Abstract
There are 4 major human-biting tick species in the northeastern United States, which include: Amblyomma americanum, Amblyomma maculatum, Dermacentor variabilis, and Ixodes scapularis. The black bear is a large mammal that has been shown to be parasitized by all the aforementioned ticks. We investigated the bacterial infections in ticks collected from Louisiana black bears (Ursus americanus subspecies luteolus). Eighty-six ticks were collected from 17 black bears in Louisiana from June 2010 to March 2011. All 4 common human-biting tick species were represented. Each tick was subjected to polymerase chain reaction (PCR) targeting select bacterial pathogens and symbionts. Bacterial DNA was detected in 62% of ticks (n=53). Rickettsia parkeri, the causative agent of an emerging spotted fever group rickettsiosis, was identified in 66% of A. maculatum, 28% of D. variabilis, and 11% of I. scapularis. The Lyme disease bacterium, Borrelia burgdorferi, was detected in 2 I. scapularis, while one Am. americanum was positive for Borrelia bissettii, a putative human pathogen. The rickettsial endosymbionts Candidatus Rickettsia andeanae, rickettsial endosymbiont of I. scapularis, and Rickettsia amblyommii were detected in their common tick hosts at 21%, 39%, and 60%, respectively. All ticks were PCR-negative for Anaplasma phagocytophilum, Ehrlichia spp., and Babesia microti. This is the first reported detection of R. parkeri in vector ticks in Louisiana; we also report the novel association of R. parkeri with I. scapularis. Detection of both R. parkeri and Bo. burgdorferi in their respective vectors in Louisiana demands further investigation to determine potential for human exposure to these pathogens.
Introduction
Since Theobald Smith’s seminal work describing ticks as the vectors for Texas cattle fever, the importance of arthropods as vectors for disease has surged to the forefront of both human and animal medicine (Kilborne, 1893). By the turn of the 20th century, many control measures were developed and deployed to combat the pests spreading these pathogens. Excellent reviews of these can be found in Hill et al. (2005) and Piesman and Eisen (2008). Even with advances in arthropod control and disease management, the world has seen an emergence and re-emergence of zoonotic vector-borne diseases within the past 30 years (Christou, 2011; Jones et al., 2008). Ticks are vectors for a plethora of zoonoses, which include the causative agents of anaplasmosis, babesiosis, ehrlichiosis, Lyme and relapsing fever borreliosis, spotted fever group (SFG) rickettsiosis, and a host of viral diseases (Dantas-Torres et al., 2012; Parola and Raoult, 2001). In the United States, ticks transmit more vector-borne diseases than any other arthropod. With over 80 known species of ticks in the US, opportunity exists for each one of the aforementioned diseases to affect humans and animals (Berrada and Telford, 2009).
Not only is the US witnessing a steady increase in reported human cases of many tick-borne diseases, new tick-borne pathogens are being discovered on a regular basis. For example in 1992, Lyme disease, Rocky Mountain spotted fever (RMSF), and tularemia were the only nationally notifiable tick-borne diseases; approximately 20 years later the number has swelled to nine (Anonymous, 2011). This increase amounts to 3 new tick-borne human diseases identified per decade. With the advent of better detection, diagnosis, and surveillance, this number is likely to increase.
Despite the increase in research on ticks and tick-borne diseases conducted elsewhere in the past several decades, comparatively less work has been done in Louisiana. With outdoor activity as the single most important risk factor for tick exposure (Piesman and Eisen, 2008) and Louisiana’s recognition as “A Sportsman’s Paradise”, there is strong risk of tick exposure for both residents and visitors. In the southern US, humans are most commonly parasitized by 4 ticks species: Amblyomma americanum, Amblyomma maculatum, Dermacentor variabilis, and Ixodes scapularis (Goddard, 2002; Merten and Durden, 2000; Felz et al., 1996). Each of these tick species have unique host preferences that, taken together, encompasses a range of host animals (Oliver, 1989). Black bears have been reported to harbor many of the same tick species as humans, and not surprisingly, pathogenic bacteria have been detected in ticks parasitizing bears (King, 1960; Rogers and Rogers, 1976; Binninger et al., 1980; Schroeder, 1987; Kazmierczak et al., 1988; Yabsley et al., 2009; Nims and Durden, 2011).
Louisiana’s state animal is the black bear, Ursus americanus subspecies luteolus, which has been listed as a threatened species by the United States Fish and Wildlife Service since 1992. The Louisiana Department of Wildlife and Fisheries carefully monitors bear populations throughout the state and has collected ectoparasites during these monitoring efforts. The objective of our study was to investigate which ticks parasitize black bears in Louisiana and to identify bacteria of interest that they harbor.
