Abstract
Rationale: The impact of modern treatments of pulmonary arterial hypertension (PAH) on pulmonary vascular pathology remains unknown.
Objectives: To assess the spectrum of pulmonary vascular remodeling in the modern era of PAH medication.
Methods: Assessment of pulmonary vascular remodeling and inflammation in 62 PAH and 28 control explanted lungs systematically sampled.
Measurements and Main Results: Intima and intima plus media fractional thicknesses of pulmonary arteries were increased in the PAH group versus the control lungs and correlated with pulmonary hemodynamic measurements. Despite a high variability of morphological measurements within a given PAH lung and among all PAH lungs, distinct pathological subphenotypes were detected in cohorts of PAH lungs. These included a subset of lungs lacking intima or, most prominently, media remodeling, which had similar numbers of profiles of plexiform lesions as those in lungs with more pronounced remodeling. Marked perivascular inflammation was present in a high number of PAH lungs and correlated with intima plus media remodeling. The number of profiles of plexiform lesions was significantly lower in lungs of male patients and those never treated with prostacyclin or its analogs.
Conclusions: Our results indicate that multiple features of pulmonary vascular remodeling are present in patients treated with modern PAH therapies. Perivascular inflammation may have an important role in the processes of vascular remodeling, all of which may ultimately lead to increased pulmonary artery pressure. Moreover, our study provides a framework to interpret and design translational studies in PAH.
Keywords: pulmonary circulation, vessel remodeling, angiogenesis, inflammation
At a Glance Commentary
Scientific Knowledge on the Subject
Severe pulmonary arterial hypertension is associated with distinct and pronounced pulmonary vascular and nonvascular lung pathology. The last reports of the pathology of severe pulmonary hypertension date back approximately 2 decades, well before use of current therapies for the disease.
What This Study Adds to the Field
The current work systematically examines the largest assortment of lungs collected from transplanted patients with idiopathic and associated pulmonary arterial hypertension in the past 2 decades. Our study reveals that in patients with advanced disease, there is a distinct spectrum of pulmonary vascular and nonvascular pathologies, including localized interstitial and perivascular inflammation. In this set of patients, who were enrolled for lung transplantation while being treated with the modern armamentarium of drug therapies, the appearance of classical pulmonary vascular lesions related to the disease was unaffected. Our results provide a unique insight into the spectrum of these pathological alterations, which can inform future translational work.
The modern treatment of pulmonary arterial hypertension (PAH) has led to substantive advancements in patients’ quality of life and survival, significantly improving on the grave prognosis historically associated with the disease (1, 2). With the restrictions regarding obtaining lung tissue for diagnosis, the endpoints for effectiveness of the current therapies are largely based on assessments of exercise performance and reported quality of life, time to clinical deterioration, and/or measured physiological performance (e.g., 6-min walk test). Whether prostacyclin and its analogs, endothelin receptor blockers, and phosphodiesterase type 5 inhibitors modify the spectrum of pulmonary vascular lesions in PAH has not been addressed with a large cohort of lungs with the disease.
Several large series, heavily reliant on autopsy samples, highlighted pulmonary vascular alterations that characterize the pulmonary vascular remodeling in idiopathic PAH (IPAH) and PAH associated with congenital heart disease (CHD) (3–14) (see Table E1 in the online supplement). Eccentric and obliterative intima thickening are largely composed of smooth muscle cells and myofibroblasts. A similar cell composition underlies media thickening, which is regarded as the signature process in pulmonary hypertension (15). In addition, most of the causes of severe pulmonary hypertension also exhibit a disorganized growth of primitive endothelial cells that form plexiform lesions. The latest comprehensive morphometric assessment of pulmonary vascular remodeling dates back almost 20 years (11). The aggregate of past pathological studies of PAH consequently lacked insights into how modern therapies may affect parameters of pulmonary vascular remodeling and perivascular inflammation.
Given the difficulty of obtaining early diseased lung tissue, analysis of explanted lungs from patients undergoing lung transplantation remains the main source of tissue for translational studies in PAH. The Pulmonary Hypertension Breakthrough Initiative (PHBI), a North American network created to accrue lung tissue for translational studies in PAH, has systematically recruited patients with PAH listed to lung transplantation for the past 5 years. This effort led to the creation of one of the largest modern banks of diseased lung tissue devoted to state of the art investigations of pulmonary vascular disease biology.
Our study describes the histopathology and pulmonary vascular morphometry of explanted lungs of patients with PAH and control subjects. Our data provide a critical framework to guide investigations of advanced pulmonary vascular disease, underscore the important clinical challenges in patients with advanced PAH, and offer key insights into the apparent heterogeneity of PAH pathological features found in the modern era of pulmonary hypertension treatment and research.
Methods
Pulmonary Hypertension Breakthrough Initiative
The organization of the PHBI, under the direction of the Cardiovascular Medical Research Fund, is outlined at http://www.ipahresearch.org/.
Enrolment of Patients and Clinical Datasets
Clinical data were obtained for the 62 patients with PAH, and 28 failed organ donors serving as normotensive control subjects, enrolled in the PHBI from April 2006 to August 2011 (for collected clinical data at enrollment, see Table E2). The present study was approved by the Colorado Multiple Institutional Review Board. Lung tissue collection was approved by each Institutional Review Board at all lung transplant sites. BMPRII and SMAD9 mutations were assessed as described in the online supplement.
Lung Tissue Processing
The PHBI implemented a standardized tissue-processing protocol detailed in the online supplement.
Histological and Morphometry Analyses
For each case, sections from 12 separate tissue blocks were stained with hematoxylin and eosin for histological analysis of pulmonary tissue. Sampling, pathological analyses, and quantification, including inflammatory score, are summarized in the online supplement, Table E3 and Figures E4 and E11.
Statistical Analysis
Statistical analyses are outlined in the online supplement.
Results
Patient Characteristics
In total, lungs from 62 patients with a diagnosis of PAH and 28 control subjects were accrued from April 2006 to August 2011 (demographics for all patients and pulmonary hemodynamics for patients with PAH are reported in Table 1 and Figure 1). Control lungs were obtained from lung donors who failed criteria for lung transplantation. The number of patients with PAH with specific clinical diagnoses of IPAH/familial PAH (FPAH), drug-related PAH, congenital heart disease (CHD) PAH, associated PAH (APAH) (due to collagen vascular disease [CVD]), venoocclusive disease-like pattern [VOD], or chronic thromboembolic pulmonary hypertension at the time of enrollment are outlined in Table E4.
TABLE 1.
