPM (N = 3,922) |
Breast cancer with OPM (N = 276) |
P value |
|---|---|---|---|
| Age at breast cancer diagnosis in years | .0004 | ||
| Median (range) | 54 (22–89) | 58 (22–88) | |
| Mean | 54.7 | 57.3 | |
| BMI | .7 | ||
| Median (range) | 27 (14–62) | 28 (18–56) | |
| Mean | 28 | 28.2 | |
| Race | .6a | ||
| White | 2,993 (76.3) | 221 (80.1) | |
| Black | 591 (15.1) | 34 (12.3) | |
| Hispanic | 253 (6.4) | 16 (5.8) | |
| Other | 85 (2.2) | 5 (1.8) | |
| Family history of cancer | .1a, b | ||
| No | 1,253 (95.1) | 124 (98.4) | |
| Yes | 65 (4.9) | 2 (1.6) | |
| Unknown | 2,604 | 150 | |
| First-degree relative with cancer | .3a, b | ||
| No | 1,268 (87.4) | 125 (90.6) | |
| Yes | 182 (12.6) | 13 (9.4) | |
| Unknown | 2,472 | 138 | |
| BRCA mutation | .001a, b | ||
| No | 54 (85.7) | 1 (16.7) | |
| Yes | 9 (14.3) | 5 (83.3) | |
| Not assessed | 3,859 | 270 | |
| Year of breast cancer diagnosis | <.0001 | ||
| 1979–1988 | 322 (8.2) | 58 (21.0) | |
| 1989–1998 | 1,114 (28.4) | 105 (38.1) | |
| 1999–2007 | 2,486 (63.4) | 113 (40.9) | |
| Clinical stage | .048 | ||
| 0 | 495 (12.6) | 32 (11.6) | |
| I | 2,264 (57.7) | 182 (65.9) | |
| II | 1,016 (25.9) | 53 (19.2) | |
| III | 147 (3.8) | 9 (3.3) | |
| Tumor histology | .3 | ||
| IDC ± DCIS | 2,922 (74.5) | 210 (76.1) | |
| ILC ± DCIS | 218 (5.6) | 10 (3.6) | |
| IDC + ILC | 172 (4.4) | 11 (4.0) | |
| DCIS | 437 (11.1) | 27 (9.8) | |
| Other | 173 (4.4) | 18 (6.5) | |
| Tumor grade | 0.1b | ||
| I | 450 (12.2) | 36 (14.8) | |
| II | 1,799 (48.8) | 128 (52.7) | |
| III | 1,435 (38.9) | 79 (32.5) | |
| Unknown | 238 | 33 | |
| Estrogen receptor status | .3b | ||
| Positive | 2,485 (71.8) | 171 (75.3) | |
| Negative | 974 (28.2) | 56 (24.7) | |
| Unknown | 463 | 49 | |
| Progesterone receptor status | .6b | ||
| Positive | 2,041 (61.0) | 133 (63.0) | |
| Negative | 1,305 (39.0) | 78 (37.0) | |
| Unknown | 576 | 65 | |
| Pathologic nodal status | .03 | ||
| Negative | 3,132 (79.9) | 235 (85.1) | |
| Positive | 790 (20.1) | 41 (14.9) | |
| 1–3 | 633 (80.1) | 31 (75.6) | .09 |
| ≥4 | 157 (19.9) | 10 (24.4) | |
| Type of surgery | <.0001 | ||
| Lumpectomy | 482 (12.3) | 45 (16.3) | |
| Lumpectomy + SLND | 1,743 (44.4) | 76 (27.5) | |
| Lumpectomy + ALND | 1,326 (33.8) | 139 (50.4) | |
| Lumpectomy + SLND + ALND | 371 (9.5) | 16 (5.8) | |
| Follow-up time | .0001c | ||
| Mean | 8.7 | 11.2 | |
| Median (range) | 7.2 (2–30.5) | 9.9 (2–30.4) | |
| Adjuvant chemotherapy | <.0001 | ||
| Yes | 1,359 (34.6) | 64 (23.2) | |
| No | 2,537 (65.4) | 212 (76.8) | |
| Adjuvant hormone therapy | .008 | ||
| Yes | 2,088 (53.2) | 124 (44.9) | |
| No | 1,834 (46.8) | 152 (55.1) | |
| Contralateral breast cancer | <.0001 | ||
| Yes | 372 (9.5) | 44 (16.0) | |
| No | 3,550 (90.5) | 231 (84.0) | |
| Local recurrence | .7a | ||
| Yes | 235 (6.0) | 18 (6.5) | |
| No | 3,684 (94.0) | 258 (93.5) | |
| Regional recurrence | .5a | ||
| Yes | 76 (1.9) | 3 (1.1) | |
| No | 3,844 (98.1) | 273 (98.9) | |
| Systemic recurrence | .7a | ||
| Yes | 314 (8.0) | 20 (7.3) | |
| No | 3,606 (92.0) | 256 (92.7) |
OPM other primary malignancies, BMI body mass index, IDC invasive ductal carcinoma, DCIS ductal carcinoma in situ, ILC invasive lobular carcinoma, SLND sentinel lymph node dissection, ALND axillary lymph node dissection
Fisher exact test
P value calculated after excluding unknown category
Rank sum test