Materials and methods
Bear sampling and tick collection
From June 2010 to March 2011, ticks were collected from 17 black bears throughout Louisiana by personnel from Louisiana’s Department of Wildlife and Fisheries. Ticks collected off bears in this study were convenience samples, as the bears consisted of relocated and accidentally or illegally killed animals encountered by the Department of Wildlife and Fisheries personnel. Bears were sampled from 7 parishes: Concordia, Iberville, Livingston, Madison, Pointe Coupee, St. Landry, and St. Mary. Exhaustive searching of bears for ectoparasites was not conducted, leading to the collection of only easily visible adult ticks. Ticks were stored in 70% ethanol for transport, then identified and sexed using standard identification keys (Sonenshine, 1979; Diamant and Strickland, 1965).
Tick DNA extraction
Ticks were surface-sterilized by first soaking in a 10% bleach solution, followed by a phosphate buffered saline (PBS) wash, next soaked in a 70% ethanol solution, followed by a PBS wash and finally a soak in molecular biology grade water (MO BIO, Carlsbad, CA). Individual ticks were then bisected with a sterile scalpel, and DNA was extracted from each tick using the GenElute DNA extraction Kit (Sigma, St. Louis, MO) following manufacturer’s instructions with minor modifications. These modifications included: Tick tissues were allowed to lyse overnight in a 55°C water bath, and DNA was eluted in 100 μl of supplied elution buffer. DNA was stored at −20°C until PCR was performed.
Polymerase chain reaction
Polymerase chain reaction (PCR) was performed on DNA extracts to detect the presence of selected bacteria. The PCR assays targeted the following genes: msp2 for Anaplasma phagocytophilum (Caspersen et al., 2002), 18s rRNA for Babesia microti (Persing et al., 1992), flaB for Borrelia burgdorferi sensu lato (Clark et al., 2005), 16s rRNA for Ehrlichia species (Anderson et al., 1992; Dawson et al., 1994), and both 17-kDa antigen gene (Carl et al., 1990) and ompA (Regnery et al., 1991) for SFG rickettsiae. Environmental controls were utilized for detection of DNA artifact contamination in all experiments. This included simultaneous extraction tubes with no tissue added for all DNA extractions and molecular biology grade water (MO BIO) used as a template in the downstream PCRs. Initial reactions contained 2.5 μL of DNA extract in a total reaction volume of 25 μL. TaKaRa Taq DNA polymerase (TaKaRa-Bio Inc, Otsu, Japan) was utilized in all reactions. Each reaction had a final concentration of 1.25 U of Taq DNA polymerase, 45 mM KCL, 2.5 mM MgCl2, 200 μM concentration of each deoxynucleoside, and 0.5 μM of the upstream and downstream primers. All reactions were carried out in a My Cycler thermal cycler (BioRad, Hercules, CA). Nested reactions contained 1 μL of product from outer reaction under the same master mix concentrations. Thermocycling protocols were followed as published for each primer set.
DNA purification/sequencing/analysis
PCR products were electrophoresed on a 2% agarose gel and stained with ethidium bromide. Bands of the correct size were purified with a QIAquick PCR purification kit (Qiagen, St. Louis, MO) and bi-directionally sequenced on an automated ABI 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA) at LSU’s Biomed Gene Lab. Sequences were aligned using ClustalX and compared with existing sequences in the National Center for Biotechnology Information (NCBI) GenBank using the Basic Local Alignment Search Tool (BLAST) (Altschul et al., 1990; Thompson et al., 1997).
PCR cloning
PCR-cloning was utilized to identify amplicon inserts in samples where multiple signals were detected in the sequencing chromatograph. Cloning reactions were performed according to manufacturer’s instructions with an Invitrogen Topo TA cloning kit (Life Technologies, Grand Island, NY). Multiple colonies were picked, and the universal primers M13F and M13R were used to sequence as previously described. The resulting sequences were aligned and analyzed as described above.
Results
Four tick species were found parasitizing Louisiana black bears
Eighty-six ticks were removed from a total of 17 bears. All ticks collected were adults; 51% (n=44) were females and 49% (n=42) were males. Am. maculatum was the most commonly collected tick, comprising 44% of all ticks collected (n=38), D. variabilis represented 29% (n=18) of the total collected, I. scapularis was the next most abundant at 21% (n=15), and the least common was Am. americanum at 6% (n=5) (Table 1).
Table 1.
