Abstract
Background
The first outbreak of H5N1 highly-pathogenic avian influenza (HPAI) virus associated with several human deaths occurred in 1997 in Hong-Kong, China. While H5N1 virus infection in poultry workers has been studied in some detail, little is known about the environmental risk factors of the H5 avian influenza virus infection in China.
Methods
A cross-sectional study was performed to evaluate the environmental load of H5 viruses in poultry-contaminated environments and to explore potential risk factors associated with infection in poultry workers between October 2010 and March 2012. Serum and environmental samples were collected in Zhejiang province, China. The hemagglutination inhibition (HI) assay was used to analyze human sera for antibodies against H5N1 virus [A/Hubei/1/2010 (H5N1) and A/Anhui/1/2005 (H5N1)]. All participants were interviewed with a standardized questionnaire to collect information on exposure to poultry. H5 Avian influenza virus in the environmental samples was detected by real time RT-PCR.
Results
One hundred and five of 3,453 environmental samples (3.0%) tested positive for H5 avian influenza virus. Fifty-five of 1,169 subjects (4.7%) tested seropositive for anti-H5N1 antibodies. A statistically significant difference in H5 virus detection rate was found among the different environments sampled (<0.001), with the highest showed in live bird markets (68.6%). Detection rate varied according to the source of samples, sewage (9.5%), drinking water (19.0%), feces (19.0%), cage surface (25.7%), and slaughtering chopping boards (15.2%), respectively. Direct or close contact with poultry (OR =5.20, 95% CI, 1.53-17.74) and breeding numerous poultry (OR =3.77, 95% CI, 1.72-8.73) were significantly associated with seroprevalence of antibodies to avian influenza virus A (H5N1).
Conclusions
The number of birds bred more than 1,000 and direct or close contact with poultry in the workplace or the environment would be a potential risk of H5N1 infection.
KEY WORDS : Avian influenza virus, occupational exposure, risk factors, environment
Background
Highly pathogenic avian influenza (HPAI) virus H5N1 causes considerable damage to the poultry industry and poses a threat to human health. In 1997, the first outbreak of H5N1 associated with several human deaths was observed in Hong-Kong, China (1). Economic losses due to AIVs vary mainly according to the pathogenicity of the virus strains (2). The highly pathogenic virus causes havoc for the poultry industry and medical care in most developing countries, such as China, Africa and Southeast Asia and beyond.
As of March 2013, 15 countries worldwide have reported a total of 622 confirmed human cases, with a high case fatality rate of 59.6% (371 deaths) (3). Although the epidemiological situation of HPAI H5N1 in China has remarkably improved over the past few years, the risk for animal and humans to be exposed to HPAI viruses may still be persistent in some sectors of the poultry industry, as sporadic human cases have continued to occur in recent years (4). In Zhejiang province, Eastern China, live bird markets are particularly important and are deeply rooted in cultural practices, traditions and consumers preferences (5). Poultry workers are therefore considered to be at greatest risk of infection with AIVs because of their higher exposure to chickens and/or waterfowl. Furthermore, China is considered to be an influenza epicenter; thus, ongoing surveillance of HPAI viruses is warranted. While H5N1 virus infection in poultry workers has been well investigated (6-8), knowledge about the environmental risk factors associated with HPAI H5N1 human infection in China, especially the eastern coast, remains limited.
Between 2010 and 2012, active surveillance was conducted on human populations and environments exposed to poultry in Zhejiang province, where a human case of H5N1 AIV occurred in 2006 (9). In this study, we analyzed surveillance data in Zhejiang province as to determine: (I) the environmental load of H5 AIVs in poultry-contaminated environments; (II) the seroprevalence of antibodies against AIVs of the H5 subtype in different categories of poultry workers; and (III) the potential risk factors for seropositivity to AIVs of the H5 subtype in poultry workers.