DEMOGRAPHIC AND HEMODYNAMIC DETAILS IN CONTROL SUBJECTS, PATIENTS WITH PULMONARY ARTERIAL HYPERTENSION, AND RELATED HISTOLOGICAL PATTERNS
| Group/Clinical Diagnosis at Enrollment | No. | Sex (M/F) | Age (yr) | Race (W/AA/A) | Genetic Mutation (Y/N) | Anticoagulated (Y/N) | Systolic PAP (mm Hg) | Diastolic PAP (mm Hg) | mPAP (mm Hg) | PVR (Wood Units) |
| Control subjects | 28 | 20/8 | 32 ± 18 (0.7–64) | 24/4/0 | NA | NA | NA | NA | NA | NA |
| Included control subjects | 22 | 16/6 | 32 ± 16 (0.7–61) | 18/4/0 | NA | NA | NA | NA | NA | NA |
| Excluded control subjects | 6 | 4/2 | 54 ± 17 (19–64) | 6/0/0 | NA | NA | NA | NA | NA | NA |
| PAH | 62 | 16/46 | 42 ± 17 (7–79) | 49/6/7 | 7*/52 | 34/28 | 81 ± 24 (44–175) | 35 ± 14 (11–84) | 46 ± 19 (30–105) | 9.3 ± 4.4 (2.5–29.8) |
| IPAH-like pattern | 48 | 12/36 | 36 ± 15 (11–62) | 35/6/7 | 7*/37 | 30/18 | 87 ± 24 (55–175) | 40 ± 13 (15–70) | 56 ± 16 (36–102) | 11.7 ± 6.7 (4.6–29.8) |
| IPAH | 27 | 9/18 | 36 ± 16 (11–62) | 17/5/5 | 1*/24 | 16/11 | 89 ± 25 (55–175) | 41 ± 14 (15–70) | 59 ± 17 (36–102) | 13.3 ± 7.1 (4.6–29.8) |
| CHD | 9 | 0/9 | 32 ± 15 (16–58) | 7/0/2 | 1*/8 | 6/3 | 108 ± 23 (80–146) | 53 ± 11 (35–68) | 74 ± 14 (50–92) | 16.0 ± 7.1 (5.6–26.9) |
| FPAH | 5 | 2/3 | 41 ± 10 (33–56) | 4/1/0 | 3*/2 | 3/2 | 84 ± 17 (65–110) | 40 ± 12 (29–55) | 56 ± 12 (48–75) | 11.7 ± 6.8 (6.4–23.3) |
| Drug exposure | 3 | 0/3 | 54 ± 6 (48–59) | 3/0/0 | 0/3 | 1/2 | 86 ± 25 (60–110) | 38 ± 7 (32–45) | 55 ± 15 (38–68) | 11.6 ± 4.8 (6.4–16.0) |
| APAH-CVD–like pattern | 12 | 4/8 | 54 ± 18 (7–79) | 12/0/0 | 0/11 | 4/8 | 81 ± 26 (52–143) | 29 ± 18 (12–82) | 48 ± 20 (30–105) | 6.8 ± 2 (2.48–9.4) |
| CTD-associated† | 5 | 0/5 | 65 ± 10 (54–79) | 5/0/0 | 0/5 | 2/3 | 66 ± 11 (52–83) | 22 ± 8 (12–29) | 40 ± 9 (30–51) | 5.4 ± 2.2 (2.5–7.7) |
| VOD-like pattern | 2 | 0/2 | 49 and 63 | 2/0/0 | 0/2 | 0/2 | 52 and 73 | 24 and 35 | 35 and 51 | 5.0 and 19.2 |
Definition of abbreviations: A = Asian; AA = African American; APAH-CVD = associated PAH–collagen vascular disease; CHD = congenital heart disease; CTD = connective tissue disease; F = female; FPAH = familial PAH; IPAH = idiopathic pulmonary arterial hypertension; M = male; NA = not available; PAH = pulmonary arterial hypertension; PAP = pulmonary arterial pressure; VOD = venoocclusive disease; W = white.
Data are shown as ±SD.
Seven cases with genetic mutations were detected: 4 BMPR2 (three FPAH and one IPAH-like pattern with a VOD clinical diagnosis) and three Smad9 (one CHD; one IPAH-diagnosed, and one IPAH-like pattern with an APAH clinical diagnosis) (details in online supplement and Table E5).
Two cases limited scleroderma, one case diffuse scleroderma, one case systemic lupus erythematosus, one case rheumatoid arthritis.
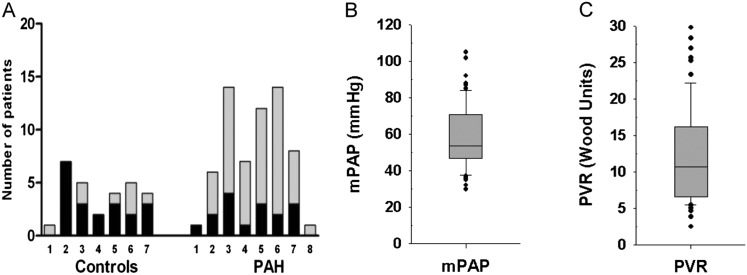
Figure 1.
Demographic characteristics of the study population (control subjects, n = 28; patients with pulmonary arterial hypertension [PAH], n = 62). (A) Distribution of sex in relation to age (displayed in decades) in control subjects and patients with PAH (solid, men; shaded, women). (B) Distribution of mean pulmonary arterial pressure (mPAP) in PAH (n = 62). (C) Distribution of pulmonary vascular resistance (PVR) in PAH (n = 60).
Control Lungs
Twenty-two (79%) control lungs had preserved alveolar architecture and morphologically normal pulmonary arteries. Six control lungs had pulmonary arteries with pronounced thickening of primarily media and without evidence of plexiform lesions (Figure E3). These lung donors were significantly older when compared with donors with normal pulmonary arteries (Table 1; P < 0.01, Student t test). Potential causes of the pulmonary vascular remodeling included one lung each with venoocclusive alterations, smoking-related changes, and thromboembolic disease; three lungs had no apparent pathological cause for vascular remodeling. Incidental pathological findings in the 28 cases of failed donor lungs included acute bronchopneumonia (11, 39%), recent thromboemboli (9, 32%), and focal emphysematous changes (9, 32%). Subsequent morphometric analyses were restricted to the 22 lungs with normal pulmonary arteries (see online supplement).
PAH
We initially interrogated whether the PAH lungs, irrespective of the underlying cause of disease, showed distinct pulmonary vascular remodeling compared with control lungs. All lungs in the PAH group (n = 62) exhibited variable degrees of arterial media and intima remodeling. Plexiform lesions were found in 56 (90%) cases. Incidental pathological findings of mild emphysematous and/or fibrotic changes were observed in eight (13%) patients, who had, however, preserved pulmonary function (Table 1).
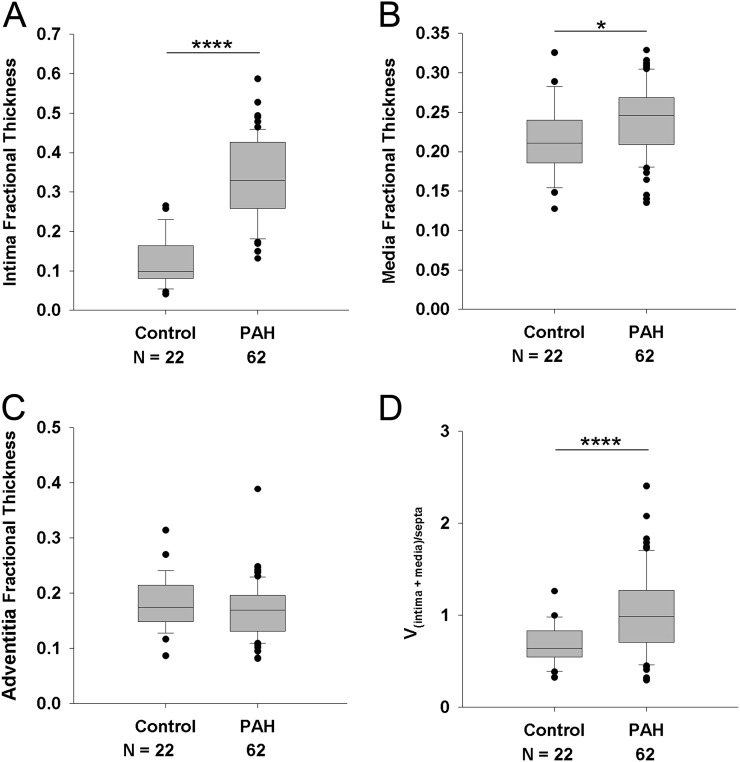
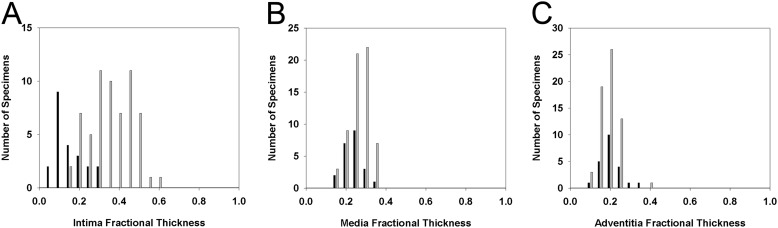
Morphometric intima and media fractional thicknesses were significantly higher in the PAH group when compared with control lungs; no differences were noted with adventitia thickness (Figure 2). These findings were also confirmed by the complementary measurements of significantly increased intima plus media volume density in relation to alveolar septa when compared with control lungs (1.4-fold, P < 0.001) (Figure 2D and Figure E4). Notably, there was a marked overlap of media (55, 88.7%) and adventitia (61, 98%) fractional thickness, but less of intima (15 of 62, 24.2%), with 95% range of the values seen in control lungs (Figure 3). In PAH lungs, no significant correlations were detected among the morphometric parameters of pulmonary vascular remodeling (i.e., media vs. intima fractional thickness, etc.; Figure E5).