Number, month sampled, species, and gender of ticks (M=male, F=female) collected from each of the bears in this study.
| Bear | Month sampled | Amblyomma americanum | Amblyomma maculatum | Dermacentor variabilis | Ixodes scapularis | Total |
|---|---|---|---|---|---|---|
| 1 | June | – | 4 (M), 4 (F) | 1 (M) | – | 9 |
| 2 | June | – | 2 (M), 4 (F) | 1 (F) | – | 7 |
| 3 | June | – | – | 2 (M), 1 (F) | – | 3 |
| 4 | June | – | 9 (M) | 2 (M), 3 (F) | – | 14 |
| 5 | June | – | – | 1 (M), 6 (F) | – | 7 |
| 6 | June | – | 3 (M), 1 (F) | 1 (M), 3 (F) | – | 8 |
| 7 | September | – | – | 1 (F) | – | 1 |
| 8 | September | – | – | 1 (F) | – | 1 |
| 9 | September | – | 1 (M) | 1 (M), 1 (F) | – | 3 |
| 10 | October | – | 9 (M), 1 (F) | – | – | 10 |
| 11 | October | – | – | – | 2 (M), 3 (F) | 5 |
| 12 | November | – | – | – | 2 (M), 1 (F) | 3 |
| 13 | December | – | – | – | 2 (F) | 2 |
| 14 | January | – | – | – | 2 (F) | 2 |
| 15 | February | – | – | – | 2 (F) | 2 |
| 16 | February | 3 (M), 2 (F) | – | – | – | 5 |
| 17 | March | – | – | – | 2 (M), 2 (F) | 4 |
| Total | 5 | 38 | 25 | 18 | 86 |
Three bacterial pathogens were detected in ticks
DNA was extracted from all 86 ticks collected from Louisiana black bears. These extracts were tested for the presence of An. phagocytophilum, Bo. burgdorferi sensu lato, Ba. microti, Ehrlichia spp., and SFG Rickettia DNA by PCR. Pathogenic bacterial DNA was detected in 44% (n=38) of all ticks. Rickettsia parkeri was detected most frequently with 25 of 38 (65.7%) Am. maculatum, 7 of 25 (28%) D. variabilis, and 3 of 18 (11.1%) I. scapularis testing positive. R. parkeri sequences were 99% homologous with sequences in GenBank (Table 2). Evidence of Bo. burgdorferi sensu stricto was found in 2 of 18 (11%) I. scapularis, while one (20%) Am. americanum had a sequence matching Bo. bissettii (Table 2). No An. phagocytophilum, Ba. microti or Ehrlichia species DNA were detected in any of the ticks.
Table 2.
Tick DNA samples PCR-positive in this study. Identical sequences are represented by identical accession numbers. Multiple infected samples are denoted by asterisks.
| Sample ID | Tick species | Accession number | Homology |
|---|---|---|---|
| S1AM1 | Amblyomma maculatum | KC003477 | 99% Rickettsia parkeri |
| S1AM3, S1AM4, S1AM5, S1AM6, S1AM8 | Am. maculatum | KC003475 | 100% Candidatus R. andeanae |
| S2AM3* | Am. maculatum | KC003475 | 100% Candidatus R. andeanae |
| S2AM3* | Am. maculatum | KC003476 | 99% R. parkeri |
| S2AM1, S2AM2, S2AM4, S2AM5, S2AM6 | Am. maculatum | KC003477 | 99% R. parkeri |
| S3DV1, S3DV2 | Dermacentor variabilis | KC003477 | 99% R. parkeri |
| S4AM1, S4AM2, S4AM3, S4AM, S4AM6, S4AM7, S4AM8, S4AM9 | Am. maculatum | KC003477 | 99% R. parkeri |
| S4DV1, S4DV2, S4DV3 | D. variabilis | KC003477 | 99% R. parkeri |
| S5DV3 | D. variabilis | KC003477 | 99% R. parkeri |
| S6AM1, S6AM3 | Am. maculatum | KC003477 | 99% R. parkeri |
| S6DV1 | D. variabilis | KC003477 | 99% R. parkeri |
| S10AM1, S10AM2, S10AM3, S10AM4, S10AM5, S10AM6, S10AM7, S10AM8, S10AM9, S10AM10 | Am. maculatum | KC003477 | 99% R. parkeri |
| S10AM5, S10AM6 | Am. maculatum | KC003475 | 100% Candidatus R. andeanae |
| S11IS3* | Ixodes scapularis | KC003478 | 99% R. parkeri |
| S11IS2, S11IS3*, S11IS4 | I. scapularis | KC003474 | 100% rickettsial endosymbiont of I. scapularis |
| S12IS1, S12IS2* | I. scapularis | KC003478 | 99% R. parkeri |
| S12IS2* | I. scapularis | KC003471 | 99% Borrelia burgdorferi |
| S13IS1 | I. scapularis | KC003470 | 99% Bo. burgdorferi |
| S14IS2 | I. scapularis | KC003472 | 100% rickettsial endosymbiont of I. scapularis |
| S16AA1, S16AA2*, S16AA4 | Am. americanum | KC003473 | 99% R. amblyommii |
| S16AA2* | I. scapularis | KC003469 | 99% Bo. bissettii |
| S17IS4 | I. scapularis | KC003472 | 100% rickettsial endosymbiont of I. scapularis |
| S17IS3 | I. scapularis | KC003474 | 100% rickettsial endosymbiont of I. scapularis |
Three rickettsial endosymbionts were detected in ticks
SFG Rickettsia primers and subsequent sequencing identified rickettsial endosymbiont DNA in 20.9% (n=18) of their respective ticks. These endosymbionts included: Candidatus Rickettsia andeanae in 8 of 38 (21%) Am. maculatum, rickettsial endosymbiont of I. scapularis in 7 of 18 (38.8%) I. scapularis, and Rickettsia amblyommii in 3 of 5 (60%) Am. americanum. Endosymbionts were 99–100% similar to their respective reference sequences in GenBank (Table 2).
