Abstract
The chronic leukemias, including chronic myeloid leukemia (CML), the Philadelphia-negative myeloproliferative neoplasms (MPNs), and chronic lymphocytic leukemia (CLL), have been characterized extensively for abnormalities of cellular signaling pathways. This effort has led to the elucidation of the central role of dysregulated tyrosine kinase signaling in the chronic myeloid neoplasms and of constitutive B-cell receptor signaling in CLL. This, in turn, has stimulated the development of small molecule inhibitors of these signaling pathways for therapy of chronic leukemia. Although the field is still in its infancy, the clinical results with these agents have ranged from encouraging (CLL) to spectacular (CML). In this review, we summarize recent studies that have helped to define the signaling pathways critical to the pathogenesis of the chronic leukemias. We also discuss correlative studies emerging from clinical trials of drugs targeting these pathways.
Keywords: Chronic myelogenous leukemia, tyrosine kinase inhibitor, BCR-ABL1, JAK2, imatinib, dasatinib, nilotinib, bosutinib, ibrutinib
Introduction
The discovery of the BCR-ABL1 fusion tyrosine kinase as the causative agent of chronic myeloid leukemia (CML) spurred the development of imatinib mesylate, an ATP-competitive tyrosine kinase inhibitor (TKI) of ABL1, for the treatment of CML [1]. Imatinib and other drugs in its class (dasatinib, nilotinib, bosutinib, ponatinib) have since revolutionized the treatment of CML. The majority of CML patients who initiate TKI therapy for their disease achieve a cytogenetic remission [2] and those who do enjoy a normal life expectancy [3], emphasizing the central role of BCR-ABL1 kinase activity in the pathogenesis of the disease. However, TKI therapy does not appear to cure CML in the majority of patients [4], and a substantial proportion develop required resistance to TKI therapy through ABL1 mutations and other mechanisms [5]. In part, the failure of TKIs to eradicate CML has been attributed to so-called leukemic stem cells (LSCs), pluripotent BCR-ABL1+ progenitors that are largely quiescent [6] and resistant to killing by ABL1 inhibitors [7]. This has rekindled interest in understanding the molecular signaling pathways that mediate the leukemia phenotype and the survival of CML stem cells. By analogy to highly active anti-retroviral therapy in HIV/AIDS, targeting multiple signaling pathways in the leukemic cell might prevent or overcome acquired TKI resistance and lead to permanent cure.
With the discovery of somatic activating mutations in the JAK2 tyrosine kinase in 2005 [8], new light was shed on the pathogenesis of the Philadelphia-negative myeloproliferative neoplasms. JAK2 mutations are found in virtually every patient with polycythemia vera (PV), and about half of patients with essential thrombocythemia (ET) and primary myelofibrosis (PMF) [9-11]. Parallel discoveries of activated fusions of PDGFR and PDGFR in some cases of chronic eosinophilic leukemia [12, 13] and chronic myelomonocytic leukemia [14], respectively, solidified the concept that dysregulated tyrosine kinase signaling underlay the pathogenesis of most of the classic MPNs and myeloid neoplasms with features overlapping MPN and myelodysplasia. While rapid progress ensued on analysis of dyregulated TK signaling in these diseases [15], the development of JAK2 inhibitors was close behind. However, the clinical debut of this class of TKIs has been somewhat disappointing. While specific JAK2 inhibitors such as ruxolitinib decrease splenomegaly and improve symptoms and quality of life in patients with myelofibrosis [16, 17], as a class they do not appear to reduce marrow fibrosis, improve cytopenias, or decrease or eliminate the malignant clone, and hence may have limited impact on survival [18]. A better understanding of the molecular pathogenesis of these diseases is a prerequisite to improving on TKI therapy, specifically to develop strategies to eliminate leukemic stem cells in CML and to reverse the pathological hematopoiesis underlying the Ph-negative MPNs.