Figure 2.
Assessment of intima fractional thickness (A), media fractional thickness (B), and adventitia fractional thickness (C) in lungs of control subjects (n = 22) and patients with pulmonary arterial hypertension (PAH) (n = 62). Measurements were made using digital imaging analysis of circular profiles of pulmonary arteries. Control cases with vascular remodeling (n = 6) were excluded from this analysis and are shown in Figure E3. (Student t test, *P < 0.05, ****P < 0.001). (D) Volume density vessel thickness (intima plus media) weighed by airway septa (Vintima+media)/septa (Wilcoxon rank-sum test, P < 0.001).
Figure 3.
Frequency distribution of intima (A), media (B), and adventitia (C) fractional thickness among control (n = 22, solid bars) and pulmonary arterial hypertension (PAH) lungs (n = 62, shaded bars) (A, Wilcoxon rank-sum test, P < 0.001; B, Student t test, P = 0.026; C, Student t test, P = 0.418).
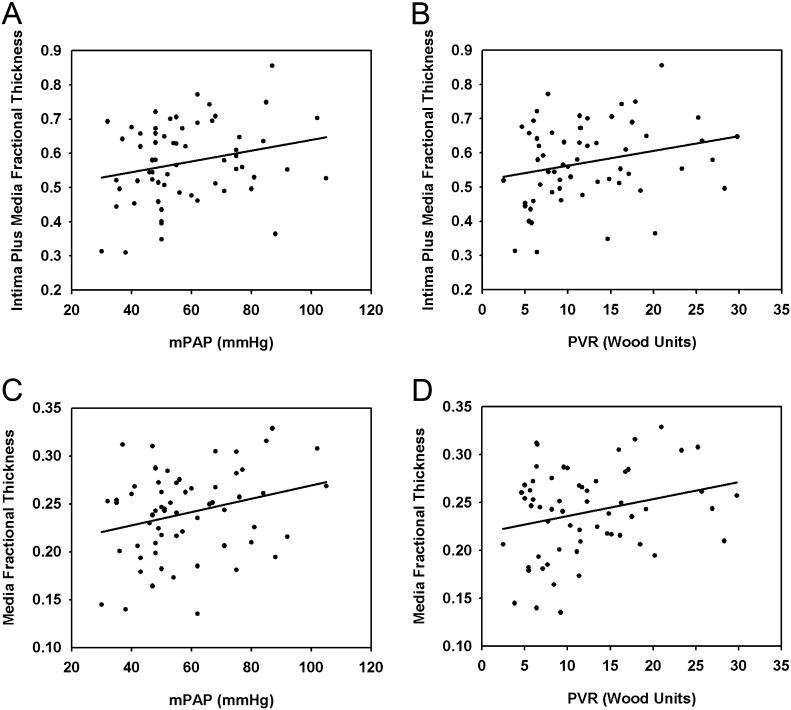
Media fractional thickness correlated with both the mean pulmonary arterial pressure (mPAP) (P < 0.05) and pulmonary vascular resistance (PVR) (P < 0.05). The intima plus media fractional thickness had a borderline correlation with mPAP and PVR in the overall PAH group (P = 0.064 and P = 0.058, respectively). In contrast, no correlations were detected between adventitia fractional thickness and pulmonary hemodynamic parameters (Figure 4). Neither the average number of profiles of plexiform lesions nor the subset of lesions fed by muscular arteries correlated with mPAP (Figure E6). Total wall thickness was lower in whites compared with Asians (P < 0.05); however, no significant correlations were detected between intima or media fractional thickness with race, nor between vascular morphometric parameters and sex. Intima plus media fractional thickness showed a negative correlation with patients’ age; on the other hand, age of patients with familial PAH showed a close correlation with mPAP and PVR (P < 0.05). Interestingly, female patients with PAH had 2.8-fold higher number of plexiform lesion profiles when compared with their male counterparts (P < 0.001) (Figure E7).
Figure 4.
Correlation of intima plus media fractional thickness in patients with pulmonary arterial hypertension (PAH) with (A) mean pulmonary arterial pressure (mPAP) (n = 62, P = 0.066, R = 0.235), and (B) pulmonary vascular resistance (PVR) (n = 60, P = 0.057, R = 0.247). Correlation of media fractional thickness in patients with PAH with (C) mPAP (n = 62, P = 0.036, R = 0.267), and (D) PVR (n = 60, P = 0.047, R = 0.258).
We detected thrombi (recent or old) in 31 of 62 (50%) of PAH lungs. Of the 31 patients with thrombi, 13 (42%) were subjected to anticoagulation with either warfarin or low molecular weight heparin. Of those without thrombi, 21 (68%) were anticoagulated (Chi-square, P < 0.05) (Table 1).
In the vast majority of nonneoplastic pulmonary diseases, a spectrum of pathological alterations in the lung can be shared among multiple lung diseases; indeed, similar pulmonary vascular lesions are seen in lungs compromised by the multiple causes of PAH (17). For subsequent analyses, PAH lungs were segregated based on the pathological pattern most suggestive of a specific clinical diagnosis (14, 16): IPAH-like pattern (muscular remodeling and endothelial cell proliferative plexiform lesions) (n = 48), APAH-CVD–like pattern (interstitial disease with marked muscularization of precapillary arteries) (n = 12), or VOD-like pattern (n = 2). The correlation between the clinical diagnosis at the time of enrollment and the histologic patterns is shown in the Table E4.
IPAH-like pattern.
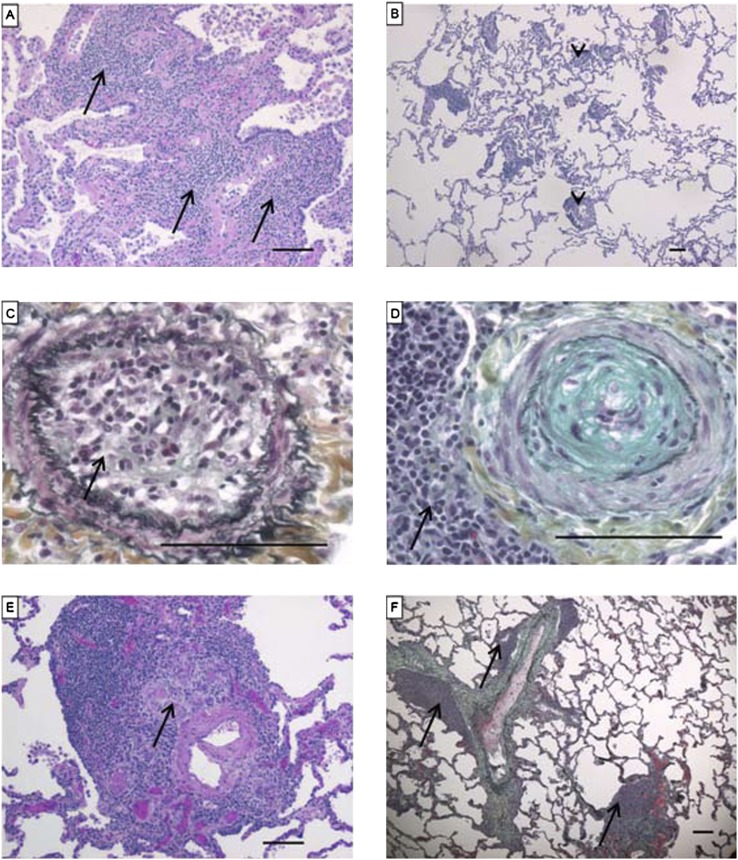
Demographics and pulmonary hemodynamics are shown in Table 1. In the cases of IPAH-like pattern, we observed variable vascular remodeling. Muscular arteries showed eccentric and concentric intima thickening, sometimes with complete occlusion of the lumen, and pronounced media thickening. The precapillary arteries showed a marked muscularization in most of the IPAH-like lungs. Plexiform lesions were encountered in all lungs (Figure 5). We found recent (n = 11), organized (n = 20), or recent and organized (n = 9) arterial thrombi in 24 (50%) patients of the IPAH-like group.