Multiple bacteria detected in ticks
Ticks with evidence of mixed SFG Rickettsia DNA after sequencing were subjected to PCR cloning and sequencing of multiple clones to identify individual amplicons. Two ticks had the presence of multiple SFG Rickettsia DNA. This included one Am. maculatum with both Candidatus R. andeanae and R. parkeri, and one I. scapularis with the rickettsial endosymbiont of I. scapularis and R. parkeri. Two additional ticks had evidence of multiple bacteria by separate PCRs. In one I. scapularis, we detected both B. burgdorferi and the rickettsial endosymbiont of I. scapularis, and one Am. americanum had evidence of both R. amblyommii and Bo. bissettii DNA (Table 2).
Discussion
In this study, we investigated which species of ticks were parasitizing Louisiana black bears and identified both pathogenic and symbiontic bacterial DNA in the arthropods. Human-biting ticks have been commonly recorded on bears in studies across the US dating back to the mid 1970s (Nims and Durden, 2011; Rogers and Rogers, 1976; Rogers, 1975; King, 1960; Yabsley et al., 2009). A previous study in Idaho detected antibodies to multiple tick-borne pathogens in black bears whilst a Wisconsin study isolated Lyme Borrelia spirochetes from these animals (Kazmierczak et al., 1988; Binninger et al., 1980). More recently, Yabsley et al. (2009) detected Ehrlichia chaffeensis and R. parkeri in ticks from black bears in southern Georgia/northern Florida. Consistent with these previous reports, black bears in Louisiana harbor many common human-biting ticks; nearly half of these had PCR evidence of bacterial pathogens.
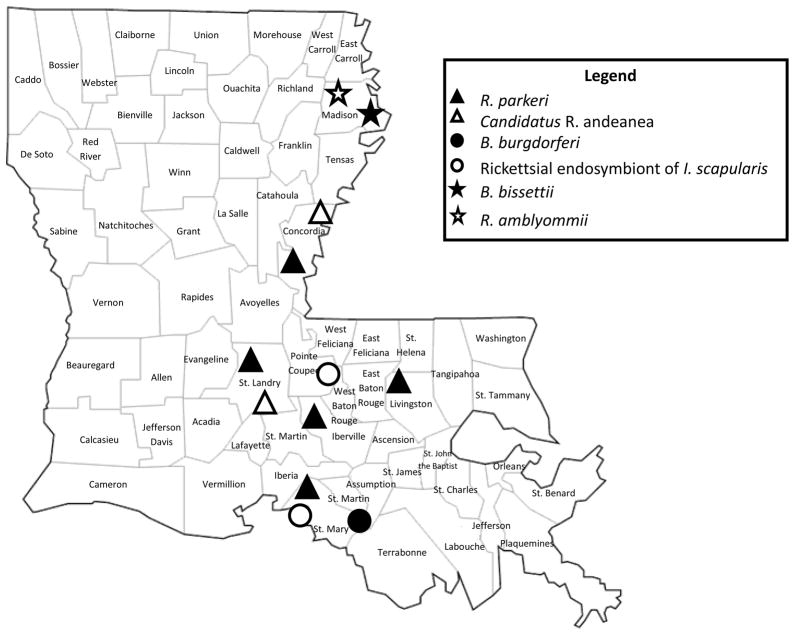
Most importantly, this is the first record of R. parkeri infection occurring in its natural vector in Louisiana, with almost 66% of the Am. maculatum we tested having evidence of R. parkeri. This coincides with a recent publication detecting R. parkeri in the blood of dogs in Louisiana (Grasperge et al., 2012). R. parkeri rickettsiosis is an emerging infectious disease. Detection of R. parkeri is becoming more common across the range of its primary vector, Am. maculatum. Published infection rates of R. parkeri range from ~10 to 40% in populations of southeastern Am. maculatum ticks (Varela-Stokes et al., 2011; Ferrari et al., 2012; Fornadel et al., 2011; Paddock et al., 2010; Sumner et al., 2007). Multiple ticks from multiple parishes in Louisiana had evidence of R. parkeri infection (Fig. 1). R. parkeri was also detected in both D. variabilis and I. scapularis. This is the first record of detection of this SFG Rickettsia in I. scapularis ticks. These ticks are not considered vectors for R. parkeri, but are known to harbor other SFG Rickettsia. Interestingly, we detected R. parkeri in 2 male I. scapularis, considering male Ixodes ticks feed sparingly if at all, this finding raises the question of pathogenic SFG Ricksttia infection in I. scapularis (Kiszewski et al., 2001). Identifying such a large number of R. parkeri-infected ticks associated with bears also proposes the question of the bear as a potential reservoir host. Lyme Borrelia spirochetes were detected in 3 ticks. Bo. burgdorferi sensu stricto was detected in 2 I. scapularis while Bo. bissettii was detected in a male A. americanum. Bo. burgdorferi sensu stricto is the leading cause of the most common vector-borne disease in the US, Lyme disease. Bo. bissettii is a closely related Lyme spirochete that shares common vectors, hosts, and geographic range and more recently has been implicated in human disease (Girard et al., 2011; Rudenko et al., 2008, 2009).
Fig. 1.
Location of ticks positive for bacterial DNA tested for in this study.