Here, we review current knowledge about the signaling pathways underlying the pathogenesis of the chronic leukemias, with a focus on chronic myeloid leukemia, the Ph-negative myeloproliferative neoplasms, and chronic lymphocytic leukemia. The emphasis will be on recent insights and implications for clinical trials of targeted therapies in these diseases.
Chronic myeloid leukemia
The BCR-ABL1 fusion kinase
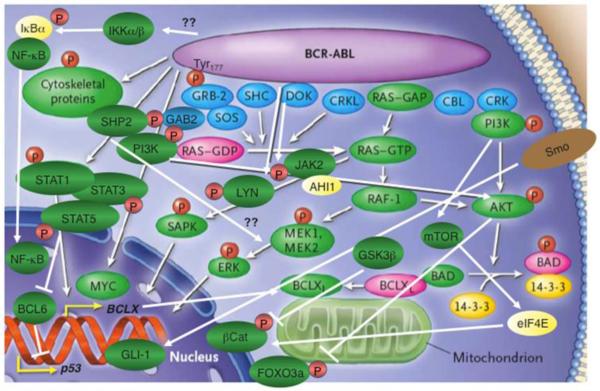
Studies in mouse models 22 years ago defined BCR-ABL1 as the direct cause of CML [19]. Since then, mutagenesis studies have clarified some of the mechanisms and pathways through which BCR-ABL1 induces the CML phenotype (Figure 1). Among the many functional domains of this fusion protein, two key motifs in the BCR portion of the protein are absolutely required for leukemogenesis. The first of these is a coiled-coil oligomerization domain at the extreme N-terminus of the protein [20], which mediates heterodimerization, cross-phosphorylation, and activation of the fusion protein, all of which are necessary to overcome the autoinhibited conformation of the kinase and stimulate cellular signaling [21]. The second key motif is Tyr177, which is stoichiometrically phosphorylated in leukemia cells and serves as binding site for the GRB2 adapter protein [22]. GRB2, in turn, couples to the GAB2 scaffolding protein and activates the phosphatidylinositol 3-kinase (PI3K) and SHP2 signaling pathways [23], both of which contribute to leukemogenesis. A recent study suggested that GAB2 signaling protects CML cells from various BCR-ABL1 TKIs by amplifying the input of BCR-ABL1 into the RAS/ERK and PI3K/AKT/mTOR pathways (discussed below). These results suggest that GAB2 could be a therapeutic target in TKI-resistant disease [24].
Figure 1. BCR-ABL1-induced signaling pathways in CML.
Schematic representation of the myriad signaling pathways that are activated in CML cells. Phosphorylation is represented by red “P” symbols. Arrowheads indicate activation of a downstream molecule, while a bar at the end of a line indicates inhibition of a downstream molecule. (Adapted from Goldman and Melo, N. Eng. J. Med 2003;349:1451).
PI3K/AKT/mTOR pathway
BCR-ABL1 activates the PI3K/AKT pathway through GAB2, and subsequently many downstream targets of the PI3K/AKT pathway have been shown to participate in BCR-ABL1-induced leukomogenesis. PI3K/AKT activation leads to BAD phosphorylation, which prevents binding to and inactivation of the antiapoptotic protein BCL-2. The FOXO transcription factors, other downstream targets of PI3K/AKT involved in cell-cycle arrest and apoptosis, are phosphorylated by AKT, inhibiting FOXO3a activity [25].