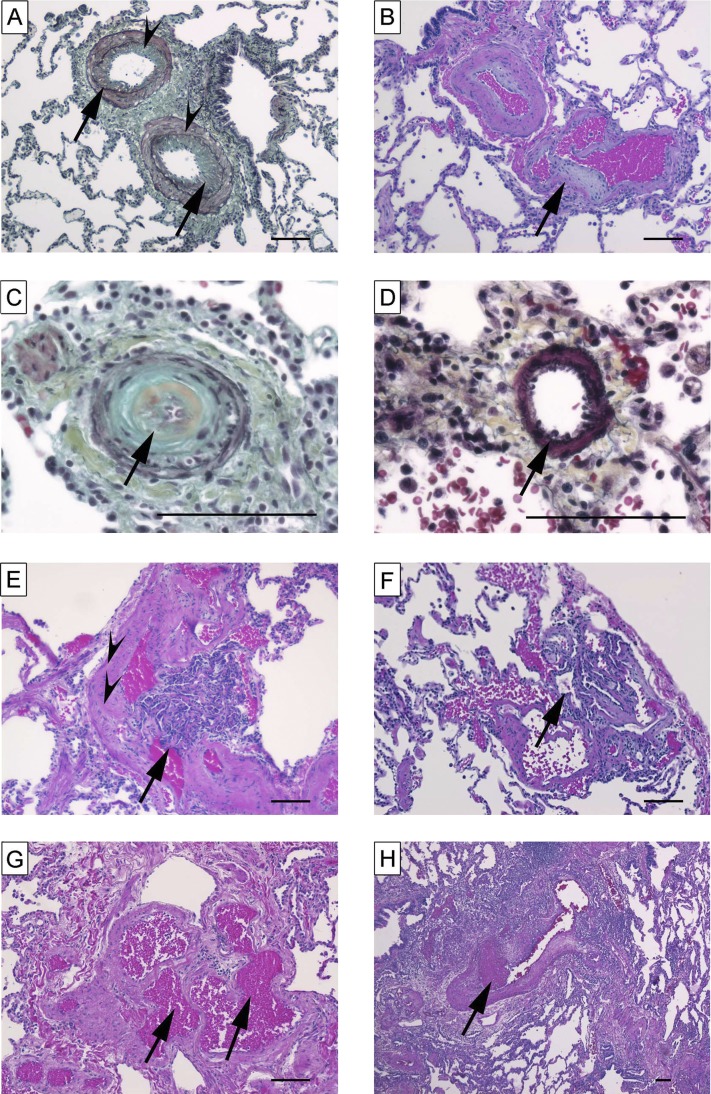
Figure 5.
Characteristic histopathological findings encountered in idiopathic pulmonary arterial hypertension–like pattern. (A) Intima (arrows) and media (arrowheads) thickening (Russel-Movat pentachrome stain). (B) Cushion-like eccentric intima thickening (arrow) in pulmonary arteries (hematoxylin and eosin [H&E]). (C) Concentric “onion-skin–like” (arrow) intima thickening with subtotal luminal occlusion (Russel-Movat pentachrome stain). (D) Muscularization of small artery (arrow) (Russel-Movat pentachrome stain). (E) Plexiform lesion (arrow) fed by muscular artery (arrowheads) (H&E). (F) Subpleural plexiform lesion (arrow) (H&E). (G) Angiomatoid lesion consisting of dilated vessels (arrows) (H&E). (H) Recent thrombus (arrow) (H&E). Scale bars = 100 μm.
Overall, there was a high concordance (45 of 48, 94%) between the pathological pattern and the clinical diagnosis. The three cases in which the histologic and clinical diagnoses differed consisted of one clinically diagnosed chronic thromboembolic disease and two VOD cases, which under microscopic examination exhibited IPAH-like pattern (Table E4).
APAH-CVD–like pattern.
The demographics and pulmonary hemodynamics for the APAH-CVD–like pattern group (n = 12) are listed in Table 1. Overall vascular remodeling of the muscular arteries was similar to the IPAH group. However, the APAH-CVD cases also showed areas with marked interstitial inflammation as well as fibrotic changes. Morphological alterations were more pronounced in the smaller-sized and precapillary arterial branches. Plexiform lesions were found in eight (66%) lungs (Figure 6). We found recent arterial thrombi in five (42%) patients with the APAH-CVD–like histological pattern.
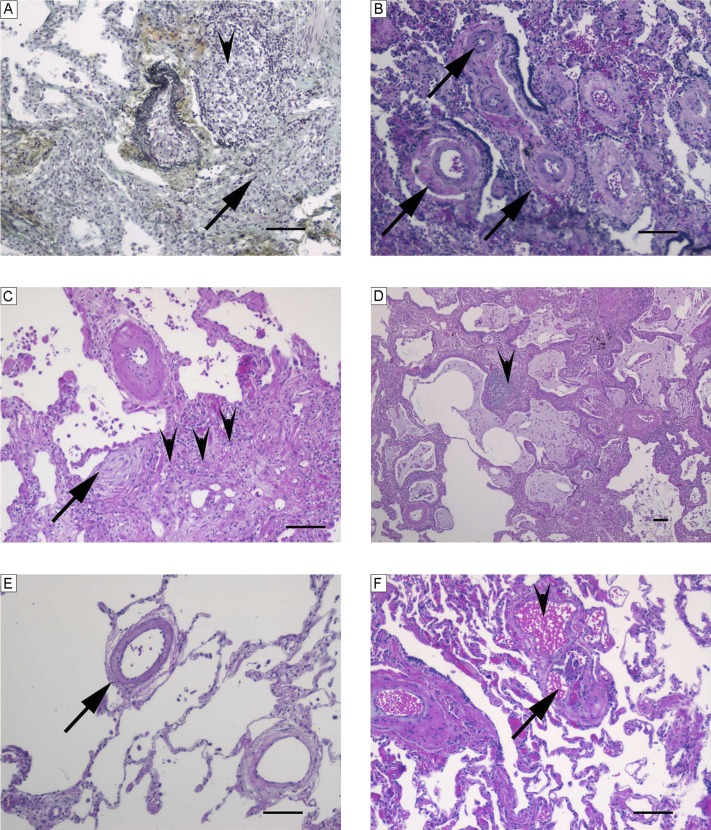
Figure 6.
Characteristic histopathological findings observed in associated pulmonary arterial hypertension–collagen vascular disease–like pattern. (A) Fibrosis (arrow) and pronounced interstitial inflammation (arrowhead) (Russel-Movat pentachrome stain). (B) Media thickening of numerous arteries (arrows) and scant interstitial inflammation (hematoxylin and eosin [H&E]). (C) Fibroblastic focus (arrow), fibrosis (arrowheads), scant interstitial inflammation, and pronounced media thickening of an artery (H&E). (D) Architectural distortion with enlarged cyst-like lung spaces lined by respiratory epithelium (honeycombing) and focal moderate interstitial inflammation (arrowhead) (H&E). (E) Muscularized small pulmonary arteries (arrow) (H&E). (F) Peripheral plexiform lesion (arrow) within angiomatoid lesion (arrowhead) (H&E). Scale bars = 100 μm.
There was agreement between the APAH-CVD pattern and the matched clinical diagnosis in five patients. Of the remaining seven patients with an APAH-CVD–like pattern, four carried a clinical diagnosis of IPAH, one VOD, and two anorexigen-associated PAH (overall discrepancy rate of 58%) (Table E4).
VOD-like pattern.
Both patients of the VOD-like pattern group (n = 2) were female and white (Table 1). Both cases displayed pronounced thickening of the venous intima and focal obliterations of the venous lumen. In some lung regions, prominent capillaries were present. The arterial changes were similar to those seen in the precapillary forms of PAH, including intimal obliteration found in some muscular arteries and focal medial hypertrophy. Plexiform lesions were absent in both cases (Figure E8). We found arterial thrombi in both patients (in the first, recent and organized, and in the second, only organized, i.e., old). Clinically, one patient had been diagnosed as having VOD and the other IPAH.