Methods
Survey site
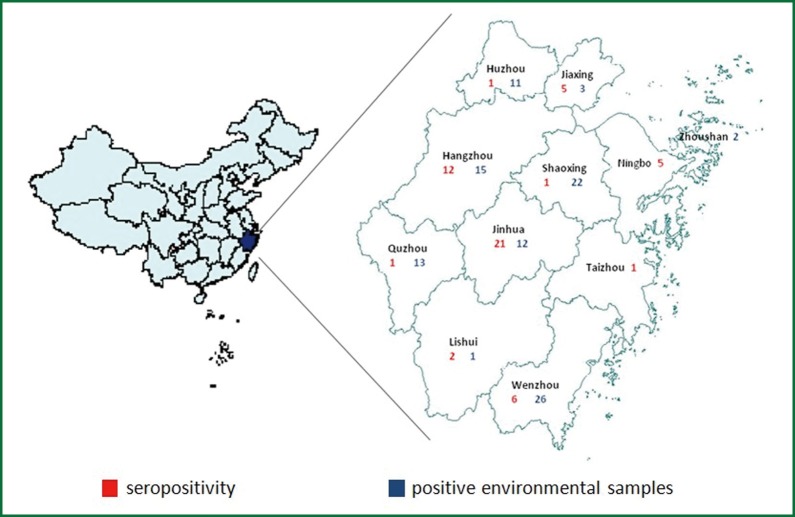
Zhejiang province encompasses eleven cities, where surveillance activities for avian influenza virus are in place through a laboratory network were included in the study between 2010 and 2012 (Figure 1). In these cities, live bird markets, large scale poultry companies, poultry backyard households, poultry slaughtering and processing plants, and wild migratory bird habitat were selected for survey site.
Figure 1.
Survey site of Zhejiang province. Zhejiang province, eastern China, encompasses eleven cities (Huzhou, Jiaxing, Hangzhou, Quzhou, Lishui, Jinhua, Shaoxing, Zhoushan, Ningbo, Taizhou, Wenzhou), where surveillance activities for avian influenza virus are in place through a laboratory network were included in the study between 2010 and 2012. A total of 55 seropositive samples (red) and 105 positive environmental samples (blue) were detected.
Subjects
Between March 2010 and December 2012, workers in direct contact with poultry from urban and rural live bird markets, large scale poultry companies, poultry slaughtering and processing plants, and household members who bred backyard poultry were invited to participate in this study. Workers were excluded if they were employed in a position in which exposure to poultry was limited such as those in administrative roles. People reporting to suffer from an immunosuppressive disease or to receive an immunosuppressive therapy were also excluded from the study. Participants were interviewed face to face by trained employees of the Zhejiang center for disease control and prevention (CDC) using a standard questionnaire in Chinese. Participants were asked about demographic data, way of occupational exposure, personal protective equipment, contact with dead or sick poultry, influenza-like symptom and influenza immunization history. All participants completed the study interview and agreed to be sampled. This study was approved by the ethical committee of Zhejiang CDC. Written informed consent was obtained from all participants.
Sample collection
All the eleven cities in Zhejiang province were selected for collecting samples. From March 2010 to December 2012, we interviewed all the poultry workers from survey site as described above in these 11 cities to collect demographic data and poultry exposure information. A single 5 mL blood sample was obtained from all participants for serological testing of H5N1 antibody. The blood was allowed to clot at room temperature then centrifuged on the same day of collection. Serum samples were aliquoted into three cryovials, labeled and preserved at –20 °C. Aliquots of serum were sent to Zhejiang CDC on dry ice for hemagglutination inhibition (HI) test.
Environmental samples were collected at the same time with collecting serum form poultry workers in the same survey site. Each survey site may include 2-3 sampling site, poultry wastewater and feces, and surfaces of cages and chopping boards used to house and slaughter poultry were collected for environmental samples. The study was conducted monthly throughout the year from 2010 to 2012. We explored the situation of the survey site a few days before collecting samples, to avoid collecting samples within two days after disinfection. The environmental samples were transported to the network laboratory at 4 °C within 48 hours, then aliquoted into three cryovials, labeled and preserved at –70 °C. Aliquots were tested for influenza A and H5N1 virus subtype by real-time RT-PCR. And all positive influenza A samples were sent to Chinese NIC of China CDC.
Real time RT-PCR detection
RNA extraction was performed as recommended by the manufacturer’s instructions, using an RNeasy mini kit (Qiagen, Germany). The RNA was eluted in 50 µL of nuclease free water and 10 µL was used as the template for real time RT-PCR.