Recent research also suggests that AKT and TGF-β signaling play a distinct role in the maintenance of LSC in CML. Whereas BCR-ABL1 activates PI3K and AKT in myeloid progenitors leading to phosphorylation and cytoplasmic retention of the FOXO3a transcription factor [26], Naka et al. recently demonstrated that the most primitive CML stem cells in the retroviral mouse model display inactive AKT, nuclear FOXO3a, and nuclear phosphor-SMAD2/3 (a hallmark of TGF-β signaling) [27]. When CML was induced in HSC from Foxo3a−/− donors, the phenotypic LSC population and efficiency of leukemia induction declined upon serial transplantation, the most rigorous test of LSC function. Their data suggest that TGF-β signaling (predominantly TGF-β1), through an unknown mechanism, may suppress AKT inhibition of FOXO3a in CML LSCs, which may be critical for LSC maintenance. Another group also described paradoxical nuclear FOXO3a in the most primitive (Lin−CD34+CD38−) human CML progenitors [28]; although this paper was subsequently retracted, the data on nuclear FOXO3a are valid (Tessa Holyoake, personal communication). Müschen et al. subsequently showed that BCL6 may mediate some of the LSC survival effects of nuclear FOXO through repression of p53, and that targeting BCL6 with a peptidomimetic drug could decrease CML LSCs in the mouse model [29].
The mTOR is a serine/threonine kinase downstream of the PI3K/AKT pathway that plays a crucial role in regulating mRNA translation in mammalian cells, controlling cell growth and proliferation. mTOR functions as the catalytic subunit/kinase for two distinct protein complexes, mTORC1 and mTORC2. Previous studies established that TORC1 and TORC2 play important roles in growth and survival of BCR-ABL1-transformed cells [30, 31]. Mohi et al. [31] demonstrated that the combination of the allosteric mTOR inhibitor rapamycin and imatinib prolonged survival in a murine CML model and was effective against disease induced by imatinib-resistant mutants of BCR-ABL1. More recently, ATP-competitive dual mTORC1/2 inhibitors (PP242 and OSI-027) have exhibited potent growth inhibition in a number of BCR-ABL1+ cell lines and primary patient samples [32, 33].
In addition to the direct inhibition of TORCs, the strategy of indirect suppression of mTOR function by modulating the AMP-activated Protein Kinase (AMPK) pathway has been explored [34]. AMPK regulates mTOR signaling both directly and indirectly. Once activated, AMPK phosphorylates and activates the TSC1/2 complex, which in turn suppresses Rheb activity, a small G-protein with regulatory functions on mTOR activation [35]. In addition, AMPK directly phosphorylates the Raptor subunit on Ser792, resulting in inactivation of the TORC1 complex [36]. Furthermore, modulation of AMPK by resveratrol, a naturally occurring substance found in grapes, has been shown to exhibit antileukemic effects in both imatinibsensitive and imatinib-resistant CML cells, including leukemic cells harboring the T315I BCR-ABL1 mutation [37]. AMPK induction causes mTOR inhibition irrespective of TKI sensitivity, raising the prospect of using AMPK activators in the treatment of Ph+ leukemias refractory to TKIs [34]. Another target of resveratrol in CML is SIRT1, a novel class of NAD(+)-dependent protein deacetylases implicated in aging [38, 39]. Pharmacologic inhibition of SIRT1 increased apoptosis in LSC of chronic phase and blast crisis CML and reduced their growth both in vitro and in vivo.
RAS/RAF/MAP kinase
As discussed above, phosphorylation of tyrosine 177 within the BCR region of BCR-ABL1 mediates the SH2-dependent binding of GRB2 [22]. In addition to GAB2, another effector of GRB2 is SOS (Son of Sevenless), a guanine nucleotide exchange factor of RAS that mediates RAS activation [40]. There is extensive biochemical evidence to suggest that BCR-ABL1 activates ERK through the activation of the RAS/RAF/MEK/ERK pathway [41]. The ERK1/2 pathway is constitutively activated in embryonic stem cells transformed by BCR–ABL1, and ERK2 activation may be involved in resistance to imatinib [32]. Inhibition of the RAS pathway by farnesyltransferase inhibitors exhibit synergy with MEK/ERK inhibitors in suppressing growth and survival in K562 CML cells and in primary chronic phase CD34+ CML cells [42].
