Abstract
Proper circuit connectivity is critical for nervous system function. Connectivity derives from the interaction of two interdependent modules: synaptic specificity and synaptic assembly. Specificity involves both targeting of neurons to specific laminar regions and the formation of synapses onto defined subcellular areas. In this review, we focus discussion on recently elucidated molecular mechanisms that control synaptic specificity and link them to synapse assembly. We use these molecular pathways to underscore fundamental cell biological concepts that underpin, and help explain, the rules governing synaptic specificity.
Introduction
Synapses are specialized connections that allow electrical or chemical communication between neurons. Synapse formation includes partner selection, synapse assembly, and maintenance of functional connections. Although these events result in unique and neuron-specific developmental outcomes, basic cell biological mechanisms including cell–cell recognition, cytoskeletal reorganization and cell polarity decisions underpin these processes.
Synaptic specificity can be conceptually broken down into three neurodevelopmental decisions: first, long-range extension of axons to target regions; second, cell–cell recognition between pre- and postsynaptic partners; and third, formation of synapses onto specific subcellular regions [1••]. The molecular mechanisms controlling long-range axon guidance, while important for circuit connectivity, have been covered elsewhere [2–4]. While synaptic specificity is also observed for electrical synapses [5], we focus our discussion on mechanisms underlying chemical synaptic specificity. In particular, we discuss recent work regarding mechanisms that instruct cell–cell recognition and subcellular targeting during synaptic specificity and the fundamental cell biological mechanisms that underpin these processes.
Receptors mediate cell–cell attraction and specificity
Since the introduction of Roger Sperry’s chemoaffinity hypothesis [6], specificity has often been hypothesized to result from molecular recognition events between a neuron and its partners, with each member expressing molecules that identify it as an appropriate or inappropriate partner. Cell adhesion molecules make attractive candidates for such a recognition mechanism, with previous work identifying a number of adhesion molecules implicated in synaptic specificity [7–14]. In this section, we focus on two major families of related cell-adhesion molecules that can direct specificity: the immunoglobulin superfamily (IgSF) and cadherins. While these are not the only families of cell adhesion molecules involved in synaptic specificity, they were selected to underscore general conceptual points arising from their mechanisms of action and which might be broadly relevant for synaptic specificity in vivo.
IgSF proteins are characterized by the presence of at least one Ig-domain and can regulate multiple neurodevelopmental processes [15,16]. The role of IgSF members in synaptic specificity was recently examined in the vertebrate retina, used because of its stereotypical laminar structure and ease of experimental access. In laminar organization, by definition, a tissue is organized into identifiable layers; in the nervous system of both vertebrates and invertebrates, layers are usually defined by the presence of certain cell types or differing patterns of cellular connectivity between layers. The presence of different synaptic targets in different laminae means that the targeting of axons and dendrites to specific laminae can regulate the specificity of synaptic connectity [16–18] (Figures 1a and 2a).
Figure 1.
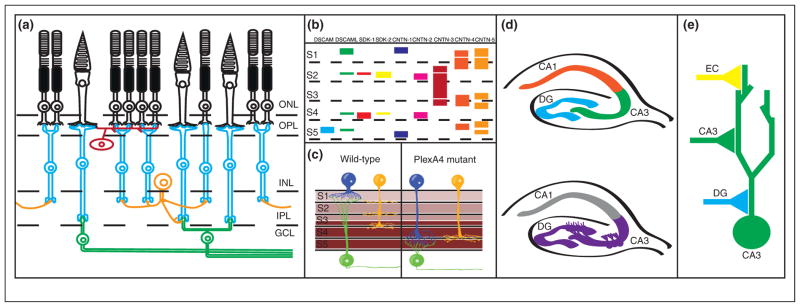
Genetic control of laminar specificity in the vertebrate retina. (a) Diagram of circuit connectivity in the vertebrate retina. Photoreceptors (rods and cones, shown in black) are located in the outer nuclear layer (ONL) of the retina. Photoreceptor axons extend to the outer plexiform layer (OPL), where they form synapses onto retinal bipolar cells (blue) and horizontal cells (red). Bipolar cell and horizontal cell bodies are located in the inner nuclear layer (INL), while bipolar cell neurites extend to the inner plexiform layer (IPL) where they form synapses with retinal ganglion cells (green) and amacrine cells (orange). (b) Diagram of immunoglobulin superfamily gene expression in the inner plexiform layer, with colored bars denoting expression of single genes. S1–S5 denote specific laminae within the inner plexiform layer. For DSCAMs and SDKs, thin bars indicate low expression levels while thick bars indicate high expression levels. For the CNTNs, bars indicate expression localization but not expression levels. Notice that each cue is expressed in a specific subset of laminae. While expression of cues may overlap in specific sublaminae, evidence in vivo suggests that cues are expressed in non-overlapping subsets of neurons. (c) PlexA4/Sema6A expression controls laminar specificity in the IPL. Sema6A is expressed in a gradient in the IPL (expression shown in brown), with high levels of expression in layers S3–S5, and lower levels of expression in S1–S2. The Sema6A receptor PlexA4 is expressed in two types of amacrine cells (blue and yellow) and retinal ganglion cells (green) forming synapses in S1 and S2; in the absence of PlexA4 both types of amacrine cells project to and form synapses in deeper layers (S3–S5). (d) Cadherin-9 regulates DG-CA3 connectivity in the hippocampus. Top panel: A diagram of the hippocampus, with the dentate gyrus (DG), CA3, and CA1 regions labeled. Neurons in the dentate gyrus project to and form synapses with CA3 neurons, which in turn form synapses onto CA1 neurons. Bottom panel: Cadherin-9 expression is restricted to the DG and CA3 regions of the hippocampus (purple), where it is thought to direct DG-CA3 hippocampal connectivity. (e) Diagram of synaptic connectivity in the CA3 neurons in the hippocampus (as in d). CA3 neurons (green) receive synapses from dentate gyrus (DG) neurons (blue), CA3 neurons (green), and entorhinal cortex (EC) neurons (yellow). Note that each type of synapse forms at a different subcellular location in the CA3 dendritic arbor.