Real time RT-PCR for identification of influenza A and H5 subtyping was performed using a fluorescently labeled TaqMan probe. The primers and probes followed WHO released primer and probe sets for lab diagnosis on of HPAI H5N1.The sequences of the following primers and probes were used: FluA forward primer: 5'-CCMAGGTCGAAACGTAYGTTCTCTCTATC-3'; FluA reverse primer: 5'-TGACAGRATYGGTCTTGTCTTTAGCCAYTCCA-3'; FluA probe: 5'-FAM-ATYTCGGCTTTGAGGGGGCCTG-MGB-3'; H5HA forward primer: 5'-CGATCTAGAYGGGGTGAARCCTC-3'; H5HA reverse primer: 5'-CCTTCTCCACTATGTANGACCATTC-3'; H5 probe-RVa: 5'-FAM-AGCCAYCCAGCTACRCTACA-MGB-3'; H5 probe-RVb: 5'-FAM-AGCCATCCCGCAACACTACA-MGB-3'; N1 forward: 5'-TAYAACTCAAGGTTTGAGTCTGTYGCTTG-3'; N1 reverse: 5'-ATGTTRTTCCTCCAACTCTTGATRGTGTC-3'; N1-Probe: 5'- FAM-TCAGCRAGTGCYTGCCATGATGGCAMGB-3' (10).
Serological testing
Serum samples were pretreated and assayed by horse red blood cell HI assay in BSL 2 laboratory at the Zhejiang CDC, in accordance with the reagent manufacturer’s instructions issued from Chinese National Influenza Center (NIC). One volume of serum was treated with four volumes of receptor destroying enzyme (RDE) at 37 °C for 18 hours, then was incubated at 56 °C for 30 minutes, and followed by absorption with horse erythrocytes. Each pretreated serum sample was further diluted with PBS to a final 1:10 dilution to test for specific antibodies against H5 virus antigen using 1% horse erythrocytes. Two-fold serial dilutions in 25 µL PBS were performed. And then, 25 μL of PBS containing four hemagglutination units (HAU) of inactivated H5N1 virus strain A/Hubei/1/2010 (H5N1) or A/Anhui/1/2005 (H5N1) was added after which 50 µL of 1% horse blood was added to each well. The V-shaped 96-well microtiter plates were incubated at room temperature for one hour before reading the results. The serum HI titer result was expressed as the reciprocal of the highest dilution of serum where haemagglutination was inhibited. According to WHO recommendations, an individual was deemed to be seropositive for H5N1 antibody if HI antibody titers of 1:160 or greater was detected (10). All the positive samples and 5% of the negative samples randomly selected were confirmed by micro-neutralization (MN) assay in Chinese NIC. Avian influenza inactivated antigen, RDE, positive control serum, horse red blood cells for the assays were provided by the Chinese NIC.
Statistical analysis
All statistical analyses were performed based on SPSS v.16.0 for windows (SPSS Inc., 2000). Questionnaire data were entered in duplicate and were verified using EpiData software. Pearson’s Chi-square test was used to compare frequencies of categorical variables. Odds ratios and associated 95% confidence intervals (CIs) were calculated. Binary logistic regression model analysis was used to identify risk factors associated with seroprevalence of antibodies to H5N1 among poultry workers. P values less than 0.05 in two-tailed test were used as a criterion of statistical significance.
Results
Prevalence of H5 avian influenza virus in the environment
A total of 3,453 environment samples were collected and tested. Examined workplaces included 1,286 (37.2%) large scale poultry companies, 1,857 (53.8%) live bird markets in urban and rural, 131 (3.8%) areas poultry backyard households, 41 (1.2%) poultry slaughtering and processing plants, 17 (0.5%) wild migratory bird habitats and 121 (3.5%) others. In 468 of 3,453 samples was detected type A influenza virus and 105 of them tested H5 subtype positive.
The positive rates of samples collected from large scale poultry companies, live bird markets, poultry slaughtering and processing plants, poultry backyard households and wild migratory bird habitats were 25.7%, 68.6%, 5.7% and 0%, respectively, with significant difference (P<0.001). Besides, there was a statistically significant difference in detection rate of H5 avian influenza virus between various types of samples (P<0.001), with surface of cages showed the highest prevalence (Table 1).
Table1. Prevalence of H5 avian influenza virus in the environment in Zhejiang, China, 2010-2012.
| Characteristics | No. of subjects (%) (n=3,453) | H5 positive (%) (n=105) | P valuea |
|---|---|---|---|
| Environment | <0.001 | ||
| Large scale poultry company | 1,286 (37.2) | 27 (25.7) | |
| Live bird markets | 1,857 (53.8) | 72 (68.6) | |
| Poultry backyard households | 131 (3.8) | 0 (0) | |
| Poultry slaughtering and processing plants | 41 (1.2) | 6 (5.7) | |
| Wild migratory bird habitat | 17 (0.5) | 0 (0) | |
| Others | 121 (3.5) | 0 (0) | |
| Samples type | <0.001 | ||
| Sewage of cleaning poultry | 224 (6.5) | 10 (9.5) | |
| Drinking water of poultry | 639 (18.5) | 20 (19.0) | |
| Feces | 865 (25.1) | 20 (19.0) | |
| Surface of cages | 1,329 (38.5) | 27 (25.7) | |
| Chopping boards used to slaughtering poultry | 228 (6.6) | 16 (15.2) | |
| Others | 168 (4.9) | 12 (11.4) |
a, α=0.05.