Unlike the MEK/ERK pathway, the role of JNK pathway in the pathogenesis of BCR-ABL1-induced leukemogenesis is still controversial. Some research suggests that activation of JNK pathway promotes pro-apoptotic signals in BCR–ABL1-expressing cells in response to various agents, such as arsenic trioxide and ceramide. Constitutive BCR–ABL1 kinase activity suppresses JNK activation while TKIs like imatinib restore phosphorylation of JNK, thus driving cells toward apoptosis [43]. However, others have reported that the activation of JNK pathway is essential for BCR–ABL-induced leukemogenesis, and that inhibition of JNK prevents BCR–ABL1-mediated transformation in vitro [44]. Furthermore, induction of autophagy in CML by resveratrol treatment involves JNK-dependent accumulation of p62, as JNK inhibition or p62 knockdown reversed resveratrol-mediated autophagy and antileukemic effects [37]. Together, these data suggest that JNK pathway promotes diverse, and possible opposing, effects in BCR-ABL1 leukomogenesis and its exact role, if any, remains to be clarified.
JAK/STAT pathway
Constitutive activation of human STAT5 has been found in many hematologic malignancies as well as in BCR-ABL1–expressing myeloid and lymphoid leukemia cells [45]. Constitutively active mutants of STAT5a induce MPN-like leukemia in primary mouse hematopoietic cells [46]. Furthermore, inhibition of STAT5 by dominant-negative mutants impairs the survival of BCR-ABL1–expressing cell lines [47], whereas siRNA against STAT5 inhibits myeloid colony formation by primary CML progenitors [48]. More recently, genetic studies have shown that loss of STAT5 abolishes the CML-like leukemia induced in mice by BCR-ABL1, validating STAT5a/b and the genes they activate as targets for therapy in CML [49, 50].
The role of JAK kinases in CML pathogenesis is less clear despite extensive study. JAK kinases, including JAK2, are clearly activated in BCR-ABL1-expressing cells but JAK2 is not the kinase responsible for STAT5 activation [45, 51]. JAK2 complexes with BCR-ABL1 via the ABL1 C-terminus and contributes to activation of the SRC kinase LYN [52, 53], but neither the JAK2 binding site on BCR-ABL1 [54] nor JAK2 itself [55] are required for induction of CML-like leukemia in mice by BCR-ABL1. Although JAK2 kinase inhibitors can decrease the proliferation and survival of cultured BCR-ABL1-expressing cells [52], JAK2-deficient progenitors are equally sensitive to these drugs [55], indicating that targets other than JAK2 are responsible. However, a role for JAK2 in maintenance of CML LSC has not been excluded, and JAK2 signaling from the BM microenvironment might contribute to resistance to TKI therapy in CML [56]. Hence, further investigation of JAK2 inhibitors in CML therapy is warranted.
Hedgehog signaling
The Hedgehog (Hh) pathway plays a critical role in the self-renewal of somatic stem cells and controls response to stress, injury, healing and regeneration [57]. Binding of Hh ligands to their receptor, Patched (PTCH), releases its inhibitory effect on Smoothened (SMO), resulting in SMO activation. SMO activation promotes the nuclear translocation of the GLI family of transcription factors (GLI1, 2, 3). The GLI family controls cell proliferation and survival through controlling the expression of genes such as Cyclin D, c-MYC and BCL2 [58].
The role of Hh pathway in CML (reviewed in [59]) was first investigated by Dierks et al. [60] who reported an increase expression of SMO, as well as the downstream targets GLI1 and PTCH1, in progenitors from mice with BCR-ABL1-induced CML-like MPN and in CML patient cells, the latter in the CML stem cell compartment as well as in BCR-ABL1+ cells in both chronic phase and the blast crisis. Subsequent studies in the retroviral mouse model of CML, using donor cells deficient in Smo and the SMO antagonist cyclopamine, showed that Smo deficiency attenuated BCR-ABL1-induced CML-like leukemia and decreased the efficiency of secondary transplantation of the disease, while cyclopamine treatment also caused significant prolongation of survival, reduction in CML stem cells and reduction of disease onset in secondary transplant recipients. Complementary to these results, Zhang et al. reported that the small-molecule SMO antagonist LDE225 (Novartis) caused a significant reduction in secondary colony formation and replating efficacy in primary CML cells in vitro and improved survival after drug discontinuation in mice [61]. Preliminary clinical results with one SMO antagonist (P-04449913, Pfizer) suggest significant activity against CML and several other myeloid neoplasms [62].