Figure 2.
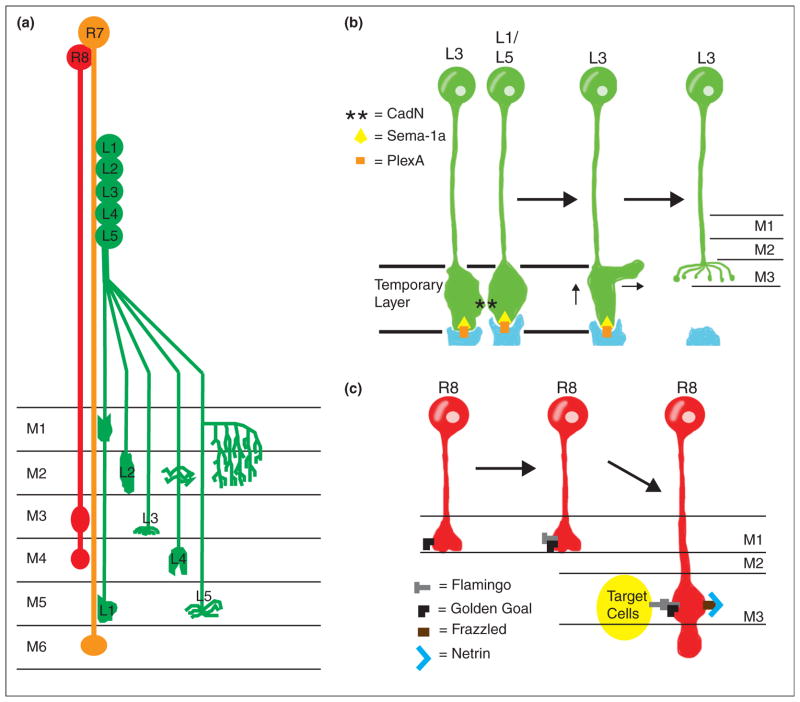
Synaptic specificity in Drosophila. (a) Neuronal projection in the Drosophila medulla. Laminae in the medulla are labeled M1–M6; M1 is the shallowest layer while M6 is the deepest. Additional deeper layers do not receive input from photoreceptor cells or laminar neurons and are not shown in the diagram. R7 (orange) and R8 (red) photoreceptors project to the M6 and the M3/M4 layers of the medulla, respectively. Lamina neurons L1–L5 (green) also project to specific layers in the medulla (indicated by labels M1–M6 in the diagram). (b) Cadherins and Plexin-Semaphorin signaling control L3 lamina neuron targeting. CadN asterisks interactions between L3 and additional laminar neurons L1 and L5 direct L3 to a temporary layer, while Sema1a (yellow triangle) and PlexA (orange bar) prevent targeting to deeper layers. Subsequently, PlexA (in orange, produced by tangential fibers labeled in blue) interacts with Sema-1a (from L3 neurons) to direct targeting to the final M3 layer via inhibitory signaling. CadN is not involved in the final targeting to the M3 layer, so L1/L5 neurons are not shown in later stages of the targeting process. (c) R8 photoreceptor targeting is dependent on interactions between two factors: the atypical cadherin flamingo (grey T) and Golden Goal (black L). Golden Goal first directs the growing R8 axon to guide to the temporary M1 layer. After the R8 axon reaches the temporary M1 layer, Flamingo antagonizes Golden Goal activity to promote R8 targeting to the deeper M3 target layer. Interactions between Flamingo and Golden Goal (expressed in R8 axons) and Flamingo (expressed on target cells in the M3 layer) then direct R8 axons to stop extending once they have reached the M3 layer. Netrin signaling is also involved in R8 targeting, as secreted Netrin (blue V) is localized in the M3 layer where it acts as an attractant for R8 axons expressing the Netrin receptor Frazzled (brown bar). Loss of the Netrin signal prevents R8 targeting to the M3 layer and instead results in premature R8 axon terminations in the M1 or M2 layers.
In the chick retina, IgSF molecules, including Down Syndrome Cell Adhesion Molecule (DSCAM), Down Syndrome Cell Adhesion Molecule-Like (DSCAML), Sidekick-1, and Sidekick-2, are expressed in non-overlapping subsets of bipolar neurons and retinal ganglion cells [19,20] (Figure 1b). Interneurons and retinal ganglion cells express single IgSF molecules, with neurons expressing different molecules projecting to different sublaminae. Loss-of-function or ectopic expression of these IgSF proteins results in laminar targeting defects. In Drosophila, DSCAMs can also direct subcellular specificity [21].
Sidekicks and DSCAM/DSCAML are expressed in approximately 60% of chick retinal neurons [22•]. Recent work examining laminar targeting resulted in the identification of a role for contactins (Cntns). Cntns comprise a set of IgSF proteins related to Sidekicks and DSCAM/DSCAML. Five cntns are expressed in non-overlapping subsets of retinal neurons, mostly amacrine cells, but possibly some retinal ganglion cells as well [22•] (Figure 1b). Similar to DSCAMs and Sidekicks, neurons expressing Cntns project to and arborize in specific sublaminae with limited degrees of overlap, and alterations in Cntn signaling disrupt laminar targeting (Figure 1b). These results, together with work from Caenorhabditis elegans [23–30], underscore the importance of the immunoglobulin superfamily in instructing synaptic specificity.