Lack of detection of Ba. microti, An. phagocytophilum, or Ehrlichia species should not be taken as evidence of their non-existence in Louisiana, as information regarding ticks and their associated diseases in Louisiana is still lacking. Surrounding states like Arkansas, Mississippi, and Texas host a range of tick-borne diseases that have been determined only after large samples and years of research (Castellaw et al., 2010; Williamson et al., 2010; Fryxell et al., 2012; Goddard et al., 2003).
Many species of ticks are associated with distinct endosymbiontic bacteria (Rounds et al., 2012). In this study, we detected multiple symbiontic SFG Rickettsia. Candidatus R. andeanae is a recently isolated SFG Rickettsia and a putative endosymbiont of Am. maculatum ticks (Luce-Fedrow et al., 2012). It has been reported from multiple regions of the US where Am. maculatum are found (Ferrari et al., 2012; Fornadel et al., 2011; Jiang et al., 2012). Our finding of Candidatus R. andeanae in 21% of Am. maculatum is the first evidence of this bacterium in Louisiana and expands the known range of this recently described SFG Rickettsia. Detection of other SFG rickettsial endosymbionts, namely R. amblyommii in Am. americanum and the rickettsial endosymbiont of I. scapularis, is not unexpected, and these are the first published records of these SFG rickettsiae in Louisiana ticks. Anecdotal reports have implicated R. amblyommii in human disease, but the majority of these conclusions have yet to be substantiated in laboratory studies (Smith et al., 2010; Billeter et al., 2007; Apperson et al., 2008; Nicholson et al., 2009; Jiang et al., 2010).
This study was conducted retrospectively on ticks collected from bears by the Louisiana Department of Wildlife and Fisheries personnel. Due to various limitations, the collected ticks probably do not comprise the total tick population on each bear. It is expected that immature stages of ticks were overlooked during sampling, as ectoparasite collection was not the focus of the Fish and Wildlife personnel’s work. In addition, many of the female ticks collected and subjected to PCR were at different stages of engorgement, yet others had no obvious signs that a blood meal had begun. These limitations directly affect the interpretation of the data presented in that, while it is possible that detection of bacteria in these ticks may indicate infection, it is just as likely that these bacteria were imbibed during feeding on the host bear either by co-feeding transmission or by an infection in the bear. Future studies should ascertain infection status in questing, unfed, ticks in Louisiana.
With the resurgence of zoonotic vector-borne diseases in the past 3 decades, the importance of disease surveillance and control has become apparent. In the US, the recent discoveries of tick-borne zoonotic pathogens like R. parkeri, Rickettsia spp. 364D, Ehrlichia muris-like (EML), Panola mountain Ehrlichia, and Powassan virus combined with the increasing frequency of tick-borne diseases like Lyme disease, SFG rickettsiosis, anaplasmosis, ehrlichiosis, and babesiosis have more than proven a need to closely monitor tick-borne diseases (Shapiro et al., 2010; Reeves et al., 2008; Pritt et al., 2011; Paddock, 2009). Since little is known about the organisms circulating in Louisiana ticks, detection of bacteria in ticks that commonly parasitize humans improves our understanding of tick-borne disease risks to human and animals in the state. The detection of R. parkeri and Bo. burgdorferi and the novel association of R. parkeri with I. scapularis in ticks collected from black bears prompts more questions on the prevalence of tick-borne pathogens in questing and other host-associated ticks across Louisiana. Surveillance of pathogens in human-biting ticks in Louisiana is essential to educating health professionals, elucidating tick-borne disease risk, and ultimately protecting the health of the public.