WNT/β-catenin
The WNT/β-catenin pathway was first connected to CML by a seminal study that discovered aberrant constitutive nuclear β-catenin in granulocyte-macrophage progenitors (GMP) in patients with CML myeloid blast crisis [63]. Normally restricted to the HSC compartment, it was hypothesized that nuclear β-catenin was associated with abnormal self-renewal in the malignant GMPs. WNT was further implicated in CML disease progression by gene expression analysis that showed increased expression of several WNT target genes, including c-MYC, cadherin, ROK13A, MDI1, prickle 1, and FZD2, in accelerated phase and blast crisis [64]. Subsequent studies in the mouse retroviral CML model demonstrated that deletion of β-catenin impaired development of CML-like MPN induced by BCR-ABL1 [65, 66], and a recent study suggested that the requirement for β-catenin is selective to CML stem cells vs normal HSC, representing a valid pharmacologic target in this disease [67].
Philadelphia-negative myeloproliferative neoplasms
The Ph-negative myeloproliferative neoplasms (MPNs) including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (MF), are prevalent blood disorders that significantly impact the health, quality of life, and overall survival of patients [15]. MPNs are clonal disorders involving the multipotent hematopoietic stem cell and characterized by increased proliferation of one or more of the myeloid, erythroid, or megakaryocytic lineages, variable progression to acute leukemia, and abnormalities of hemostasis and thrombosis [68]. In 2005, the discovery of a somatic mutation in the JAK2 tyrosine kinase in most patients with Ph-negative MPN [8-11, 69] offered a unifying hypothesis about the pathogenesis of MPNs as resulting from dysregulated tyrosine kinase (TK) signaling [70]. While there is abundant evidence that mutations in other genes, including epigenetic regulators such as TET2 and ASXL1, contribute to the clinical course of MPN [71] and perhaps predisposition [72] to these diseases, it is clear that TK signaling is a central and common feature of MPNs that can be mined for potential targets for therapy.
JAK2 and MPL
JAK2 is a key regulator of signaling from many cytokine receptors in the myeloid lineages such as erythropoietin (EPO) receptor, thrombopoietin (TPO) receptor and G-CSF receptor. The JAK2V617F mutation occurs in exon 14 and abrogates the inhibitory effect of the pseudokinase domain, causing constitutively active JAK2. Although the pseudokinase domain was presumed to be catalytically inactive and function as a negative regulator, recent studies have shown that the pseudokinase domain has a dual Ser/Tyr kinase activity that modulates the basal activity and signaling of JAK2 through autophosphorylation [73].
JAK2V617F is detected in more than 95% of patients with PV, 50%-70% with ET, and 40%-50% with MF, as well as in some cases of atypical MPN [74]. Subsequently, another mutation was detected in exon 12 of JAK2 in some of the JAK2V617F-negative PV patients [75]. Several mutations of the thrombopoietin receptor (MPL) have also been detected in 6-7% of patients with JAK2V617F-negative ET or MF, resulting in the substitution of Trp 515 to Ala, Asp, Leu, or Lys that causes constitutive activation of MPL signaling [76].