Pathological Pattern Correlations with Hemodynamic and Morphometric Parameters
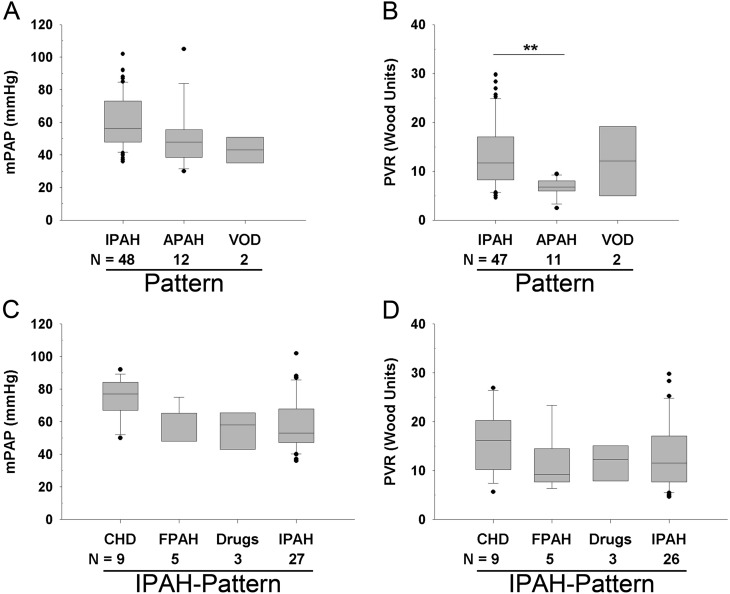
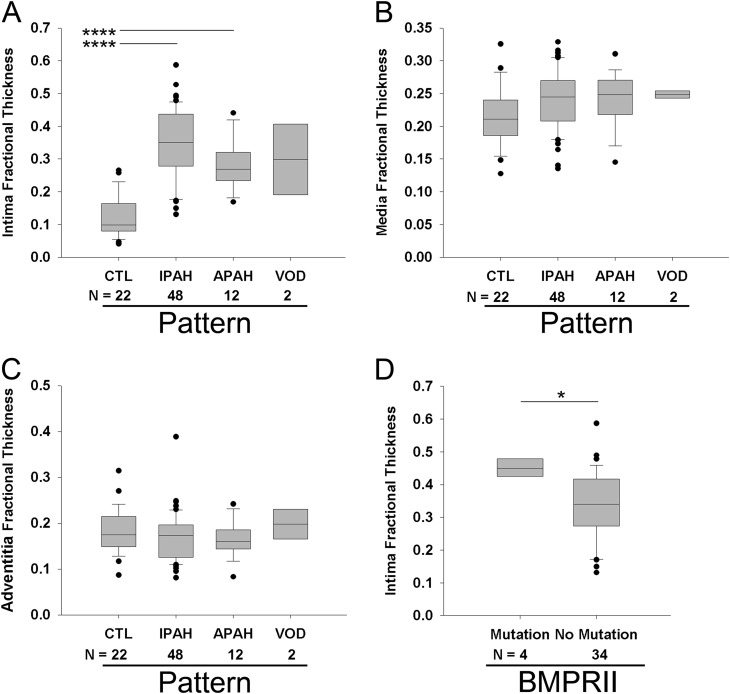
Mean pulmonary arterial pressure did not differ among the main histological patterns (Figure 7A), but there was a significant difference between IPAH-like pattern and APAH-CVD–like pattern for PVR (Figure 7B, P < 0.01). In the IPAH-like pattern group, there was no significant difference in mPAP after stratification into different causes based on clinical data, yet cases due to CHD tended to have higher pulmonary artery pressures (Figures 7C and 7D). Statistically significant differences in intima and intima plus media fractional thickness were observed between control lungs and all PAH pathological subgroups (Figure E8). In contrast to the comparison of control lungs versus PAH lungs (Figure 2), no significant differences were detected in media fractional thickness among PAH subgroups when compared with control lungs (Figures 8A–8C). FPAH and IPAH lungs with BMPRII mutations (n = 4) had increased intima thickness compared with those without mutations (n = 34, P < 0.05) (with no differences in media or adventitia thickness) (Figure 8D, Table E5). Stratifying the IPAH-like pattern group into clinical subgroups, we found no statistically significant differences in morphometric measurements or profiles of plexiform lesions among these groups (Figure E9).
Figure 7.
Comparison of hemodynamics with histologic pattern: (A) mean pulmonary arterial pressure (mPAP) (analysis of variance [ANOVA], P = not significant [NS]), and (B) pulmonary vascular resistance (PVR) (ANOVA, P = 0.008). Comparison of hemodynamics within the idiopathic pulmonary arterial hypertension (IPAH)-like group according to the underlying cause: (C) mPAP (ANOVA, P = NS), and (D) PVR (ANOVA, P = NS). **P < 0.01. APAH = associated PAH; CHD = congenital heart disease; FPAH = familial PAH; VOD = venoocclusive disease.
Figure 8.
Measurements of (A) intima fractional thickness (analysis of variance [ANOVA], P < 0.001), (B) media fractional thickness (ANOVA, P = NS), and (C) adventitia fractional thickness (ANOVA, P = NS) in control subjects (CTL) and in patients with pulmonary arterial hypertension (PAH) on stratification into different histologic subgroups (idiopathic PAH [IPAH]-like pattern, associated PAH [APAH]–collagen vascular disease [CVD]-like pattern, or venoocclusive disease [VOD]-like pattern). Measurements were made using digital imaging of circular profiles of pulmonary arteries. (D) Measurement of intima fractional thickness in familial PAH and IPAH lungs of patients with (n = 4) or without identified BMPRII mutations (n = 34; Student t test). *P < 0.05, ****P < 0.001.
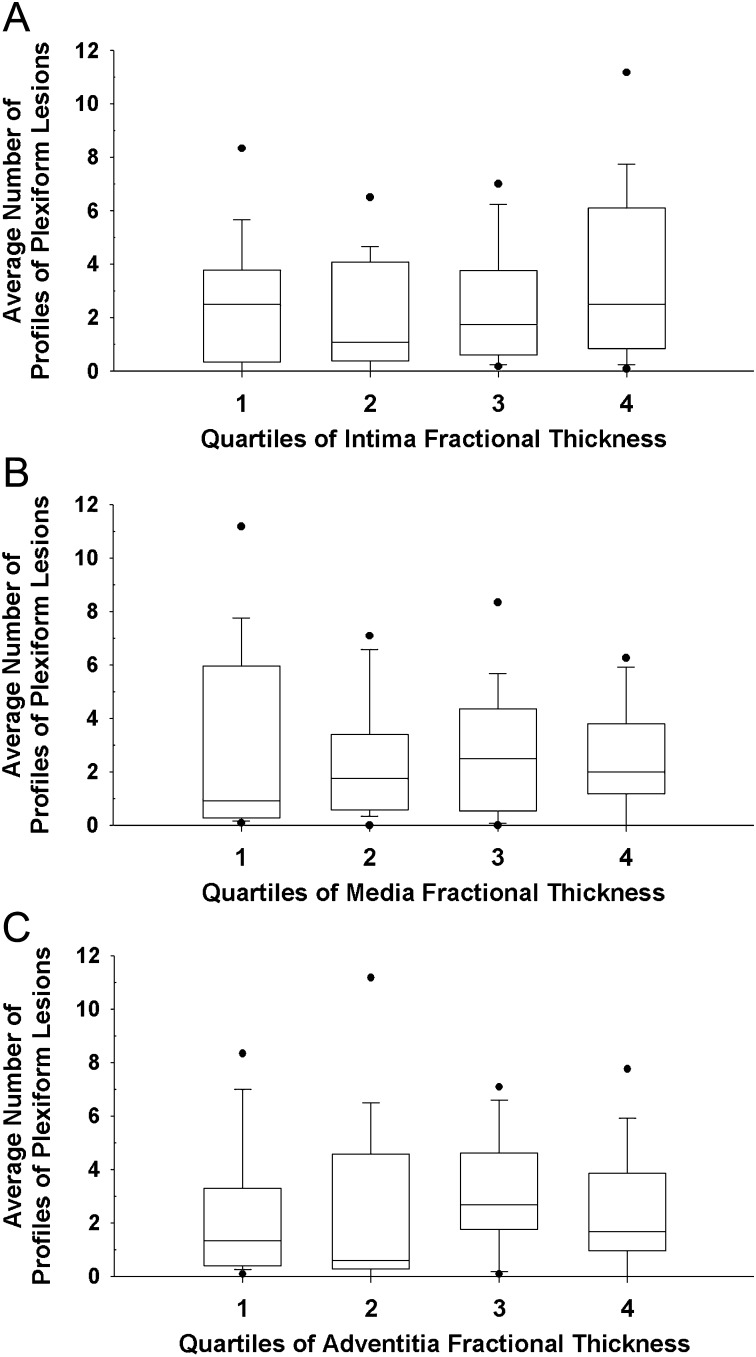
PAH cases were next stratified into quartiles based on the degree of vascular remodeling measured morphometrically. Interestingly, a similar number of profiles of plexiform lesions were detected in the lungs of IPAH- and APAH-CVD–like patterns irrespective of the degree of morphometric remodeling observed in the intima, media, or adventitia fractional thickness (Figure 9).