The hippocampus also has a well-defined organization useful in examining synaptic specificity (Figure 1d,e) [18]. A series of type II cadherins show unique expression patterns among hippocampal neurons, including Cadherin-9, which is selectively expressed on DG and CA3 neurons. In culture, Cadherin-9 shRNA-mediated knockdown specifically reduced DG-CA3 synapse formation and decreased mossy fiber bouton size and complexity [31••], suggesting that it is involved in determining synaptic connectivity between DG and CA3 neurons (Figure 1d). Cadherins may also act to control target specificity in other regions of the nervous system [32].
Together, these studies indicate that cell adhesion molecules, like IgSF molecules and cadherins, can mediate cellular recognition, laminar targeting, and synaptic specificity. These molecules can act as instructive cues directing the development of synapses. One might expect that because expression of specific adhesion molecules is deterministic in establishing synaptic connectivity, the transcriptional programs that instruct the expression of these receptors are also important in directing specificity. Indeed, there is a growing body of literature demonstrating that coordinated expression of transcription factors in both vertebrates and invertebrates is key in regulating the specificity of synaptic connections, both during development and during synaptic remodeling [33–41].
Beyond the homophilic interaction: matching levels of receptor expression directs synaptic specificity
Teneurins are a set of transmembrane proteins expressed in the nervous system that interact homophilically in vivo. In the Drosophila olfactory circuit, ten-m and ten-a direct matching of projection neurons and olfactory receptor neurons [42••]. Interestingly, neuronal matching was dependent on Teneurin levels; high-expressing neurons formed connections with high-expressing partners, while low-expressing neurons formed connections with low-expressing partners. Teneurins directed this activity through homophilic interactions, with neurons expressing either ten-m or ten-a forming connections with partners expressing the same molecule [42••]. Teneurins have also been implicated in Drosophila NMJ formation where they interact heterophilically, with ten-m expressed postsynaptically on muscles and ten-a expressed presynaptically on neurons [43].
The use of expression levels to match synaptic partners and to specify connectivity as seen above could be a generalizable neurodevelopmental mechanism. Semaphorins are a family of cell-adhesion molecules that can instruct synaptic specificity through Plexin and Neuropilin receptors. In the fly olfactory system, the semaphorin Sema-1a controls projection neuron dendritic targeting in the antennal lobe in a gradient fashion, with high expression in dorsolateral regions and low expression in ventromedial regions. Neurons with high levels of Sema-1a normally target to separate regions of the antennal lobe than neurons expressing low levels of Sema-1a. However, Sema-1a loss caused neurons which normally have high Sema-1a expression to mistarget to the same areas as neurons which normally contain low levels of Sema-1a [44•]. While the ligand for Sema-1a in this paradigm has not been identified yet, this study suggests that expression levels of Sema-1a can direct synaptic specificity.
These studies illustrate an important concept: neuronal targeting does not simply depend on the presence or absence of a specific cue, but can depend on expression level. A combination of the identity and level of expression of a given receptor can add greater complexity to the control of synaptic specificity.
Repulsive cues contribute to target specificity
While recognition of correct targets can instruct specificity, equally important is the capacity to avoid inappropriate targets. Similar to attractive molecules, Semaphorins show differential expression patterns in the retina and can control synaptic specificity. Sema6A is expressed in a subset of amacrine cells and RGCs, and acts non-cell autonomously to direct laminar specificity of PlexA4-expressing neurons [45••]. Mice missing PlexA4 and Sema6A showed mistargeting of amacrine cells and retinal ganglion cells in the inner plexiform layer (Figure 1c). Sema6A acts as a repulsive cue to non-cell-autonomously direct laminar termination away from inappropriate sublaminae (Figure 1c).
The role for Semaphorins in directing synaptic specificity by inhibiting inappropriate target selection could be a generalizable concept conserved throughout evolution. Semaphorins instruct synaptic specificity in C. elegans [46•], and were recently shown to regulate synapse formation in multiple vertebrate circuits including spinal cord neurons, the striatum, the hippocampus, and different areas of the retina [47–50].
Can molecular recognition signals and repulsive cues cooperate to refine target selection? In Drosophila, L3 lamina neurons are directed to the proper lamina through Sema-1a and CadN activity [51••]. L3 neurons first project to a temporary region in a Sema-1a/PlexA and CadN-dependent manner. This is achieved by Sema-1a mediated repulsion from deeper medulla layers and CadN mediated homophilic adhesion with other CadN expressing neurons. Next, a Sema-1a/PlexA-dependent remodeling process regulates growth cone retraction from PlexA-expressing cells through a mechanism probably involving repulsive Sema-1a activity [51••] (Figure 2b). Similarly, Flamingo (an atypical cadherin) and the transmembrane protein Golden Goal control R8 photoreceptor cell laminar targeting [52••] (Figure 2c). These studies suggest that a combination of multiple cell-recognition events and repulsive cues can cooperate in directing synaptic specificity [53].
Cell-recognition cues might inhibit synapse formation to inappropriate targets or regions (such as semaphorin-dependent inhibitory signaling), or promote the formation of synapses via receptor interactions and cellular recognition events. We note that, in this regard, the specification of synapse assembly is reminiscent of the mechanisms described for long-range axon guidance, in that a combination of attractant and repellent cues are used to provide spatial information, which can be relayed by intermediate targets (guidepost cells) [54]. The analogies between axon guidance and synaptic specificity also extend to the molecular realm, as both processes are sometimes guided by similar cues, including semaphorins, cadherins, IgSF molecules, ephrins, netrins, leucine-rich repeat domain proteins, and secreted factors like Wnt and FGF [1••,55].