Mouse models of Ph-negative MPN
As they have done with CML, mouse models of the Ph-negative MPNs have yielded important insights into the signaling mechanisms underlying these diseases. Shortly after the discovery of the JAK2V617F mutation, several groups expressed this mutant kinase in mouse hematopoietic stem cells using the retroviral BM transduction/transplantation approach [77-80]. Expression of JAK2V617F, but not JAK2WT, induced non-fatal polycythemia characterized by increased hematocrit and hemoglobin, reticulocytosis, splenomegaly, low plasma erythropoietin (Epo), and Epo-independent erythroid colonies. JAK2V617F also induced leukocytosis and neutrophilia that was much more prominent in Balb/c mice than in B6 [77, 79]. Platelet counts were not affected in either strain despite expression of JAK2V617F in megakaryocytes and markedly prolonged tail bleeding times [79]. The polycythemia tended to resolve after several months and could progress to frank anemia, coincident with increased spleen and marrow fibrosis resembling postpolycythemia MF. These findings demonstrated that JAK2V617F induces EPO-independent expansion of the erythroid lineage in vivo, and the fact that the central erythroid features of PV are recapitulated by expression of JAK2V617F argued that it is the primary and direct cause of human PV. Similar studies also demonstrated that retroviral expression of the mutant MPL receptor in BM of Balb/c mice caused a fulminant and rapidly fatal MPN characterized by marked leukocytosis and thrombocytosis, hepatosplenomegaly, and marrow fibrosis [76, 81].
Following the development of the retroviral JAK2 models of MPN, several groups reported mouse models wherein JAK2V617F was expressed from a chromosomal transgene. These fell into two general classes, those where JAK2 is expressed from an exogenous promoter, and those where JAK2 is expressed from the native mouse Jak2 gene promoter (“knock-in” models). Currently, there are there three published traditional transgenic strains [82-84] and four knock-in strains [85-88]. All of these strains exhibit some clinicopathological features of MPN, but there are numerous differences between the individual models that collectively make it difficult to draw definitive conclusions about some aspects of MPN pathogenesis (for an excellent review, see [89]). Some important pathogenetic information has certainly emerged from these JAK2V617F transgenic models. For example, several models support the hypothesis that the ratio of expression of the mutant JAK2 kinase to endogenous JAK2 determines whether the phenotype will be predominantly one of polycythemia (JAK2V617F ≥ endogenous JAK2) or thrombocythemia (JAK2V617F < endogenous JAK2) [82, 87], which fits with the clinical observation of frequent homozygosity of the JAK2V617F mutation in PV but not ET [90, 91]. In addition, studies in one of the JAK2V617F knock-in models confirm that the disease-initiating cell population in PV is the phenotypic HSC [88]. However, there are also many discrepancies between the various models. One knock-in model demonstrated that expression of JAK2V617F has a negative effect on self-renewal and repopulation by HSCs[87], another suggested a positive effect [85], while a third found no effect on HSC frequency or function [88] (of note, a recent study of human MPN patients found no effect of JAK2 mutations on the size or in vitro function of HSCs [92]). Furthermore, two knock-in strains exhibited progressive development of MF [85, 86], another developed MF with very low penetrance [87], while a fourth strain did not develop MF at all [88]. The reasons for these differences are not clear, but may include variable use of human or mouse JAK2.
Downstream pathways: STAT5 and TGF-β
Mouse models have been used to better define the signaling pathways downstream of JAK2 in the Ph-negative MPNs. For JAK2V617F, genetic deletion of Stat5a/b in the mouse retroviral model reversed the polycythemia phenotype in recipient mice, with normalization of hematocrit, reticulocytes, and spleen size. However, unlike with BCR-ABL1, where loss of Stat5 normalized the histology of BM, liver, and spleen, Stat5 deletion did not eliminate completely myelofibrosis of the BM or infiltration of the liver/spleen with myeloerythroid progenitors in JAK2V617F recipients. Hence, STAT5-independent signaling pathways contribute to the pathogenesis of MPN and MF associated with mutant JAK2. By contrast, Mohi et al. found no residual MF at 6 months following Stat5 deletion in a knock-in transgenic JAK2V617F model, but these investigators did not study JAK2V617F homozygotes, which may better reflect human PV patients [93].