Figure 9.
Comparison between plexiform lesions and vascular wall remodeling. Average number of profiles of plexiform lesions in correlation with quartiles of (A) intima fractional thickness, (B) media fractional thickness, and (C) adventitia fractional thickness in idiopathic PAH (IPAH)-like pattern and associated PAH (APAH)–collagen vascular disease (CVD)-like pattern (n = 60, n = 15/quartile).
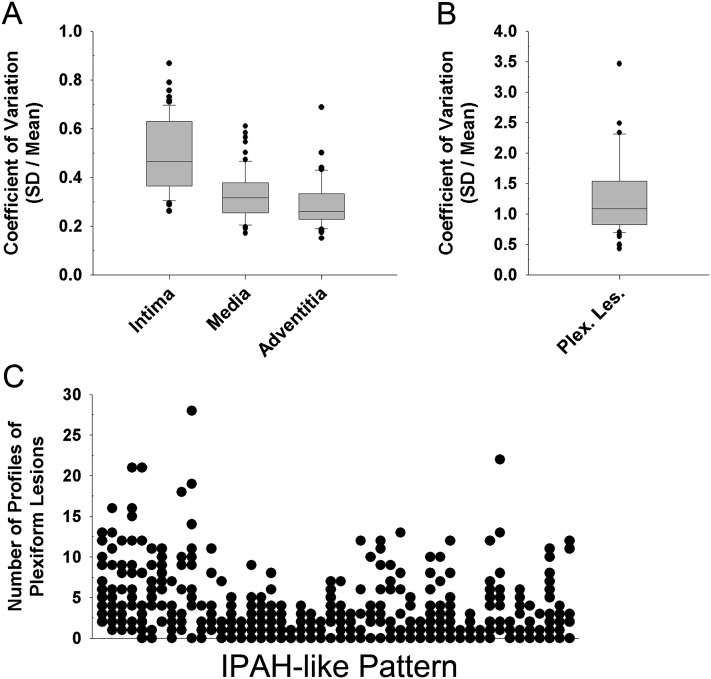
Specific histopathological features, such as intima, media, and adventitia fractional thickness and the number of profiles of plexiform lesions, varied remarkably among PAH cases and within the pathological-pattern groups. This marked variability was also seen in different areas sampled within any particular lung. The coefficient of variance was calculated to express the heterogeneity encountered (Figure 10, Figure E10). The variability was greatest for the intima layer thickness. No correlations were detected between the coefficient of variance for average number of profiles of plexiform lesions compared with the coefficients of variance of intima or media fractional thickness.
Figure 10.
(A) Coefficient of variation (CV), calculated as the standard deviation divided by the mean, as a measurement of variability of intima fractional thickness, media fractional thickness, and adventitia fractional thickness per slide in diseased patients (n = 62). (B) CV of profiles of plexiform lesions per slide in patients with plexiform lesions (n = 56). (C) Distribution of profiles of plexiform lesions per slide in idiopathic PAH (IPAH)-like pattern, each column on the x axis representing one patient (n = 48). Each column projecting from the x axis represents one individual patient; the respective y-dots represents the count of plexiform lesion profiles (i.e., outlines) in each of the 12 slides examined in each patient (P = not significant for all).
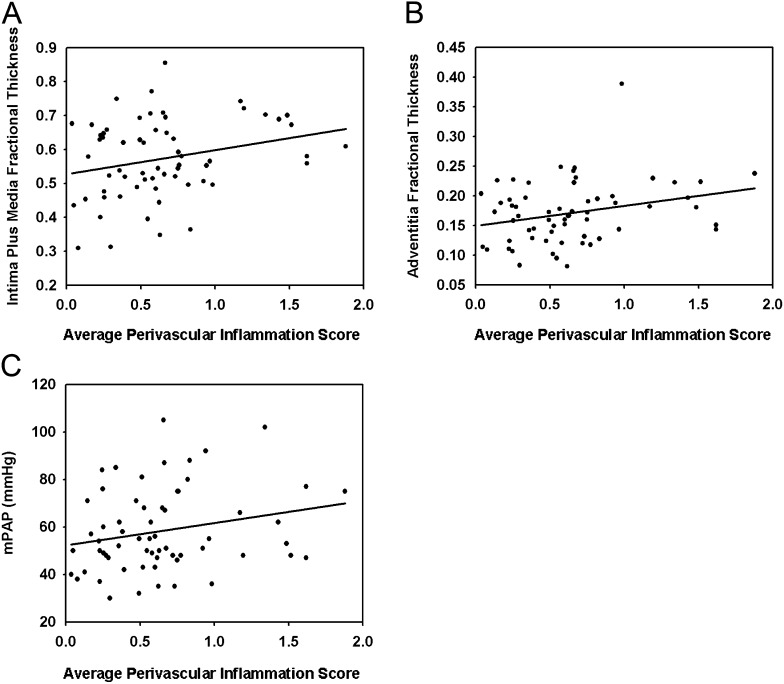
Perivascular and interstitial inflammatory infiltrates were observed in all pathological patterns of PAH (Figure 11). These infiltrates were predominantly composed by mononuclear cells, consistent with lymphocytes, with a minority of macrophages and neutrophils, as previously reported (17). Perivascular inflammation score (Figure E11) correlated with intima plus media (P < 0.05) and adventitia (P < 0.05) fractional thickness and a trend toward correlating with mPAP (P = 0.075) (Figure 12). We found no correlation of perivascular inflammation score with PVR, media fractional thickness, or with the number of profiles of plexiform lesions (Figure E12). Interstitial inflammation did not correlate with any of key hemodynamic and morphometric parameters (data not shown).
Figure 11.
Examples of inflammation in pulmonary arterial hypertension (PAH) lungs. (A) Diffuse pronounced interstitial inflammation (arrows) (hematoxylin and eosin [H&E]). (B) Localized moderate perivascular inflammation (arrowheads) (H&E). (C) Occluded artery with dense inflammatory infiltrate (arrow) (Russel-Movat pentachrome stain). (D) Artery with concentric intima proliferation, neighbored by a dense inflammatory infiltrate (arrow) (Russel-Movat pentachrome stain). (E) Artery with plexiform lesion (arrow), embedded in inflammatory infiltrate (H&E). (F) Arteries focally cuffed by dense inflammation (arrows) (Russel-Movat pentachrome stain). Scale bars = 100 μm.
Figure 12.
Correlation of the average perivascular inflammation score with (A) intima plus media fractional thickness (P = 0.042, R = 0.259), (B) adventitia fractional thickness (P = 0.030, R = 0.275), and (C) mean pulmonary arterial pressure (mPAP) (P = 0.075, R = 0.228) in pulmonary arterial hypertension (n = 62). The measurement of the average perivascular inflammation score is described in the online supplement.