In this study, no distinction was made between neoadjuvant and adjuvant chemotherapy. In patients matched by clinical stage, chemotherapy was more frequently given to patients with breast cancer who did not have OPM. Patients with OPM were less likely to receive hormonal therapy than patients without OPM. The local, regional, and systemic recurrence rates were similar in the two groups. In this cohort, patients with OPM had the highest proportion of contralateral breast cancers (16.0 % in patients with OPM compared with 9.5 % of patients without OPM, P < .0001).
Risk Factors for OPM after Breast Cancer Treatment
A backward stepwise logistic regression analysis was used to identify factors that were associated with OPM. The factors included in the final model were age, contralateral cancer, race, BMI, family history of cancer, BRCA mutation, chemotherapy, and hormone therapy. Table 2 shows results from the multivariate analysis for the factors associated with OPM. Older patients (age >55 years) were more likely to have OPM [odds ratio (OR) 1.44, P = .006], as well as contralateral breast cancer (OR 1.75, P = .001). Patients who did not receive chemotherapy or hormone therapy were more likely to have OPM.
TABLE 2.
Multivariate analysis for the factors associated with post breast cancer OPM
| Odds ratio | P value | 95 % CI | |
|---|---|---|---|
| Age (years) | |||
| >55 vs. ≤55 | 1.44 | .006 | 1.11–1.86 |
| Contralateral breast cancer | |||
| Yes vs. no | 1.75 | .001 | 1.25–2.47 |
| Adjuvant chemotherapy | |||
| No vs. yes | 1.63 | .001 | 1.21–2.19 |
| Adjuvant hormone therapy | |||
| No vs. yes | 1.47 | .002 | 1.15–1.89 |
The final multivariate model with all of the covariates listed in Table 1 and those factors included in the model that were not significant are not shown
Non-Breast Malignancies
The specific types of OPM are reported in Table 3. Of note, the total number of OPM is greater than the number of patients identified with OPM because some of the patients had more than 1 OPM. The most commonly diagnosed OPM in our patients were gynecological malignancies, which included uterine, ovarian, cervical, and vaginal cancer. Gastrointestinal and lung malignancies were the second most common cancer type. Hematologic and endocrine cancers, melanoma, genitourinary cancer, head and neck cancer, and sarcoma followed in decreasing order. There was no evidence that the higher incidence of lung cancer after breast cancer treatment was associated with radiation, as contralateral lung cancers were as common as ipsilateral lung cancers. The SIR for all OPM sites combined after a first primary breast cancer was 2.91 [95 % confidence interval (95 % CI) 2.57–3.24] (Table 4). Significantly elevated risks were seen for numerous cancer sites, with SIRs ranging from 1.84 for lung cancer to 5.69 for ovarian cancer.
TABLE 3.
Comparisons of types of other primary malignancy (OPM)
| Other primary malignancy | OPM post breast cancer N = 286 (%) |
|---|---|
| Head and neck | 11 |
| Gastrointestinal | 38 |
| Gastric | 8 (3.0) |
| Colon/rectal | 26 |
| Liver | 4 |
| Lung | 38 (13.3) |
| Brain | 5 |
| Endocrine | 28 |
| Pancreas | 7 |
| Thyroid | 21 |
| Melanoma | 24 |
| Genitourinary | 21 |
| Renal cell | 17 |
| Bladder | 4 |
| Gynecologic | 73 (25.5) |
| Ovarian | 29 |
| Uterine | 30 |
| Cervical | 12 (4.0) |
| Vagina | 2 |
| Sarcoma (soft tissue and bone) | 4 |
| Hematologic | 40 |
| Leukemia | 15 |
| Lymphoma | 23 |
| Myeloma | 2 |
| Others | 4 |
TABLE 4.