Acknowledgments
The authors would like to thank personnel at the Louisiana Department of Wildlife and Fisheries involved in Louisiana black bear research. Many thanks go to Dr. Britton Grasperge for his input on this manuscript. This work was supported in part by the National Institutes of Health (AI077733). Brian Leydet Jr. is a recipient of a Louisiana Board of Regent’s Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anderson BE, Sumner JW, Dawson JE, Tzianabos T, Greene CR, Olson JG, Fishbein DB, Olsen-Rasmussen M, Holloway BP, George EH, Azad AF. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992;30:775–780. doi: 10.1128/jcm.30.4.775-780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. List of Notafiable Conditions, Historically by Year (1992–2011) Centers for Disease Control; 2011. http://wwwn.cdc.gov/nndss/document/NNDSS_history_spreadsheet_2011_for_web_v3.pdf. [Google Scholar]
- Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. Tick-borne diseases in North Carolina: Is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- Berrada ZL, Telford SR., 3rd Burden of tick-borne infections on American companion animals. Topics in Companion Animal Medicine. 2009;24:175–181. doi: 10.1053/j.tcam.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter SA, Blanton HL, Little SE, Levy MG, Breitschwerdt EB. Detection of Rickettsia amblyommii in association with a tick bite rash. Vector Borne Zoonotic Dis. 2007;7:607–610. doi: 10.1089/vbz.2007.0121. [DOI] [PubMed] [Google Scholar]
- Binninger C, Beecham J, Thomas L, Winward L. A serologic survey for selected infectious diseases of black bears in Idaho. Journal of Wildlife Diseases. 1980;16:423–430. doi: 10.7589/0090-3558-16.3.423. [DOI] [PubMed] [Google Scholar]
- Carl M, Tibbs CW, Dobson ME, Paparello S, Dasch GA. Diagnosis of acute typhus infection using the polymerase chain reaction. J Infect Dis. 1990;161:791–793. doi: 10.1093/infdis/161.4.791. [DOI] [PubMed] [Google Scholar]
- Caspersen K, Park JH, Patil S, Dumler JS. Genetic variability and stability of Anaplasma phagocytophila msp2 (p44) Infect Immun. 2002;70:1230–1234. doi: 10.1128/IAI.70.3.1230-1234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellaw AH, Showers J, Goddard J, Chenney EF, Varela-Stokes AS. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J Med Entomol. 2010;47:473–476. doi: 10.1603/me09263. [DOI] [PubMed] [Google Scholar]
- Christou L. The global burden of bacterial and viral zoonotic infections. Clin Microbiol Infect. 2011;17:326–330. doi: 10.1111/j.1469-0691.2010.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K, Hendricks A, Burge D. Molecular identification and analysis of Borrelia burgdorferi sensu lato in lizards in the southeastern United States. Appl Environ Microbiol. 2005;71:2616–2625. doi: 10.1128/AEM.71.5.2616-2625.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a One Health perspective. Trends in parasitology. 2012;28:437–446. doi: 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Dawson JE, Stallknecht DE, Howerth EW, Warner C, Biggie K, Davidson WR, Lockhart JM, Nettles VF, Olson JG, Childs JE. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Clin Microbiol. 1994;32:2725–2728. doi: 10.1128/jcm.32.11.2725-2728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant G, Strickland RK. Manual on Livestock Ticks for Animal Disease Eradication Division Personnel. 1965. [Google Scholar]
- Felz MW, Durden LA, Oliver JH., Jr Ticks parasitizing humans in Georgia and South Carolina. J Parasitol. 1996;82:505–508. [PubMed] [Google Scholar]