In 20 cases (32%) of PAH, we noted focal remodeling of intraseptal veins consisting of thickening of media and/or intima, including one case with focal occlusion of the lumen (Figure E13). There were no correlations between venous remodeling with intima, media, or adventitia fractional thickness or the number of profiles of plexiform lesions (Figure E14).
Correlation of Morphometric Assessment of Pulmonary Vascular Remodeling with PAH Therapies
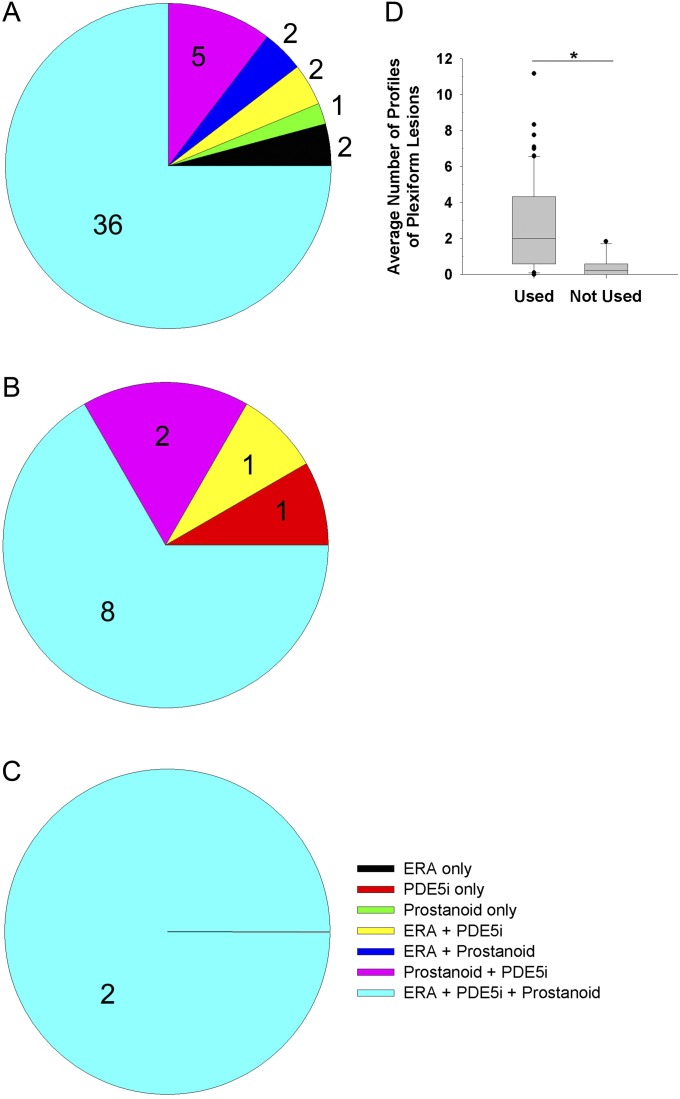
All patients with PAH were treated with at least one of the currently recommended medications for PAH. Most patients with PAH (including within histopathological pattern groups) received combination therapy (Figure 13). Prostacyclin treatment correlated positively with the average number of profiles of plexiform lesions (Figure 13D). No further correlations were observed when comparing specific medications and the other morphometric measurements, including perivascular inflammation (Figure E15).
Figure 13.
Use of pulmonary arterial hypertension (PAH)-specific medication in (A) idiopathic PAH–like pattern, (B) associated PAH–collagen vascular disease–like pattern, and (C) venoocclusive disease–like pattern. (D) Average number of profiles of plexiform lesions in PAH (n = 62) in correlation with use of prostanoids (Wilcoxon rank-sum test, P = 0.010). ERA = endothelin receptor antagonist. PDE5i = phosphodiesterase type 5 inhibitor.
Discussion
We show that the largest collection of lungs with PAH studied in the era of modern therapeutics for pulmonary hypertension shares many of the pathological features of pulmonary vascular remodeling as described over the past 20 to 30 years. Our relatively large sample size allowed us to quantitatively assess key morphological parameters despite the extensive degree of morphological heterogeneity observed within individual lungs and among lungs with IPAH and APAH-CVD–like patterns. Our study also reveals important new findings, including the potential for pathological subphenotypes and marked interstitial and, most importantly, a significant perivascular inflammation in numerous PAH lungs. Our data therefore provide a window into the pathology of pulmonary hypertensive disease in a large cohort of patients with advanced disease, treated with modern medical therapeutics, followed in specialized centers and subjected to lung transplantation.
Our sampling and morphometric approaches to quantify key pulmonary vascular alterations are unique when compared with prior studies. The latter lacked consistent sampling strategies, analyzed only a few fragments of tissue, and/or did not apply quantitative measures to assess pulmonary vascular remodeling (3, 4, 6, 9, 10). Our approach revealed that the primary differences between the PAH and control groups reside largely in the intima and in the intima plus media thickening; moreover, plexiform lesions were only detected in PAH lungs (in 90% of the PAH cases). Of note, media fractional thickness, albeit statistically increased in PAH lungs compared with control lungs and correlated with PAP and PVR, was largely superimposable with the range of media fractional thickness measured in normal pulmonary arteries. In a study by Chazova and colleagues (11), the closest study to match our approach, a 25 to 30% media fractional thickness was reported in IPAH lungs as compared with 5% in the control group. As medical therapy for these patients was not listed, it is unclear whether our findings represent a true effect of PAH medications or if it can be explained by our experimental design, including systematic sampling or detailed morphometric approaches, leading to a more thorough assessment of pulmonary media remodeling.
In addition to comparing all PAH lungs representative of the World Health Organization group 1 classification with control lungs, we performed a detailed analysis of histopathological patterns, subsequently correlated with the underlying clinical causes of that specific pattern. No morphometric differences were noted among IPAH, PAH-CHD, and drug-related IPAH. Despite a low number of patients with BMPRII mutations, we noted that these lungs had a more pronounced intima remodeling than those without the mutations; these results are in agreement with the findings of increased pulmonary artery pressures in patients with BMPRII mutations (18). Of note, the occurrence of BMPR2 mutation was lower (1 in 35) in IPAH with no family history than the 10 to 20% incidence previously reported in sporadic IPAH (19).
Our data indicate the possibility that patients present with subphenotypes of pulmonary vascular remodeling, all of which are associated with systemic levels of pulmonary artery pressures and elevated pulmonary vascular resistance. This was supported by the finding that a significant number of lungs with IPAH- and APAH-like patterns showed intima, media, and adventitia fractional thickness overlapping with the control range, yet with a similar number of plexiform lesion profiles as those with more severe remodeling. Conversely, we found that 6 of 28 normal lungs had pulmonary vascular remodeling similar to PAH lungs, albeit without plexiform lesions. Two important diagnostic and pathogenic implications can be inferred from these observations. The fact that plexiform lesions may occur in patients with IPAH and APAH despite the lack of alterations involving vascular compartments occupied by smooth muscle cells (intima and media) does not support that the sequence of remodeling outlined by Heath and Edwards for PAH associated with congenital heart malformations applies to IPAH or APAH (3). Notwithstanding their advanced clinical stage, patients with PAH had marked variability in their manifestations of smooth muscle cell-based remodeling process in the pulmonary arteries. These findings suggest that PAH may be heterogeneous in the relative involvement of the pulmonary vascular cells and the types of vascular lesions underlying the disease.
Our findings support that perivascular inflammation may significantly impact pulmonary hemodynamics in PAH, as the extent of inflammation was found to be more exuberant than reported in earlier series (17, 20). One hypothesis is that perivascular inflammation may be affected by the therapies instituted in the last 20 years, notably of prostanoids (21, 22). We were, however, unable to distinguish any single therapy as significantly associated with altered interstitial or perivascular inflammation (see online supplement).