Total number of cancers, standardized incidence rates, and standardized incidence ratios (SIR) relative to U.S. population, in a cohort of 4,198 2-year breast cancer survivors
| Cancer sites | Cases | Expecteda | SIR | 95 % CI |
|---|---|---|---|---|
| All sitesb | 286 | 98 | 2.91 | 2.57–3.24 |
| Lung | 38 | 21 | 1.84 | 1.25–2.42 |
| Brain | 5 | 2 | 2.54 | .31–4.76 |
| Thyroid | 21 | 6 | 3.72 | 2.13–5.30 |
| Melanoma | 24 | 6 | 4.30 | 2.58–6.02 |
| Ovarian | 29 | 5 | 6.05 | 3.84–8.25 |
| Uterine | 30 | 9 | 3.38 | 2.17–4.58 |
| Cervical | 12 | 3 | 3.98 | 1.73–6.24 |
| Sarcoma (soft tissue and bone) | 4 | 1 | 3.07 | .06–6.08 |
| Leukemia | 15 | 4 | 4.16 | 2.05–6.26 |
| Lymphoma | 23 | 7 | 3.27 | 1.93–4.61 |
| Bladder | 4 | 8 | .52 | .01–1.03 |
| Renal cell | 17 | 4 | 4.35 | 2.28–6.42 |
95 % CI 95 %confidence interval
Expected cases were calculated using incidence rates based on NAACCR publication cancer incidence in North America, 2002–2006, cancer incidence rate for females in United States25
Incidence rates for breast cancer are deduced from total incidence rates
Survival
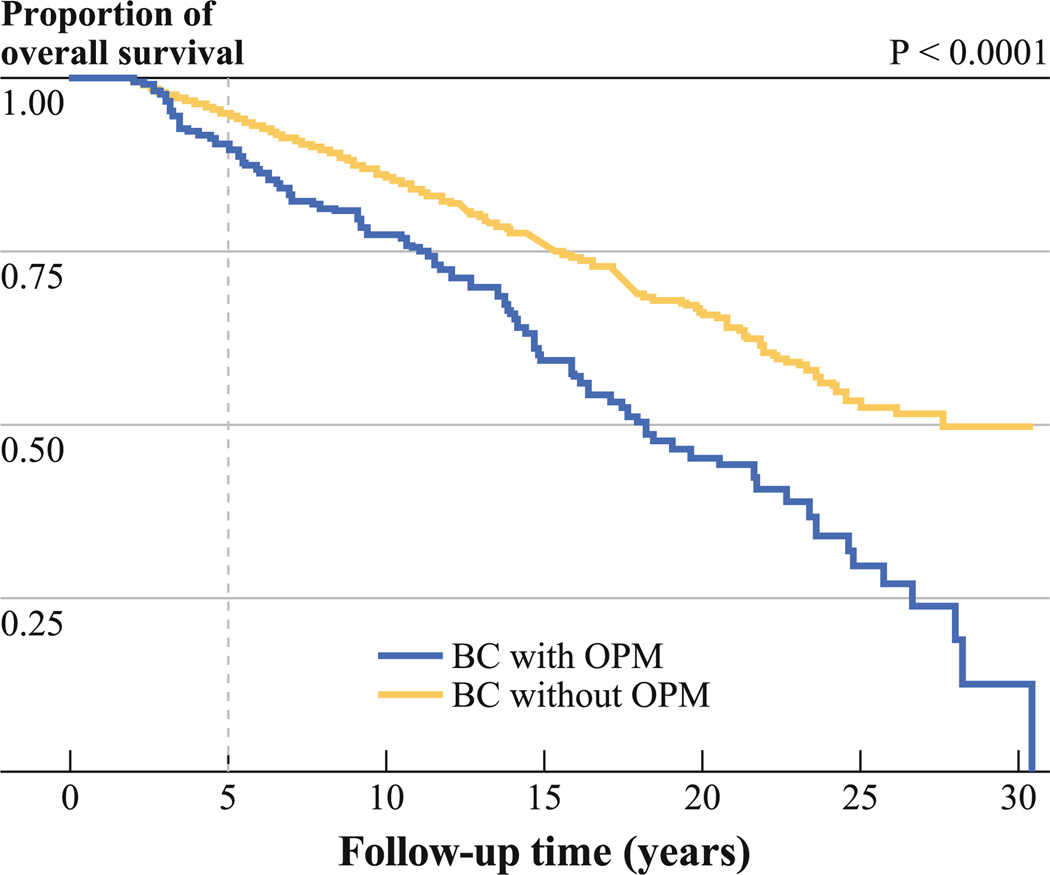
The 5-year actuarial overall survival rate was 94.9 % for patients without OPM, 90.2 % for patients with OPM (P < .0001; Fig. 1). Patient, clinical, and pathological factors affecting overall survival stratified by clinical TNM stage and age are presented in Table 5. Patients who were African American, or had negative ER status, or had positive lymph nodes had shorter overall survival. Patients with OPM had a worse overall survival compared with patients without OPM.