H5N1 seroprevalence among poultry workers
The study population consisted of 1,169 participants: 241 from live bird markets (20.6% of participants), 537 from large scale poultry companies (45.9% of participants), 36 from poultry slaughtering and processing plants (3.1% of participants), and 355 from household members who bred backyard poultry (30.4% of participants). Their median age was 48 (range, 15-94) years, and 54.6% were male. The 1,169 participants were enrolled by random selection. 97 (8.3%) reported direct or close contact with sick or dead poultry, 884 (75.6%) reported direct or close contact with poultry, and 21 (1.8%) reported have fever a month before investigation (Table 2). A total of 55 participants were seropositive for influenza virus (H5N1) HI antibodies (Table 3). The positive control sera had titers of 1:1,280 and the negative control sera tested negative.
Table 2. Characteristics of the study population, Zhejiang, China, 2010-2012.
| Characteristic | Seropositive sample (%) (n=55) | Seronegative sample (%) (n=1,114) | P value | Total sample No. (%) (n=1,169) |
|---|---|---|---|---|
| Age group, years | 0.87 | |||
| 15-30 | 6 (10.9) | 89 (8.0) | 95 (8.1) | |
| 31-46 | 21 (38.2) | 413 (37.1) | 434 (37.1) | |
| 47-62 | 23 (41.8) | 507 (45.5) | 530 (45.3) | |
| 63-78 | 5 (9.1) | 97 (8.7) | 102 (8.7) | |
| 79-95 | 0 (0.0) | 8 (0.7) | 8 (0.7) | |
| Sex | 0.41 | |||
| Male | 33 (60.0) | 605 (54.3) | 638 (54.6) | |
| Female | 22 (40.0) | 509 (45.7) | 531 (45.4) | |
| Source of poultry workers | 0.56 | |||
| Live bird markets | 13 (23.6) | 228 (20.5) | 241 (20.6) | |
| Large scale poultry companies | 25 (45.5) | 512 (46.0) | 537 (45.9) | |
| Poultry slaughtering and processing plants | 0 (0.0) | 36 (3.2) | 36 (3.1) | |
| Household members bred backyard poultry | 17 (30.9) | 338 (30.3) | 355 (30.4) | |
| Had fever | 0.44 | |||
| Yes | 0 (0.0) | 21 (1.9) | 21 (1.8) | |
| No | 38 (69.1) | 805 (72.3) | 843 (72.1) | |
| Missing | 17 (30.9) | 288 (25.9) | 305 (26.1) | |
| Exposure history | ||||
| Direct or close contact with poultry | 52 (94.5) | 832 (74.7) | 884 (75.6) | |
| Direct or close contact with sick or dead poultry | 10 (18.2) | 87 (7.8) | 97 (8.3) |
Table 3. Avian influenza virus A (H5N1) HI antibody titers among study participants (n=1,169), Zhejiang, China, 2010-2012.
| Source of poultry workers | No. of workers | No. of workers by antibody titer |
|||||
|---|---|---|---|---|---|---|---|
| <80 | 80 | 160 | 320 | 640 | 1,280 | ||
| Live bird markets | 241 | 190 | 38 | 13 | 0 | 0 | 0 |
| Large scale poultry companies | 537 | 474 | 38 | 19 | 5 | 0 | 1 |
| Poultry slaughtering and processing plants | 36 | 31 | 5 | 0 | 0 | 0 | 0 |
| Household members bred backyard poultry | 355 | 318 | 20 | 15 | 2 | 0 | 0 |
| Total | 1,169 | 1,013 | 101 | 47 | 7 | 0 | 1 |
Unconditional logistic regression model analysis was performed to identify the independent risk factors for seroprevalence of antibodies to H5N1 in poultry workers, Zhejiang, China. Direct or close contact with poultry was significantly associated (OR =5.203, 95% CI, 1.526-17.736) with an increased risk of being H5N1-seropositive.And the number of poultry bred more than 1,000 was also found to be associated with a 3.774 fold increased risk (95% CI, 1.721-8.726) (Table 4).