Secreted factors direct synaptic specificity
Specificity is often thought of in the context of contact-mediated interactions, but secreted factors also direct specificity. Sonic Hedgehog, a secreted cue important for nervous system patterning [56–59], regulates synaptic connectivity. After its involvement in nervous system patterning, Sonic Hedgehog is expressed by postsynaptic, deep-layer cortical projection neurons while its receptor Brother of CDO (Boc) is expressed by presynaptic callosal projection neurons [60••]. Loss of Sonic Hedgehog or Boc specifically disrupts synapse formation between these two neuronal populations [60••]. The roles of Sonic Hedgehog and Boc illustrate a larger point relevant to other chemotrophic factors, including Wnts and BDNFs (recently reviewed [55,61]): chemotrophic factors are playing multiple roles in nervous system development, including the determination of specificity during synaptogenesis.
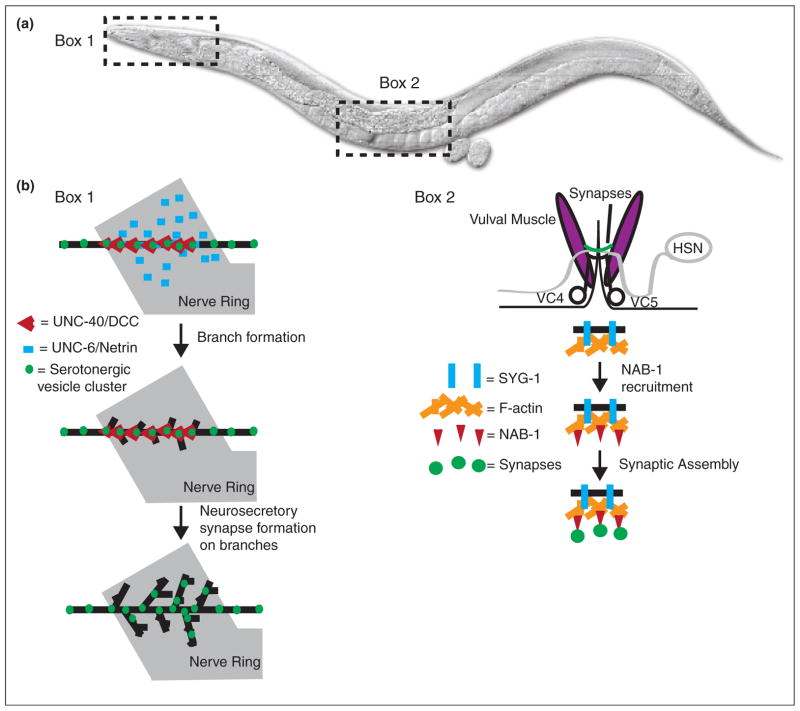
Netrin is a chemotrophic factor best known for its role in axon guidance. In C. elegans, UNC-6/Netrin promotes synapse assembly [27,62] and directs cell polarity by preventing presynaptic protein recruitment to dendrites [63]. Recent work also demonstrated that Netrin can promote release site specificity in serotonergic neurosecretory neurons (Figure 3b) [64•]. Netrin’s capacity to instruct synaptic specificity is conserved in Xenopus and Drosophila. In Xenopus, retinal axons terminate in the optic tectum, where they undergo axon branching and synapse formation with their tectal neuron partners. Retinal axons express the Netrin receptor DCC/UNC-40/Frazzled while Netrin itself is found near the cell bodies of tectal neurons [65•]. In the Drosophila visual system, Netrins act to direct R8 photoreceptor targeting (Figure 2c) [66•].
Figure 3.
Control of synaptic specificity in C. elegans. (a) A diagram of C. elegans, with boxes showing two regions described in the review. (b) Box 1: Netrin/UNC-6 and its receptor DCC/UNC-40/Frazzled control neurosecretory arbor formation in the NSM neuron. The Netrin receptor DCC/UNC-40/Frazzled (red V) localizes to serotonergic vesicle clusters (green) in the main axon shaft (black) of NSM. Following receptor localization and response to Netrin/UNC-6 (blue bar) secreted by neurons in the nerve ring (gray), axon arbor branch growth starts at sites of DCC/UNC-40/Frazzled localization. Following branch growth, new neurosecretory synapses form in the branches. Box 2: NAB-1 links the actin cytoskeleton to synapse formation in the HSN neurons. The HSN neuron (gray) forms synapses (green) onto vulval muscles (purple). VC4 and VC5 are ventral cord neurons also involved in synapse formation. The adhesion molecule SYG-2 (not shown) is expressed in vulval epithelial cells that act as guideposts. Presynaptic SYG-1 (blue bar) interacts with SYG-2 and directs F-actin (orange) assembly in HSN. NAB-1 (red triangle) binds to F-actin, where it subsequently acts to recruit synaptic assembly proteins and form synapses (green circles).
How can secreted chemotrophic factors provide positional information? In C. elegans, Netrin is either expressed by the postsynaptic partner [62] or by guide-post cells [27,63,64•] to provide positional information. In both C. elegans and Drosophila, DCC/UNC-40/Frazzled is able to capture secreted Netrin [67] and display it to provide positional information [66•,67,68•]. Therefore, localized release of Netrin by specific tissues and binding to the Netrin receptor can limit secreted Netrin to a target area [66•]. The extracellular matrix provides another substrate capable of binding to and retaining secreted specificity cues. In the zebrafish optic tectum, tectal neurons secrete Slit1a, which binds to Dragnet, a basement membrane type IV Collagen [69••]. The binding of Slit1a to Dragnet is thought to restrict its distribution and provide positional information important for laminar targeting.