Fig. 1.
Actuarial survival curves showing longer overall survival time in breast cancer patients without other primary malignancy compared with patients with other primary malignancy. BC breast cancer, OPM other primary malignancies
TABLE 5.
Multivariate stratified Cox proportional hazards model of overall survival
| HRa | P value | 95 % CIa | |
|---|---|---|---|
| OPM | |||
| Yes | Referent | ||
| No | .6 | <.0001 | .5–.8 |
| ER | |||
| Positive | Referent | ||
| Negative | 1.5 | .004 | 1.1–1.9 |
| Race | |||
| White | Referent | ||
| Black | 1.4 | .01 | 1.1–1.8 |
| Hispanic | .9 | .4 | .6–1.3 |
| Other | .5 | .1 | .2–1.2 |
| Pathologic nodal status | |||
| Negative | Referent | ||
| 1–3 | 1.3 | .01 | 1.1–1.7 |
| ≥4 | 2.6 | <.0001 | 1.8–3.6 |
| Year of breast cancer diagnosis | NS | ||
| Chemotherapy | NS | ||
| Contralateral breast cancer | NS | ||
| Family history of cancer | NS | ||
| Progesterone receptor status | NS | ||
| Tumor grade | NS |
Whether patients had hormone therapy is highly correlated with ER status, so it is not included in the model
HR hazard ratio, 95 % CI 95 % confidence interval, OPM other primary malignancy, NS not significant
Stratified by clinical TNM stage and age
DISCUSSION
Our study shows that breast cancer patients have an increased risk of developing OPM compared with the general population. The use of systemic therapy was not associated with increased risk of OPM in our patient cohort. The 6.6 % incidence OPM after breast cancer treatment in our study is comparable to the incidence reported in other studies.6,26,27 The most common types of OPM identified in our cohort were, in decreasing order, lung, endometrial, ovarian, and colorectal cancers, and melanoma, a list that is very similar to that reported by Galper et al.26
We did note some significant differences between the patients with and without OPM. Patients with OPM were older, had more stage I and lower grade tumors and less nodal involvement, were more likely to have undergone lumpectomy with ALND, and were more likely to have a first-degree relative with cancer compared with those without OPM.
We noted that older patients (>55 years old), those with contralateral breast cancer, and those who did not receive adjuvant chemotherapy and hormone therapy had a higher risk of developing post breast cancer OPM. Fowble et al.28 published their experience on 1,253 women with early-stage breast cancer who underwent BCT and found that older age was associated with an increased cumulative risk of non-breast primary malignancy. An earlier report from our center showed that among postmenopausal women (age ≥ 50 years) the incidence of OPM was lower in those who received chemotherapy.29 Rubagotti et al.30 studied the risk of new primaries after chemotherapy for early-stage breast cancer patients treated between 1983 and 1991 and found that the incidence of OPM was similar among chemotherapy-treated patients and untreated patients (2.6 vs. 2 %, respectively); however, a 2-fold risk of OPM was observed among the breast cancer patients compared with the expected rate in the general population.