Table 4. Risk factors for seroprevalence of antibodies to avian influenza virus A (H5N1) among poultry workers, as determined by logistic regression model analysis, Zhejiang, China, 2010-2012.
| Variables | Wald χ2 | P | OR | 95% CI |
|---|---|---|---|---|
| Number of poultry bred >1,000 | 10.993 | 0.001 | 3.774 | (1.721, 8.726) |
| Direct or close contact with poultry | 6.946 | 0.008 | 5.203 | (1.526, 17.736) |
Discussion
A number of serological studies have examined AIV infection in occupationally exposed populations, including poultry workers (11,12), healthcare workers (13) and veterinarians (14,15). These studies have indeed concluded that they are at increased risk of infection with AIVs (7,16-18). Our study is an attempt towards examination of H5 influenza viruses both in poultry workers and in poultry-contaminated environments in Eastern China, Zhejiang. Enquiries didn’t indicate respiratory illness in the two weeks before the interview, suggesting the probable subclinical or mild H5 AIV infection, which was consistent with previous studies (19,20).
In the present study, the prevalence of H5 virus in the environment was 3.0% (105/3,453) and the seroprevalence of antibodies to H5N1was 4.7% (55/1,169), indicating evidence of infection with H5 both in workplace environment and human. It seems that more number of AIV H5 in workplace environment may lead to much high H5N1 antibody positive rate in poultry workers. Further homology analysis need to do between environment sources of H5N1 HA gene and human source and avian source H5N1 virus isolated from Zhejiang province in recent years. Although we found that there was a statistically significant difference in detection rate of H5 avian influenza virus between various workplace, there was no statistically significance between seroprevalence of them. Our results demonstrated that H5 avian influenza virus detection rate was significantly higher in live bird markets than other workplaces, which is not noticed by Wang et al. (8). It was probably because of the large number of birds from different location that are collected together in live bird markets before slaughtering. It has been reported that exposure to high risk environment, such as live bird market, may provide the opportunity for human infections and the possibility of reassortment with the existing poultry AIV (21). We also found H5 virus positive rate significantly higher on the surface of cages. Therefore it was suggested that surface of cages in high risk workplaces such as live bird markets and large scale poultry company would be potential risks of infection with H5 avian influenza virus. However, further analysis and surveillance are needed to adequately address this.
A previous study (7) identified a significant association between increasing poultry number and risk of human infection with avian influenza H5. In this study, the number of birds bred was also identified as an independent risk factor associated with antibodies to H5N1 avian influenza virus infection in the logistic regression model. The elevated H5 positive rate in poultry-contaminated environments strongly suggested that close contact with poultry would be an important risk factor for H5N1 infection. Our presented logistic model also suggested that direct or close contact with poultry was significantly associated with an increased risk of being H5N1-seropositive. Contact with infected but probably asymptomatic birds would be more risk than the environmental viral load.
In this study, we applied HI assay using horse erythrocytes to detect human sera for antibody against H5 virus and confirmed by MN assay, which has high sensitivity and specificity in detecting human antibodies against avian influenza viruses (22). In comparison with HI assay using chicken or turkey erythrocytes, HI assay using horse erythrocytes has increased sensitivity, which may be explained by the fact that horse erythrocytes express a higher proportion of sialic acid containing N-acetylneuraminic acid α2,3-galactose (SAα2,3Gal) linkages which avian specific influenza viruses preferentially bind.
The present survey’s findings are subject to several limitations. Firstly, it’s really the intrinsic limitation of this study design based on survey data and there was lack of follow-up data for the infected poultry workers. Secondly, we only detected fifty-five subjects with antibody against H5N1 virus; therefore, it was probably underpowered as the small sample size to detect other potentially significant risk factors for previous infection. Finally, information regarding the exposures of participants to poultry was all self-report, therefore it is subject to recall bias.
Conclusions
In conclusion, our results provide the evidence, to our knowledge, the number of birds bred more than 1,000 and direct or close contact with poultry in the workplace or environment would be potential risks of H5 avian influenza virus infection.
Acknowledgements
We thank the staff of the Hangzhou, Ningbo, Huzhou, Shaoxing, Jiaxing, Quzhou, Taizhou, Zhoushan, Wenzhou, Jinhua, Lishui Centers for Disease Control and Prevention for their assistance with specimens’ acquisition. Their cooperation was invaluable to this research.
This work was partly supported by grants from the Research Foundation of China Hospital Infection Control (ZHYY2013-023).
Disclosure: The authors declare no conflict of interest.