- Ferrari FA, Goddard J, Paddock CD, Varela-Stokes AS. Rickettsia parkeri and Candidatus Rickettsia andeanae in Gulf Coast Ticks, Mississippi, USA. Emerg Infect Dis. 2012;18:1705–1707. doi: 10.3201/eid1810.120250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornadel CM, Zhang X, Smith JD, Paddock CD, Arias JR, Norris DE. High rates of Rickettsia parkeri infection in Gulf Coast ticks (Amblyomma maculatum) and identification of “Candidatus Rickettsia andeanae” from Fairfax County, Virginia. Vector Borne Zoonotic Dis. 2011;11:1535–1539. doi: 10.1089/vbz.2011.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryxell RT, Steelman CD, Szalanski AL, Kvamme KL, Billingsley PM, Williamson PC. Survey of borreliae in ticks, canines, and white-tailed deer from Arkansas, U.S.A. Parasit Vectors. 2012;5:139. doi: 10.1186/1756-3305-5-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard YA, Fedorova N, Lane RS. Genetic diversity of Borrelia burgdorferi and detection of B. bissettii-like DNA in serum of north-coastal California residents. J Clin Microbiol. 2011;49:945–954. doi: 10.1128/JCM.01689-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. A ten-year study of tick biting in Mississippi: implications for human disease transmission. Journal of Agromedicine. 2002;8:25–32. doi: 10.1300/J096v08n02_06. [DOI] [PubMed] [Google Scholar]
- Goddard J, Sumner JW, Nicholson WL, Paddock CD, Shen J, Piesman J. Survey of ticks collected in Mississippi for Rickettsia, Ehrlichia, and Borrelia species. J Vector Ecol. 2003;28:184–189. [PubMed] [Google Scholar]
- Grasperge BJ, Wolfson W, Macaluso KR. Rickettsia parkeri infection in domestic dogs, Southern Louisiana, USA, 2011. Emerg Infect Dis. 2012;18:995–997. doi: 10.3201/eid1806.120165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Kafatos FC, Stansfield SK, Collins FH. Arthropod–borne diseases: vector control in the genomics era. Nat Rev Microbiol. 2005;3:262–268. doi: 10.1038/nrmicro1101. [DOI] [PubMed] [Google Scholar]
- Jiang J, Stromdahl EY, Richards AL. Detection of Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Gulf Coast ticks collected from humans in the United States. Vector Borne Zoonotic Dis. 2012;12:175–182. doi: 10.1089/vbz.2011.0614. [DOI] [PubMed] [Google Scholar]
- Jiang J, Yarina T, Miller MK, Stromdahl EY, Richards AL. Molecular detection of Rickettsia amblyommii in Amblyomma americanum parasitizing humans. Vector Borne Zoonotic Dis. 2010;10:329–340. doi: 10.1089/vbz.2009.0061. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak J, Amundson T, Burgess E. Borreliosis in free-ranging black bears from Wisconsin. Journal of Wildlife Diseases. 1988;24:366–368. doi: 10.7589/0090-3558-24.2.366. [DOI] [PubMed] [Google Scholar]
- Kilborne FL. Investigations into the nature, causation, and prevention of Texas or southern cattle fever. US Dept. of Agriculture, Bureau of Animal Industry; 1893. [Google Scholar]
- King JM. Pathology, parasitology, and hematology of the black bear in New York. New York Fish and Game J. 1960;7:99–l. [Google Scholar]
- Kiszewski AE, Matuschka FR, Spielman A. Mating strategies and spermiogenesis in ixodid ticks. Annu Rev Entomol. 2001;46:167–182. doi: 10.1146/annurev.ento.46.1.167. [DOI] [PubMed] [Google Scholar]
- Luce-Fedrow A, Wright C, Gaff HD, Sonenshine DE, Hynes WL, Richards AL. In vitro propagation of Candidatus Rickettsia andeanae isolated from Amblyomma maculatum. FEMS Immunol Med Microbiol. 2012;64:74–81. doi: 10.1111/j.1574-695X.2011.00905.x. [DOI] [PubMed] [Google Scholar]
- Merten HA, Durden LA. A state-by-state survey of ticks recorded from humans in the United States. J Vector Ecol. 2000;25:102–113. [PubMed] [Google Scholar]
- Nicholson WL, Masters E, Wormser GP. Preliminary serologic investigation of Rickettsia amblyommi in the aetiology of Southern tick-associated rash illness (STARI) Clin Microbiol Infect. 2009;15(Suppl 2):235–236. doi: 10.1111/j.1469-0691.2008.02155.x. [DOI] [PubMed] [Google Scholar]
- Nims TN, Durden LA. Ticks and lice of the black bear, Ursus americanus Pallas, in northern Georgia, USA, including a new state record for the chewing louse, Trichodectes euarctidos (Phthiraptera: Trichodectidae) Journal of Entomological Science. 2011;46:345. [Google Scholar]
- Oliver JH., Jr Biology and systematics of ticks (Acari: Ixodida) Annual Review of Ecology and Systematics. 1989;20:397–430. [Google Scholar]
- Paddock CD. The science and fiction of emerging rickettsioses. Ann NY Acad Sci. 2009;1166:133–143. doi: 10.1111/j.1749-6632.2009.04529.x. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Fournier PE, Sumner JW, Goddard J, Elshenawy Y, Metcalfe MG, Loftis AD, Varela-Stokes A. Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl Environ Microbiol. 2010;76:2689–2696. doi: 10.1128/AEM.02737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- Persing DH, Mathiesen D, Marshall WF, Telford SR, Spielman A, Thomford JW, Conrad PA. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Eisen L. Prevention of tick-borne diseases. Annu Rev Entomol. 2008;53:323–343. doi: 10.1146/annurev.ento.53.103106.093429. [DOI] [PubMed] [Google Scholar]