The role of tamoxifen as an adjuvant therapy is well documented in patients who have ER-positive disease; however, whether or not tamoxifen is associated with an increased risk of non-breast malignancies is not clear. A study by Fowble et al.28 reported a significant increase in the cumulative incidence of endometrial and gastrointestinal cancers among breast cancer patients treated with tamoxifen. Similar findings were observed by Rutqvist et al.31 Conversely, a study conducted in Japan by Katase et al.32 showed that there was no increase in the incidence of subsequent endometrial cancers among tamoxifen-treated patients who underwent annual screening for gynecological malignancy. Several other investigators have found no significant increase in the development of OPM among breast cancer patients treated with tamoxifen.33–36 Rubagotti et al.30 found that tamoxifen-treated patients had a significantly lower incidence of OPM compared with those treated with chemotherapy (.95 vs. 2.6 %). They concluded that tamoxifen use appeared to have a protective effect against OPM. The use of hormonal therapy was slightly different for the two groups: patients with OPM were less likely to receive hormonal therapy (44.9 vs. 53.2 %, P = .008) in our study, but the most common type of OPM was gynecological cancers.
A number of studies have examined the incidence of OPM in breast cancer patients treated with radiation therapy, chemotherapy, and hormonal therapy.4,26,37–42 Our study shows that women with breast cancer treated with BCT had an SIR of more than 2.9 for developing a new primary non-breast malignancy compared with women without breast cancer. Rubino et al.17 observed a slightly increased incidence of OPM in breast cancer patients and reported a SIR of 1.4 when compared with the general population in France. Another study showed that women with a first primary breast cancer had only a 25 % increase in the risk of developing a new primary non-breast cancer in comparison with women without cancer.7 Both a National Cancer Institute early breast cancer trial of comparing lumpectomy, axillary dissection, and radiation with modified radical mastectomy, with a median follow-up period of 10 years, and the Institute Gustave-Roussy trial of conservative treatment versus mastectomy in early breast cancer, with a minimum follow-up of 14 years, failed to show a significant increase in second non-breast malignancies from radiation therapy.41,43 Obedian et al.22 compared lumpectomy and radiation therapy with mastectomy for early-stage breast cancer patients at a single institution. The 15-year risk of any second malignancy was nearly identical in both cohorts. However, Roychoudhuri et al.44 suggested that the radiation therapy seemed to pose an increased risk for OPM, especially for lung cancer, myeloid leukemia, second breast cancer, and esophageal cancer. The increased incidence of lung cancer in the NSABP B0-4 trial patients who received radiation therapy was also seen in the studies by Deutsch et al. and by Zablotska et al.45,46 It is interesting but difficult to claim a cause-effect relationship between the radiation therapy and lung cancer development in our study since we did not account for the patient’s smoking history, genetic predisposition, and environmental factors. In addition, patients with a history of breast cancer undergo more frequent imaging studies including chest X-rays, leading to a selection bias. Despite the clinical importance of the consequence of radiation therapy exposure, little is known about predisposing risk factors. A recent study found that two genetic variants were strongly associated with an increased risk of a second cancer in children with Hodgkin lymphoma who received radiation therapy as part of their treatment.47 This study may be crucial for understanding the etiology of OPM in those Hodgkin lymphoma survivors, as well as in other cancer patients treated with radiation therapy.
Our study has a number of limitations, including those inherent to any single-institution, retrospective study. First, our study is limited by the sample size, the lack of a nonradiation therapy control group, and missing data regarding genetic information, lifestyle choices, and other risk factors. The strengths of the study include a long follow-up interval and inclusion of patients from a single institution that has used standardized treatment regimens over decades.
In conclusion, our study shows that breast cancer patients are at increased risk for the development of OPM compared with the general population. Systemic therapy use was not associated with increased risk of OPM in our cohort. In addition to screening for development of a contralateral breast cancer and recurrences, routine screening for the development of second primary malignancies should be encouraged. Breast cancer patients, especially younger ones, those who are obese, and those with a smoking history, should be encouraged to follow the primary prevention advice related to tobacco use, diet, and weight control. In addition, all breast cancer survivors should have ongoing screening for contralateral breast cancers and be educated regarding the signs and symptoms of recurrent breast cancer. Physicians must also be aware of the increased risk of non-breast malignancies and should incorporate this information into surveillance strategies. The early detection of OPM should be a priority in order to improve the prognosis of breast cancer survivors.