References
- 1.Claas EC, Osterhaus AD, van Beek R, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 1998;351:472-7 [DOI] [PubMed] [Google Scholar]
- 2.Kelso JK, Halder N, Postma MJ, et al. Economic analysis of pandemic influenza mitigation strategies for five pandemic severity categories. BMC Public Health 2013;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization: Avian influenza update. Available online: http://www.who.int/influenza/human_animal_interface/en/
- 4.Wan XF, Dong L, Lan Y, et al. Indications that live poultry markets are a major source of human H5N1 influenza virus infection in China. J Virol 2011;85:13432-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin V, Zhou X, Marshall E, et al. Risk-based surveillance for avian influenza control along poultry market chains in South China: the value of social network analysis. Prev Vet Med 2011;102:196-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang P, Ma C, Shi W, et al. A serological survey of antibodies to H5, H7 and H9 avian influenza viruses amongst the duck-related workers in Beijing, China. PLoS One 2012;7:e50770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huo X, Zu R, Qi X, et al. Seroprevalence of avian influenza A (H5N1) virus among poultry workers in Jiangsu Province, China: an observational study. BMC Infect Dis 2012;12:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Fu CX, Zheng BJ. Antibodies against H5 and H9 avian influenza among poultry workers in China. N Engl J Med 2009;360:2583-4 [DOI] [PubMed] [Google Scholar]
- 9.Yan J, Lu Y, Mao H, et al. Pathogenic and molecular characterization of the H5N1 avian influenza virus isolated from the first human case in Zhejiang province, China. Diagn Microbiol Infect Dis 2007;58:399-405 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization: recommendations and laboratory procedures for detection of avian influenza A (H5N1) virus in specimens from suspected humancases. Available online: http://www.who.int/influenza/resources/documents/h5n1_laboratory_procedures/en/
- 11.Ortiz JR, Katz MA, Mahmoud MN, et al. Lack of evidence of avian-to-human transmission of avian influenza A (H5N1) virus among poultry workers, Kano, Nigeria, 2006. J Infect Dis 2007;196:1685-91 [DOI] [PubMed] [Google Scholar]
- 12.Leibler JH, Silbergeld EK, Pekosz A, et al. No evidence of infection with avian influenza viruses among US poultry workers in the Delmarva Peninsula, Maryland and Virginia, USA. J Agromedicine 2011;16:52-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Alessandro E, Soula G, Jaffré Y, et al. Preparedness for influenza A/H5N1 pandemic in Niger: a study on health care workers' knowledge and global organization of health activities. Bull Soc Pathol Exot 2012;105:68-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers KP, Setterquist SF, Capuano AW, et al. Infection due to 3 avian influenza subtypes in United States veterinarians. Clin Infect Dis 2007;45:4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su S, Ning Z, Zhu W, et al. Lack of evidence of avian-to-human transmission of avian influenza A (H5N1) virus among veterinarians, Guangdong, China, 2012. J Clin Virol 2013;56:365-6 [DOI] [PubMed] [Google Scholar]
- 16.Di Trani L, Porru S, Bonfanti L, et al. Serosurvey against H5 and H7 avian influenza viruses in Italian poultry workers. Avian Dis 2012;56:1068-71 [DOI] [PubMed] [Google Scholar]
- 17.Uyeki TM, Nguyen DC, Rowe T, et al. Seroprevalence of antibodies to avian influenza A (H5) and A (H9) viruses among market poultry workers, Hanoi, Vietnam, 2001. PLoS One 2012;7:e43948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon D, Lee JY, Choi W, et al. Avian influenza a (H5N1) virus antibodies in poultry cullers, South Korea, 2003-2004. Emerg Infect Dis 2012;18:986-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khuntirat BP, Yoon IK, Blair PJ, et al. Evidence for subclinical avian influenza virus infections among rural Thai villagers. Clin Infect Dis 2011;53:e107-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vong S, Coghlan B, Mardy S, et al. Low frequency of poultry-to-human H5NI virus transmission, southern Cambodia, 2005. Emerg Infect Dis 2006;12:1542-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fournié G, Guitian J, Desvaux S, et al. Identifying live bird markets with the potential to act as reservoirs of avian influenza A (H5N1) virus: a survey in northern Viet Nam and Cambodia. PLoS One 2012;7:e37986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayali G, Setterquist SF, Capuano AW, et al. Testing human sera for antibodies against avian influenza viruses: horse RBC hemagglutination inhibition vs. microneutralization assays. J Clin Virol 2008;43:73-8 [DOI] [PMC free article] [PubMed] [Google Scholar]