Although varying mechanisms are used to restrict chemotrophic factors to specific regions, common among these pathways is the concept that chemotrophic factors are playing multiple roles in the development of the nervous system by providing positional information. How the same chemotrophic factor can instruct cell migration, guidance or synaptic specificity in different contexts is a central question in the field and key to understanding how coordinated and precise neurodevelopment ensues in vivo.
Intracellular factors direct synapse specificity
Extracellular cues have to be transduced into spatial and target specificity information by cells. The presence of specific receptors determines a neuron’s response to its environment and the positioning of its synapses. Intracellular mechanisms that regulate these receptors and downstream pathways also control synaptic targeting. For example, in the Drosophila visual system, Rich regulates laminar targeting of photoreceptor and laminar neuron axons. Rich acts as a GEF for the GTPase Rab6, regulating laminar targeting through CadN trafficking [70•]. This study indicates that intracellular mechanisms regulating trafficking of specific adhesion molecules or receptors to their appropriate destinations have dramatic yet specific effects on synaptic specificity. This study also raises the possibility that synaptic specificity could be physiologically regulated, not only at the level of receptor expression (discussed in [33–41]), but also at the level of trafficking.
Extracellular cues convey polarity information to the developing neuron by regulating the actin cytoskeleton. For example, SynCAM, an adhesion molecule that induces synapse formation in co-cultured neurons [71,72] and controls excitatory synapse number in vivo [73], promotes postsynaptic spine formation through the RhoGEF FARP1 and the actin cytoskeleton [74••]. Similarly, the Ephrin receptor EphB2 interacts with Tiam1 and Rac1 to remodel the actin cytoskeleton and induce spine formation [75,76,82]. The actin cytoskeleton is also linked to presynaptic assembly, as disruption of F-actin in C. elegans can affect presynaptic assembly in vivo [77•] (Figure 3b). In the Drosophila visual system, the cytoskeletal regulatory protein Ghengis Khan (GEK) is required for columnar targeting specificity [78•]. The actin cytoskeleton underlies other neurodevelopmental programs besides synaptic specificity, including morphogenesis, cell migration and guidance. The identification of molecules such as Ghengis Khan reveal the existence of specific pathways that modulate the cytoskeleton to regulate very specific aspects of neurodevelopment, such as synapse formation.
An apparent paradox emerging from the discussed studies is that fact that different neurodevelopmental processes depend on similar molecules, yet interference with these molecular pathways alters very specific neurodevelopmental outcomes, depending on the cellular context. For example, in C. elegans, the Netrin pathway can regulate axon outgrowth, axon arborization or presynaptic assembly in different neurons. A comparison between these neurons determined that underlying these different neurodevelopmental outcomes was the actin cytoskeleton [79,80,81••]. Interestingly, the adaptor molecule MIG-10/Lamellipodin plays an important role in conferring the cell-specific developmental response to the Netrin signal. Different protein isoforms of MIG-10/Lamellipodin all share the capacity to regulate the actin cytoskeleton, but differentially localize to presynaptic sites, growing arbors or growth cones. The subcellular localization of the isoforms, in response to the Netrin signal, is crucial to instruct the specific cellular response to Netrin [81••]. These studies demonstrate that basic cell biological mechanisms such as subcellular localization can influence the activation of diverse and developmentally specific downstream signaling pathways, and play important roles in directing the assembly of the nervous system in a spatially appropriate manner.
Conclusions
Nervous systems can be extremely complex, with billions of cells forming trillions of specific connections. Synaptic specificity results from polarity signals and rearrangements of the cellular cytoskeleton in response to those signals. The recent work discussed here underscores the fact that governing the development of the bewildering complexity of the nervous system are basic cell biological mechanisms that can be conceptually categorized to provide an understanding of the rules of synaptic specificity.
Acknowledgments
We apologize to colleagues whose work we did not cite due to lack of space or unintentional oversight. We thank T. Biederer and the Colón-Ramos lab for thoughtful comments on the manuscript. This work was supported by a March of Dimes Research Grant and an R01 from the National Institutes of Health (NS076558).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1••.Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. A comprehensive review of mechanisms directing synaptic specificity. [DOI] [PubMed] [Google Scholar]
- 2.Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2010;2:a001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starich TA, Xu J, Skerrett IM, Nicholson BJ, Shaw JE. Interactions between innexins UNC-7 and UNC-9 mediate electrical synapse specificity in the Caenorhabditis elegans locomotory nervous system. Neural Dev. 2009;4:16. doi: 10.1186/1749-8104-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci U S A. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akins MR, Biederer T. Cell–cell interactions in synaptogenesis. Curr Opin Neurobiol. 2006;16:83–89. doi: 10.1016/j.conb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 9.Giagtzoglou N, Ly CV, Bellen HJ. Cell adhesion, the backbone of the synapse: “vertebrate” and “invertebrate” perspectives. Cold Spring Harb Perspect Biol. 2009;1:a003079. doi: 10.1101/cshperspect.a003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson DL, Colman DR, Huntley GW. Molecules, maps and synapse specificity. Nat Rev Neurosci. 2001;2:899–909. doi: 10.1038/35104078. [DOI] [PubMed] [Google Scholar]