ACKNOWLEDGMENT
The authors wish to acknowledge Walter J. Pagel, Department of Scientific Publications, The University of Texas MD Anderson Cancer Center, for editorial assistance. Supported in part by the National Cancer Institute through Cancer Center Support Grant, CA016672 to MD Anderson.
Footnotes
DISCLOSURES There are no disclosures from any authors.
REFERENCES
- 1.Colditz GA, Baer HJ, Tamimi RM. Breast cancer. In: Schottenfeld D, Fraumeni JFJ, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press; 2006. [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Montague ED. Conservation surgery and radiation therapy in the treatment of operable breast cancer. Cancer. 1984;53:700–704. doi: 10.1002/1097-0142(19840201)53:3+<700::aid-cncr2820531318>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Rayter Z, Gazet JC, Ford HT, Easton DF, Coombes RC. Comparison of conservative surgery and radiotherapy with mastectomy in the treatment of early breast cancer. Eur J Surg Oncol. 1990;16:486–492. [PubMed] [Google Scholar]
- 5.Cancer Facts and Figures 2011. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 6.Brown LM, Chen BE, Pfeiffer RM, Schairer C, Hall P, Storm H, et al. Risk of second non-hematological malignancies among 376,825 breast cancer survivors. Breast Cancer Res Treat. 2007;106:439–451. doi: 10.1007/s10549-007-9509-8. [DOI] [PubMed] [Google Scholar]
- 7.Mellemkjaer L, Friis S, Olsen JH, Scelo G, Hemminki K, Tracey E, et al. Risk of second cancer among women with breast cancer. Int J Cancer. 2006;118:2285–2292. doi: 10.1002/ijc.21651. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein L, Deapen D, Cerhan JR, Schwartz SM, Liff J, McGann-Maloney E, et al. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91:1654–1662. doi: 10.1093/jnci/91.19.1654. [DOI] [PubMed] [Google Scholar]
- 9.Brady MS, Garfein CF, Petrek JA, Brennan MF. Post-treatment sarcoma in breast cancer patients. Ann Surg Oncol. 1994;1:66–72. doi: 10.1007/BF02303543. [DOI] [PubMed] [Google Scholar]
- 10.Curtis RE, Boice JD, Jr, Shriner DA, Hankey BF, Fraumeni JF., Jr Second cancers after adjuvant tamoxifen therapy for breast cancer. J Natl Cancer Inst. 1996;88:832–834. doi: 10.1093/jnci/88.12.832. [DOI] [PubMed] [Google Scholar]
- 11.Del Mastro L, Garrone O, Guenzi M, Cafiero F, Nicolo G, Rosso R, et al. Angiosarcoma of the residual breast after conservative surgery and radiotherapy for primary carcinoma. Ann Oncol. 1994;5:163–165. doi: 10.1093/oxfordjournals.annonc.a058770. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim NK. Leukemogenic effect of chemotherapy in patients with breast carcinoma: is it a real concern? Cancer. 2004;101:1479–1481. doi: 10.1002/cncr.20525. [DOI] [PubMed] [Google Scholar]
- 13.Inskip PD, Stovall M, Flannery JT. Lung cancer risk and radiation dose among women treated for breast cancer. J Natl Cancer Inst. 1994;86:983–988. doi: 10.1093/jnci/86.13.983. [DOI] [PubMed] [Google Scholar]
- 14.Levi F, Randimbison L, Te VC, La Vecchia C. Cancer risk after radiotherapy for breast cancer. Br J Cancer. 2006;95:390–392. doi: 10.1038/sj.bjc.6603235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matesich SM, Shapiro CL. Second cancers after breast cancer treatment. Semin Oncol. 2003;30:740–748. doi: 10.1053/j.seminoncol.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Peters MH, Sonpal IM, Batra MK. Breast cancer in women following mantle irradiation for Hodgkin’s disease. Am Surg. 1995;61:763–766. [PubMed] [Google Scholar]
- 17.Rubino C, de Vathaire F, Diallo I, Shamsaldin A, Le MG. Increased risk of second cancers following breast cancer: role of the initial treatment. Breast Cancer Res Treat. 2000;61:183–195. doi: 10.1023/a:1006489918700. [DOI] [PubMed] [Google Scholar]