The pathophysiology of IPAH and APAH remains unclear. Vasoconstriction is an important element in pulmonary hypertension, yet its role as the inciting event or as complication of pulmonary vascular remodeling has not been elucidated. The prevailing hypothesis underlying the pathogenesis of pulmonary hypertension supports a key role for the uncontrolled growth of vascular cells leading to pulmonary vascular remodeling (14). It is difficult, however, to establish a mechanistic hierarchy among the elements of pulmonary vascular remodeling, as we found a lack of correlation among quantitative parameters of pulmonary vascular remodeling, including number of profiles of plexiform lesions, in PAH. It is possible that, with advanced disease, maximum vascular remodeling is achieved, notably involving intima, obscuring differences in the frequency of specific vascular lesions that may indicate potential involvements in the initiation and progression of disease.
It is important to highlight specific aspects and limitations of our experimental design, which may have impacted on the overall significance and interpretation of our data. Most importantly, our patient cohort may be in a stage of disease unresponsive to current therapies. Although the use of current pharmacotherapies for PAH did not lead to significant segregation of the pathology in our cohort, it is conceivable that patients who respond to these therapies may show effects of specific pulmonary vascular remodeling not captured by our study. The patients in our study were enrolled in well-established lung transplant programs for highly variable intervals and therefore subjected to recruitment bias. Our lung sampling approach was designed to maximize tissue harvesting for histology and, importantly, for modern molecular studies reliant on high-quality preservation of macromolecules. The findings of this study cannot be extended to pulmonary vascular and interstitial alterations present in the whole lung because our sampling was not random. Furthermore, as our sampling was not randomized in three dimensions, we were not able to assess remodeling parameters related to length and surface, therefore limiting our assessment of pulmonary vascular remodeling. Moreover, these approaches may allow answering if there is true vascular rarefaction in PAH, as suggested by casting or imaging studies. It is therefore pressing that insights into early disease be developed and future studies incorporate sound stereological approaches using systematic, uniform, and random sampling for correct mapping of the vascular alterations present in PAH (23) (see online supplement).
In conclusion, our study underscores key aspects of pathological heterogeneity and important findings of intima thickening and perivascular inflammation in PAH lungs. It also provides a dataset that may guide investigators in the field on how to understand potential caveats in using human tissue for translational studies in PAH research, notably of tissue and patient heterogeneity, which may impact the data and their interpretation. Furthermore, the pathological subphenotypes may inform of potentially relevant pathogenic processes and novel therapeutic interventions that may advance standard of care for PAH.
Acknowledgments
The authors thank the investigators and personnel participating in the PHBI, notably those involved in the transplant and preparation centers: Allegheny University of the Health Sciences (PI: Raymond L. Benza, M.D.); Baylor College of Medicine (George Noon, M.D.); Cleveland Clinic (PI: Serpil Erzurum, M.D.); Duke University (PI: Pang-Chieh Jerry Eu, M.D.); Stanford University-UCSF (PI: Marlene Rabinovitch, M.D.); University of Alabama (PI: Keith Wille, M.D.; prior PI: Raymond L. Benza, M.D.); University of California, San Diego (PI: Patricia Thistlethwaite, M.D., Ph.D); Vanderbilt University (Barbara Meyrick, Ph.D.). They also thank Dr. Irina Petrache, M.D., for reviewing the manuscript.
Footnotes
Supported by Cardiovascular Medical Research Fund grants (R.M.T., W.E.G., V.V.M.); National Institutes of Health (NIH) grant RC1HL100849 (R.M.T.); and a Parker B. Francis fellowship grant and NIH grant K08HL105536 (B.B.G.).
Author Contributions: Concept and design: R.M.T; acquisition of data: E.S., B.B.G., J.M.H., C.D.C., A.G., S.D.G., V.V.M., M.J., M.A.A, R.M.T.; analysis and interpretation: E.S., B.B.G., R.M.T.; drafting the manuscript: E.S., B.B.G., J.M.H., W.E.G., R.M.T.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201201-0164OC on June 7, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004;351:1425–1436 [DOI] [PubMed] [Google Scholar]
- 2.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343–349 [DOI] [PubMed] [Google Scholar]
- 3.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 1958;18:533–547 [DOI] [PubMed] [Google Scholar]
- 4.Wagenvoort CA, Wagenvoort N. Primary pulmonary hypertension: a pathologic study of the lung vessels in 156 clinically diagnosed cases. Circulation 1970;42:1163–1184 [Google Scholar]
- 5.Rabinovitch M, Haworth SG, Castaneda AR, Nadas AS, Reid LM. Lung biopsy in congenital heart disease: a morphometric approach to pulmonary vascular disease. Circulation 1978;58:1107–1122 [DOI] [PubMed] [Google Scholar]
- 6.Wagenvoort CA. Lung biopsy specimens in the evaluation of pulmonary vascular disease. Chest 1980;77:614–625 [DOI] [PubMed] [Google Scholar]
- 7.Wagenvoort CA. The pathology of primary pulmonary hypertension. J Pathol 1970;101:i [DOI] [PubMed] [Google Scholar]
- 8.Yamaki S, Horiuchi T, Takahashi T. Pulmonary changes in congenital heart disease with Down’s syndrome: their significance as a cause of postoperative respiratory failure. Thorax 1985;40:380–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjornsson J, Edwards WD. Primary pulmonary hypertension: a histopathologic study of 80 cases. Mayo Clin Proc 1985;60:16–25 [DOI] [PubMed] [Google Scholar]
- 10.Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation 1989;80:1198–1206 [DOI] [PubMed] [Google Scholar]
- 11.Chazova I, Loyd JE, Newman JH, Belenkov Y, Meyrick B. Pulmonary artery adventitial changes and venous involvement in primary pulmonary hypertension. Am J Pathol 1995;146:389–397 [PMC free article] [PubMed] [Google Scholar]
- 12.Overbeek MJ, Vonk MC, Boonstra A, Voskuyl AE, Vonk-Noordegraaf A, Smit EF, Dijkmans BA, Postmus PE, Mooi WJ, Heijdra Y, et al. Pulmonary arterial hypertension in limited cutaneous systemic sclerosis: a distinctive vasculopathy. Eur Respir J 2009;34:371–379 [DOI] [PubMed] [Google Scholar]
- 13.Yi ES, Kim H, Ahn H, Strother J, Morris T, Masliah E, Hansen LA, Park K, Friedman PJ. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension. A morphometric and immunohistochemical study. Am J Respir Crit Care Med 2000;162:1577–1586 [DOI] [PubMed] [Google Scholar]
- 14.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 2007;28:23–42 (vii.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuder RM. Pathology of pulmonary arterial hypertension. Semin Respir Crit Care Med 2009;30:376–385 [DOI] [PubMed] [Google Scholar]
- 16.Tuder RM, Groshong SD, Cool CD. General features of non-neoplastic lung diseases. : Mason RJ, Broaddus VC, Martin TR, King TE, Jr, Schraufnagel DE, Murray JF, Nadel JA, Murray & Nadel’s textbook of respiratory medicine, 5th ed Philadelphia: Saunders; 2009. pp. 314–329 [Google Scholar]
- 17.Tuder RM, Groves BM, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994;144:275–285 [PMC free article] [PubMed] [Google Scholar]
- 18.Girerd B, Montani D, Eyries M, Yaici A, Sztrymf B, Coulet F, Sitbon O, Simonneau G, Soubrier F, Humbert M. Absence of influence of gender and BMPR2 mutation type on clinical phenotypes of pulmonary arterial hypertension. Respir Res 2010;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 2002;105:1672–1678 [DOI] [PubMed] [Google Scholar]
- 20.Caslin AW, Heath D, Madden B, Yacoub M, Gosney JR, Smith P. The histopathology of 36 cases of plexogenic pulmonary arteriopathy. Histopathology 1990;16:9–19 [DOI] [PubMed] [Google Scholar]
- 21.Achcar RO, Yung GL, Saffer H, Cool CD, Voelkel NF, Yi ES. Morphologic changes in explanted lungs after prostacyclin therapy for pulmonary hypertension. Eur J Med Res 2006;11:203–207 [PubMed] [Google Scholar]
- 22.Perros F, Dorfmuller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012;185:311–321 [DOI] [PubMed] [Google Scholar]
- 23.Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 2010;181:394–418 [DOI] [PMC free article] [PubMed] [Google Scholar]