- Pritt BS, Sloan LM, Johnson DK, Munderloh UG, Paskewitz SM, McElroy KM, McFadden JD, Binnicker MJ, Neitzel DF, Liu G, Nicholson WL, Nelson CM, Franson JJ, Martin SA, Cunningham SA, Steward CR, Bogumill K, Bjorgaard ME, Davis JP, McQuiston JH, Warshauer DM, Wilhelm MP, Patel R, Trivedi VA, Eremeeva ME. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med. 2011;365:422–429. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves WK, Loftis AD, Nicholson WL, Czarkowski AG. The first report of human illness associated with the Panola Mountain Ehrlichia species: a case report. Journal of Medical Case Reports. 2008;2:139. doi: 10.1186/1752-1947-2-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L. Parasites of black bears of the Lake Superior region. Journal of Wildlife Diseases. 1975;11:189–192. doi: 10.7589/0090-3558-11.2.189. [DOI] [PubMed] [Google Scholar]
- Rogers LL, Rogers SM. Parasites of bears: a review. Bears: Their Biology and Management. 1976:411–430. [Google Scholar]
- Rounds MA, Crowder CD, Matthews HE, Philipson CA, Scoles GA, Ecker DJ, Schutzer SE, Eshoo MW. Identification of endosymbionts in ticks by broad-range polymerase chain reaction and electrospray ionization mass spectrometry. J Med Entomol. 2012;49:843–850. doi: 10.1603/me12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko N, Golovchenko M, Mokracek A, Piskunova N, Ruzek D, Mallatova N, Grubhoffer L. Detection of Borrelia bissettii in cardiac valve tissue of a patient with endocarditis and aortic valve stenosis in the Czech Republic. J Clin Microbiol. 2008;46:3540–3543. doi: 10.1128/JCM.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko N, Golovchenko M, Ruzek D, Piskunova N, Mallatova N, Grubhoffer L. Molecular detection of Borrelia bissettii DNA in serum samples from patients in the Czech Republic with suspected borreliosis. FEMS Microbiol Lett. 2009;292:274–281. doi: 10.1111/j.1574-6968.2009.01498.x. [DOI] [PubMed] [Google Scholar]
- Schroeder MT. Bears: Their Biology and Management. 1987. Blood chemistry, hematology, and condition evaluation of black bears in northcoastal California; pp. 333–349. [Google Scholar]
- Shapiro MR, Fritz CL, Tait K, Paddock CD, Nicholson WL, Abramowicz KF, Karpathy SE, Dasch GA, Sumner JW, Adem PV, Scott JJ, Padgett KA, Zaki SR, Eremeeva ME. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis. 2010;50:541–548. doi: 10.1086/649926. [DOI] [PubMed] [Google Scholar]
- Smith MP, Ponnusamy L, Jiang J, Ayyash LA, Richards AL, Apperson CS. Bacterial pathogens in ixodid ticks from a Piedmont County in North Carolina: prevalence of rickettsial organisms. Vector Borne Zoonotic Dis. 2010;10:939–952. doi: 10.1089/vbz.2009.0178. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE. Ticks of Virginia (Acari, Metastigmata) Virginia Polytechnic Institute and State University; 1979. [Google Scholar]
- Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, Paddock CD. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis. 2007;13:751–753. doi: 10.3201/eid1305.061468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Stokes AS, Paddock CD, Engber B, Toliver M. Rickettsia parkeri in Amblyomma maculatum ticks, North Carolina, USA, 2009–2010. Emerg Infect Dis. 2011;17:2350–2353. doi: 10.3201/eid1712.110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson PC, Billingsley PM, Teltow GJ, Seals JP, Turnbough MA, Atkinson SF. Borrelia, Ehrlichia, and Rickettsia spp. in ticks removed from persons, Texas, USA. Emerg Infect Dis. 2010;16:441–446. doi: 10.3201/eid1603.091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabsley MJ, Nims TN, Savage MY, Durden LA. Ticks and tick-borne pathogens and putative symbionts of black bears (Ursus americanus floridanus) from Georgia and Florida. Journal of Parasitology. 2009;95:1125–1128. doi: 10.1645/GE-2111.1. [DOI] [PubMed] [Google Scholar]