- 18.Rubino C, de Vathaire F, Shamsaldin A, Labbe M, Le MG. Radiation dose, chemotherapy, hormonal treatment and risk of second cancer after breast cancer treatment. Br J Cancer. 2003;89:840–846. doi: 10.1038/sj.bjc.6601138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swerdlow AJ, Jones ME. Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case-control study. J Natl Cancer Inst. 2005;97:375–384. doi: 10.1093/jnci/dji057. [DOI] [PubMed] [Google Scholar]
- 20.Taghian A, de Vathaire F, Terrier P, Le M, Auquier A, Mouriesse H, et al. Long-term risk of sarcoma following radiation treatment for breast cancer. Int J Radiat Oncol Biol Phys. 1991;21:361–367. doi: 10.1016/0360-3016(91)90783-z. [DOI] [PubMed] [Google Scholar]
- 21.Travis LB, Hill DA, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 22.Obedian E, Fischer DB, Haffty BG. Second malignancies after treatment of early-stage breast cancer: lumpectomy and radiation therapy versus mastectomy. J Clin Oncol. 2000;18:2406–2412. doi: 10.1200/JCO.2000.18.12.2406. [DOI] [PubMed] [Google Scholar]
- 23.Hunt K, Singletary SE, Smith TL, Ross MI, Strom EA, McNeese MD, et al. The breast: comprehensive management of benign and malignant diseases. Philadelphia: W.B. Saunders; 1998. Conservation surgery and radiation: The MD Anderson Cancer Center experience; pp. 1179–1182. [Google Scholar]
- 24.Buzdar AUHG, Kau SW. Breast Cancer Adjuvant Therapy at the MD Anderson Cancer Center: Results of Four Prospective Studies. Philadelphia: J.B. Lippincott; 1993. [Google Scholar]
- 25.NAACCR. Section III: Average Annual Age-adjusted NAACCR Combined Cancer Incidence Rates for the United States. 2002–2006 [Google Scholar]
- 26.Galper S, Gelman R, Recht A, Silver B, Kohli A, Wong JS, et al. Second nonbreast malignancies after conservative surgery and radiation therapy for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2002;52:406–414. doi: 10.1016/s0360-3016(01)02661-x. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez L, Lana A, Hidalgo A, Rodríguez JM, Del Valle Mdel O, Cueto A, et al. Risk factors for second primary tumours in breast cancer survivors. Eur J Cancer Prev. 2008;17:406–413. doi: 10.1097/CEJ.0b013e3282f75ee5. [DOI] [PubMed] [Google Scholar]
- 28.Fowble B, Hanlon A, Freedman G, Nicolaou N, Anderson P. Second cancers after conservative surgery and radiation for stages I–II breast cancer: identifying a subset of women at increased risk. Int J Radiat Oncol Biol Phys. 2001;51:679–690. doi: 10.1016/s0360-3016(01)01665-0. [DOI] [PubMed] [Google Scholar]
- 29.Herring MK, Buzdar AU, Smith TL, Hortobagyi GN, Blumenschein GR. Second neoplasms after adjuvant chemotherapy for operable breast cancer. Am J Clin Oncol. 1986;9:269–275. doi: 10.1097/00000421-198606000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Rubagotti A, Perrotta A, Casella C, Boccardo F. Risk of new primaries after chemotherapy and/or tamoxifen treatment for early breast cancer. Ann Oncol. 1996;7:239–244. doi: 10.1093/oxfordjournals.annonc.a010566. [DOI] [PubMed] [Google Scholar]
- 31.Rutqvist LE, Johansson H, Signomklao T, Johansson U, Fornander T, Wilking N. Adjuvant tamoxifen therapy for early stage breast cancer and second primary malignancies. Stockholm Breast Cancer Study Group. J Natl Cancer Inst. 1995;87:645–651. doi: 10.1093/jnci/87.9.645. [DOI] [PubMed] [Google Scholar]
- 32.Katase K, Sugiyama Y, Hasumi K, Yoshimoto M, Kasumi F. The incidence of subsequent endometrial carcinoma with tamoxifen use in patients with primary breast carcinoma. Cancer. 1998;82:1698–1703. [PubMed] [Google Scholar]