- 11.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colón-Ramos DA. Synapse formation in developing neural circuits. Curr Top Dev Biol. 2009;87:53–79. doi: 10.1016/S0070-2153(09)01202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- 14.Juttner R, Rathjen FG. Molecular analysis of axonal target specificity and synapse formation. Cell Mol Life Sci. 2005;62:2811–2827. doi: 10.1007/s00018-005-5299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams AF, Barclay AN. The immunoglobulin superfamily —domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- 16.Rougon G, Hobert O. New insights into the diversity and function of neuronal immunoglobulin superfamily molecules. Annu Rev Neurosci. 2003;26:207–238. doi: 10.1146/annurev.neuro.26.041002.131014. [DOI] [PubMed] [Google Scholar]
- 17.Huberman AD, Clandinin TR, Baier H. Molecular and cellular mechanisms of lamina-specific axon targeting. Cold Spring Harb Perspect Biol. 2010;2:a001743. doi: 10.1101/cshperspect.a001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams ME, de Wit J, Ghosh A. Molecular mechanisms of synaptic specificity in developing neural circuits. Neuron. 2010;68:9–18. doi: 10.1016/j.neuron.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- 20.Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- 21.Millard SS, Lu Z, Zipursky SL, Meinertzhagen IA. Drosophila dscam proteins regulate postsynaptic specificity at multiple-contact synapses. Neuron. 2010;67:761–768. doi: 10.1016/j.neuron.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Yamagata M, Sanes JR. Expanding the Ig superfamily code for laminar specificity in retina: expression and role of contactins. J Neurosci. 2012;32:14402–14414. doi: 10.1523/JNEUROSCI.3193-12.2012. In this paper, the authors show evidence for contactins in directing laminar specificity in the vertebrate retina. This work also extends previous findings by the same authors describing the role of IgSF proteins in directing retinal laminar specificity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003;112:619–630. doi: 10.1016/s0092-8674(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 24.Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 25.Patel MR, Lehrman EK, Poon VY, Crump JG, Zhen M, Bargmann CI, Shen K. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat Neurosci. 2006;9:1488–1498. doi: 10.1038/nn1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao DL, Shen K. Functional dissection of SYG-1 and SYG-2, cell adhesion molecules required for selective synaptogenesis in C. elegans. Mol Cell Neurosci. 2008;39:248–257. doi: 10.1016/j.mcn.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colon-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godenschwege TA, Kristiansen LV, Uthaman SB, Hortsch M, Murphey RK. A conserved role for Drosophila Neuroglian and human L1-CAM in central-synapse formation. Curr Biol. 2006;16:12–23. doi: 10.1016/j.cub.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 29.Baines RA, Seugnet L, Thompson A, Salvaterra PM, Bate M. Regulation of synaptic connectivity: levels of Fasciclin II influence synaptic growth in the Drosophila CNS. J Neurosci. 2002;22:6587–6595. doi: 10.1523/JNEUROSCI.22-15-06587.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashley J, Packard M, Ataman B, Budnik V. Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J Neurosci. 2005;25:5943–5955. doi: 10.1523/JNEUROSCI.1144-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Williams ME, Wilke SA, Daggett A, Davis E, Otto S, Ravi D, Ripley B, Bushong EA, Ellisman MH, Klein G, et al. Cadherin-9 regulates synapse-specific differentiation in the developing hippocampus. Neuron. 2011;71:640–655. doi: 10.1016/j.neuron.2011.06.019. The authors demonstrate that Cadherin-9 is capable of controlling subcellular specificity in the hippocampus, as they show that Cadherin-9 is required for the development of DG-CA3 synapses, but not CA3-CA3 or EC-CA3 synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, Meister M, Sanes JR. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci. 2011;31:7753–7762. doi: 10.1523/JNEUROSCI.0907-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morey M, Yee SK, Herman T, Nern A, Blanco E, Zipursky SL. Coordinate control of synaptic-layer specificity and rhodopsins in photoreceptor neurons. Nature. 2008;456:795–799. doi: 10.1038/nature07419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrovic M, Hummel T. Temporal identity in axonal target layer recognition. Nature. 2008;456:800–803. doi: 10.1038/nature07407. [DOI] [PubMed] [Google Scholar]
- 35.Tripodi M, Arber S. Regulation of motor circuit assembly by spatial and temporal mechanisms. Curr Opin Neurobiol. 2012;22:615–623. doi: 10.1016/j.conb.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Thompson-Peer KL, Bai J, Hu Z, Kaplan JM. HBL-1 patterns synaptic remodeling in C. elegans. Neuron. 2012;73:453–465. doi: 10.1016/j.neuron.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen K, Scheiffele P. Genetics and cell biology of building specific synaptic connectivity. Annu Rev Neurosci. 2010;33:473–507. doi: 10.1146/annurev.neuro.051508.135302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller DM, Shen MM, Shamu CE, Burglin TR, Ruvkun G, Dubois ML, Ghee M, Wilson L. C. elegans unc-4 gene encodes a homeodomain protein that determines the pattern of synaptic input to specific motor neurons. Nature. 1992;355:841–845. doi: 10.1038/355841a0. [DOI] [PubMed] [Google Scholar]
- 39.Von Stetina SE, Fox RM, Watkins KL, Starich TA, Shaw JE, Miller DM., 3rd UNC-4 represses CEH-12/HB9 to specify synaptic inputs to VA motor neurons in C. elegans. Genes Dev. 2007;21:332–346. doi: 10.1101/gad.1502107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider J, Skelton RL, Von Stetina SE, Middelkoop TC, van Oudenaarden A, Korswagen HC, Miller DM., 3rd UNC-4 antagonizes Wnt signaling to regulate synaptic choice in the C. elegans motor circuit. Development. 2012;139:2234–2245. doi: 10.1242/dev.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen SC, Watson JD, Richmond JE, Sarov M, Walthall WW, Miller DM., 3rd A transcriptional program promotes remodeling of GABAergic synapses in Caenorhabditis elegans. J Neurosci. 2011;31:15362–15375. doi: 10.1523/JNEUROSCI.3181-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Hong W, Mosca TJ, Luo L. Teneurins instruct synaptic partner matching in an olfactory map. Nature. 2012;484:201–207. doi: 10.1038/nature10926. This paper demonstrates that teneurins are required for CNS target selectivity through homophilic interactions, with target selectivity mediated by expression level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosca TJ, Hong W, Dani VS, Favaloro V, Luo L. Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature. 2012;484:237–241. doi: 10.1038/nature10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Komiyama T, Sweeney LB, Schuldiner O, Garcia KC, Luo L. Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell. 2007;128:399–410. doi: 10.1016/j.cell.2006.12.028. The authors demonstrate that a Sema-1a gradient is responsible for directing peripheral neuron dendritic targeting. [DOI] [PubMed] [Google Scholar]