- 33.Fornander T, Rutqvist LE, Cedermark B, Glas U, Mattsson A, Silfverswärd C, et al. Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet. 1989;1:117–120. doi: 10.1016/s0140-6736(89)91141-0. [DOI] [PubMed] [Google Scholar]
- 34.Matsuyama Y, Tominaga T, Nomura Y, Koyama H, Kimura M, Sano M, et al. Second cancers after adjuvant tamoxifen therapy for breast cancer in Japan. Ann Oncol. 2000;11:1537–1543. doi: 10.1093/oxfordjournals.annonc.a010406. [DOI] [PubMed] [Google Scholar]
- 35.Newcomb PA, Solomon C, White E. Tamoxifen and risk of large bowel cancer in women with breast cancer. Breast Cancer Res Treat. 1999;53:271–277. doi: 10.1023/a:1006117220284. [DOI] [PubMed] [Google Scholar]
- 36.Peters-Engl C, Frank W, Danmayr E, Friedl HP, Leodolter S, Medl M. Association between endometrial cancer and tamoxifen treatment of breast cancer. Breast Cancer Res Treat. 1999;54:255–260. doi: 10.1023/a:1006126411210. [DOI] [PubMed] [Google Scholar]
- 37.Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 2000;355:1757–1770. [PubMed] [Google Scholar]
- 38.Curtis RE, Boice JD, Jr, Stovall M, Bernstein L, Greenberg RS, Flannery JT, et al. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med. 1992;326:1745–1751. doi: 10.1056/NEJM199206253262605. [DOI] [PubMed] [Google Scholar]
- 39.Doherty MA, Rodger A, Langlands AO, Kerr GR. Multiple primary tumours in patients treated with radiotherapy for breast cancer. Radiother Oncol. 1993;26:125–131. doi: 10.1016/0167-8140(93)90093-n. [DOI] [PubMed] [Google Scholar]
- 40.Fisher B, Rockette H, Fisher ER, Wickerham DL, Redmond C, Brown A. Leukemia in breast cancer patients following adjuvant chemotherapy or postoperative radiation: the NSABP experience. J Clin Oncol. 1985;3:1640–1658. doi: 10.1200/JCO.1985.3.12.1640. [DOI] [PubMed] [Google Scholar]
- 41.Jacobson JA, Danforth DN, Cowan KH, d’Angelo T, Steinberg SM, Pierce L, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995;332:907–911. doi: 10.1056/NEJM199504063321402. [DOI] [PubMed] [Google Scholar]
- 42.Neugut AI, Robinson E, Lee WC, Murray T, Karwoski K, Kutcher GJ. Lung cancer after radiation therapy for breast cancer. Cancer. 1993;71:3054–3057. doi: 10.1002/1097-0142(19930515)71:10<3054::aid-cncr2820711027>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 43.Arriagada R, Le MG, Rochard F, Contesso G. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol. 1996;14:1558–1564. doi: 10.1200/JCO.1996.14.5.1558. [DOI] [PubMed] [Google Scholar]
- 44.Roychoudhuri R, Evans H, Robinson D, Moller H. Radiation-induced malignancies following radiotherapy for breast cancer. Br J Cancer. 2004;91:868–872. doi: 10.1038/sj.bjc.6602084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deutsch M, Land SR, Begovic M, Wieand HS, Wolmark N, Fisher B. The incidence of lung carcinoma after surgery for breast carcinoma with and without postoperative radiotherapy. Results of National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trials B-04 and B-06. Cancer. 2003;98:1362–1368. doi: 10.1002/cncr.11655. [DOI] [PubMed] [Google Scholar]
- 46.Zablotska LB, Neugut AI. Lung carcinoma after radiation therapy in women treated with lumpectomy or mastectomy for primary breast carcinoma. Cancer. 2003;97:1404–1411. doi: 10.1002/cncr.11214. [DOI] [PubMed] [Google Scholar]
- 47.Best T, Li D, Skol AD, Kirchhoff T, Jackson SA, Yasui Y, et al. Variants at 6q21 implicate PRDM1 in the etiology of therapy-induced second malignancies after Hodgkin’s lymphoma. Nat Med. 2011;17:941–943. doi: 10.1038/nm.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]