- 45••.Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263. doi: 10.1038/nature09675. This paper demonstrates a functional role for semaphorin signaling in controlling amacrine cell targeting in the retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Mizumoto K, Shen K. Interaxonal interaction defines tiled presynaptic innervation in C. elegans. Neuron. 2013;77:655–666. doi: 10.1016/j.neuron.2012.12.031. The authors demonstrate the importance of interaxonal interactions in controlling synaptic placement. Plexin-semaphorin signaling between axons is important in preventing overlap and restricting synapse localization to appropriate regions of neighboring axons in C. elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, Chedotal A, Peachey NS, et al. Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron. 2011;71:460–473. doi: 10.1016/j.neuron.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD, Kolodkin AL. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462:1065–1069. doi: 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding JB, Oh WJ, Sabatini BL, Gu C. Semaphorin 3E-Plexin-D1 signaling controls pathway-specific synapse formation in the striatum. Nat Neurosci. 2011;15:215–223. doi: 10.1038/nn.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51••.Pecot MY, Tadros W, Nern A, Bader M, Chen Y, Zipursky SL. Multiple interactions control synaptic layer specificity in the Drosophila visual system. Neuron. 2013;77:299–310. doi: 10.1016/j.neuron.2012.11.007. This paper demonstrates that laminar targeting of L3 neurons in the Drosophila visual system occurs through a two-step process whereby L3 neurons first target to a laminar domain shared with L1 and L5 neurons, and then undergo a retraction from the common domain and extension to its final M3 target layer. Targeting to the common domain is mediated by both CadN and Sema-1a/PlexA interactions, while retraction from the common domain is mediated solely by Sema-1a/PlexA interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Hakeda-Suzuki S, Berger-Muller S, Tomasi T, Usui T, Horiuchi SY, Uemura T, Suzuki T. Golden Goal collaborates with Flamingo in conferring synaptic-layer specificity in the visual system. Nat Neurosci. 2011;14:314–323. doi: 10.1038/nn.2756. The authors describe a mechanism regulating laminar targeting first to a temporary domain, and then to a final target layer. The transmembrane protein Golden Goal mediates targeting to the temporary M1 layer, while the atypical cadherin Flamingo antagonizes Golden Goal-dependent adhesion in M1 and directs, with Golden Goal, R8 targeting to the M3 layer. [DOI] [PubMed] [Google Scholar]
- 53.Nose A. Generation of neuromuscular specificity in Drosophila: novel mechanisms revealed by new technologies. Front Mol Neurosci. 2012;5:62. doi: 10.3389/fnmol.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chao DL, Ma L, Shen K. Transient cell–cell interactions in neural circuit formation. Nat Rev Neurosci. 2009;10:262–271. doi: 10.1038/nrn2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2010;2:a001842. doi: 10.1101/cshperspect.a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- 57.Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- 58.Xu Q, Wonders CP, Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development. 2005;132:4987–4998. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- 59.Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, Anderson SA. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 2010;65:328–340. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Harwell CC, Parker PR, Gee SM, Okada A, McConnell SK, Kreitzer AC, Kriegstein AR. Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation. Neuron. 2012;73:1116–1126. doi: 10.1016/j.neuron.2012.02.009. The authors show that Sonic Hedgehog is repurposed to direct dendritic growth and synapse formation in corticofugal projection neurons, while the Sonic Hedgehog receptor BOC is expressed in local and callosal projection neurons where it promotes synapse formation onto Sonic Hedgehog-expressing corticofugal projection neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maeder CI, Shen K. Genetic dissection of synaptic specificity. Curr Opin Neurobiol. 2011;21:93–99. doi: 10.1016/j.conb.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park J, Knezevich PL, Wung W, O’Hanlon SN, Goyal A, Benedetti KL, Barsi-Rhyne BJ, Raman M, Mock N, Bremer M, et al. A conserved juxtacrine signal regulates synaptic partner recognition in Caenorhabditis elegans. Neural Dev. 2011;6:28. doi: 10.1186/1749-8104-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poon VY, Klassen MP, Shen K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature. 2008;455:669–673. doi: 10.1038/nature07291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Nelson JC, Colon-Ramos DA. Serotonergic neurosecretory synapse targeting is controlled by netrin-releasing guidepost neurons in Caenorhabditis elegans. J Neurosci. 2013;33:1366–1376. doi: 10.1523/JNEUROSCI.3471-12.2012. The authors demonstrate that Netrin/UNC-6 promotes axon arbor formation at a specific spatial location along the axons of NSM neurosecretory neurons. The Netrin receptor DCC/UNC-40/Frazzled localizes to neurosecretory release sites in the main axon shaft, where it responds to Netrin/UNC-6 secreted by neurons in the C. elegans nerve ring to promote branch formation and subsequent neurosecretory synapse formation in branches. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Manitt C, Nikolakopoulou AM, Almario DR, Nguyen SA, Cohen-Cory S. Netrin participates in the development of retinotectal synaptic connectivity by modulating axon arborization and synapse formation in the developing brain. J Neurosci. 2009;29:11065–11077. doi: 10.1523/JNEUROSCI.0947-09.2009. This paper uses Xenopus retinal ganglion cells (RGCs) to demonstrate a role for Netrin in instructing synaptic specificity. RGC axons depend on Netrin secreted by their tectal targets to induce RGC axon branching and synapse formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Timofeev K, Joly W, Hadjieconomou D, Salecker I. Localized netrins act as positional cues to control layer-specific targeting of photoreceptor axons in Drosophila. Neuron. 2012;75:80–93. doi: 10.1016/j.neuron.2012.04.037. The authors describe a mechanism by which secreted cues can provide specific spatial information to direct laminar specificity. The secreted cue Netrin is retained in a specific spatial location through localized secretion, and localized capture by the receptor DCC/UNC-40/Frazzled expressed in R8 neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hiramoto M, Hiromi Y, Giniger E, Hotta Y. The Drosophila Netrin receptor Frazzled guides axons by controlling Netrin distribution. Nature. 2000;406:886–889. doi: 10.1038/35022571. [DOI] [PubMed] [Google Scholar]
- 68•.Smith CJ, Watson JD, VanHoven MK, Colon-Ramos DA, Miller DM., 3rd Netrin (UNC-6) mediates dendritic self-avoidance. Nat Neurosci. 2012;15:731–737. doi: 10.1038/nn.3065. This paper demonstrates that in C. elgans, the PVD neurons use the diffusable cue Netrin to prevent inappropriate dendritic contact. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69••.Xiao T, Staub W, Robles E, Gosse NJ, Cole GJ, Baier H. Assembly of lamina-specific neuronal connections by slit bound to type IV collagen. Cell. 2011;146:164–176. doi: 10.1016/j.cell.2011.06.016. The basement menbrane can also serve as a substrate for retention of secreted cues in specific spatial locations. In this paper, the authors demonstrated that secreted Slit1a binds to the type IV Collagen Dragnet in the basement membrane, where the receptor Robo2 expressed in retinal ganglion cells can respond to it and direct axonal targeting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tong C, Ohyama T, Tien AC, Rajan A, Haueter CM, Bellen HJ. Rich regulates target specificity of photoreceptor cells and N-cadherin trafficking in the Drosophila visual system via Rab6. Neuron. 2011;71:447–459. doi: 10.1016/j.neuron.2011.06.040. This paper shows that CadN trafficking in Drosophila photoreceptor cells appears to be dependent on a mechanism involving Rab6 and a previously uncharacterized protein known as Rich, which binds to and regulates Rab6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sara Y, Biederer T, Atasoy D, Chubykin A, Mozhayeva MG, Sudhof TC, Kavalali ET. Selective capability of SynCAM and neuroligin for functional synapse assembly. J Neurosci. 2005;25:260–270. doi: 10.1523/JNEUROSCI.3165-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 73.Robbins EM, Krupp AJ, Perez de Arce K, Ghosh AK, Fogel AI, Boucard A, Sudhof TC, Stein V, Biederer T. SynCAM 1 adhesion dynamically regulates synapse number and impacts plasticity and learning. Neuron. 2010;68:894–906. doi: 10.1016/j.neuron.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74••.Cheadle L, Biederer T. The novel synaptogenic protein Farp1 links postsynaptic cytoskeletal dynamics and transsynaptic organization. J Cell Biol. 2012;199:985–1001. doi: 10.1083/jcb.201205041. SynCAM is one of only two molecules able to induce synapse formation in cultured neurons. The authors describe a novel synaptogenic protein, FARP1, which acts to link SynCAM to synaptic assembly through F-actin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, Greenberg ME. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc Natl Acad Sci U S A. 2007;104:7265–7270. doi: 10.1073/pnas.0702044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 77•.Chia PH, Patel MR, Shen K. NAB-1 instructs synapse assembly by linking adhesion molecules and F-actin to active zone proteins. Nat Neurosci. 2012;15:234–242. doi: 10.1038/nn.2991. This paper demonstrates how the specificity-determining adhesion molecule SYG-1 is coupled to the recruitment of synaptic assembly proteins through F-actin and the actin-binding protein NAB-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gontang AC, Hwa JJ, Mast JD, Schwabe T, Clandinin TR. The cytoskeletal regulator Genghis khan is required for columnar target specificity in the Drosophila visual system. Development. 2011;138:4899–4909. doi: 10.1242/dev.069930. The authors examine how control of cytoskeletal activity can regulate the development of circuit specificity. Column-specific targeting of a subset of photoreceptor (R) cells in the Drosophila eye is dependent on proper regulation of the cytoskeleton through a mechanism involving the GTPase effector Ghengis Khan (GEK) and most likely CDC42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adler CE, Fetter RD, Bargmann CI. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stavoe AK, Colon-Ramos DA. Netrin instructs synaptic vesicle clustering through Rac GTPase, MIG-10, and the actin cytoskeleton. J Cell Biol. 2012;197:75–88. doi: 10.1083/jcb.201110127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81••.Stavoe AK, Nelson JC, Martinez-Velazquez LA, Klein M, Samuel AD, Colon-Ramos DA. Synaptic vesicle clustering requires a distinct MIG-10/Lamellipodin isoform and ABI-1 downstream from Netrin. Genes Dev. 2012;26:2206–2221. doi: 10.1101/gad.193409.112. Netrin signals through MIG-10/Lamellipodin and the WAVE complex component ABI-1 to promote vesicle clustering through SNN-1/Synapsin. Interestingly, the role of MIG-10/Lamellipodin in vesicle clustering is isoform-specific; only one isoform of MIG-10 localizes to sites of vesicle clustering and is required for this activity to occur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hruska M, Dalva MB. Ephrin regulation of synapse formation, function, and plasticity. Mol Cell Neurosci. 2012;50:35–44. doi: 10.1016/j.mcn.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]