In human MF, the fibroblasts are polyclonal, do not carry the JAK2V617F mutation, and are not part of the malignant clone [94]. However, considerable evidence suggests that one or more cytokines secreted by the clonal megakaryocytes are the cause of fibroblast proliferation and fibrosis in MF, including PDGF, FGF, and TGF-β. Human MF patients have consistent increases in TGF-β1 in their sera, CD34+ cells, and megakaryocytes [95]. Overexpression of TPO in mouse BM through retroviral transduction and transplantation induces fatal MF [96, 97] with increased plasma levels of TGF-β1 and PDGF [98], whereas donor cells with mutations in the Tgfb1 gene do not cause MF following overexpression of TPO [99]. These results suggest a possible role for TGF-β1 in the pathogenesis of MF.
Lessons from JAK2 inhibitors
There is great interest in whether molecularly targeted drugs aimed at the JAK2 signaling pathway may be effective treatments for MPN and MF. Several groups have reported preclinical testing of potent and relatively selective JAK2 inhibitors in mouse models of Ph-negative MPN. Three structurally different JAK2 inhibitors, CYT387 [100], TG101348 (now called SAR302503) [88], and AZD1480 [101] all reversed completely the polycythemia phenotype induced by JAK2V617F, with normalization of leukocyte count and hematocrit, reduction in splenomegaly, and substantial restoration of normal splenic and hepatic histology. However, JAK2 inhibitor treatment failed to impact several clinicopathological features of the MPN induced by JAK2V617F, as there was no significant decrease in overall extent of marrow fibrosis, in the percentage of GFP+ cells in blood, spleen and BM, and EPO-independent endogenous erythroid colonies were detected at similar frequency to vehicle-treated mice. Early clinical experience with the sole FDA-approved JAK2 inhibitor ruxolitinib (INCB018424) in patients with MF confirm that this drug is relatively ineffective at reducing MF or JAK2V617F allele burden [17]. Ruxolitinib was shown to decrease MF in the mouse retroviral model of MPN induced by MPLW515, but this was a short-term study of 2 weeks of treatment beginning 2 weeks post-transplant [102], and it is likely that the results reflect prevention of MF rather than reversal of established MF.
One of the central questions in contemporary MPN research is why JAK2 inhibitors are so different from ABL1 inhibitors, which are very effective at decreasing the BCR-ABL1 allele burden in CML patients and in mouse models. Levine and colleagues recently described heterodimeric JAK1-JAK2 and TYK2-JAK2 complexes in cell lines selected for cross-resistance to multiple JAK2 inhibitors and in granulocytes from patients treated chronically with ruxolitinib on clinical trials [103]. For unclear reasons, although ruxolitinib is a potent JAK1 inhibitor, inhibition of the JAK1-JAK2 complex by ruxolitinib in vitro was inefficient, and the authors proposed that such heterodimeric JAK complexes may contribute to persistence of JAK2V617F–positive cells in treated patients. Although interesting, several features of this model are difficult to reconcile. First, it is clear that JAK2 inhibitors dramatically reduce the total burden of JAK2V617F–positive cells in treated patients, as the spleens are returned to near normal size, but some physiologic response prevents further specific reduction in the malignant cells with continued treatment. Calling this persistence may beg the question, as the production of JAK2V617F–positive neutrophils in ruxolitinib-treated patients is nearly normal and represents an enormous and ongoing proliferation of malignant cells in the marrow. Second, it is unclear what stimulus triggers formation of the active heterodimeric JAK complexes, as Levine et al. observed no difference in total JAK1-JAK2 heterodimers in treated patients, only an increase of phospho-JAK2 in the complex [103]. Hence, addition investigation into the molecular mechanisms regulating the formation, catalytic activity, and inhibitor sensitivity of heterodimeric JAK complexes is needed.
Chronic lymphocytic leukemia
Chronic lymphocytic leukemia (CLL) is the most common lymphoid malignancy in adults over the age of 65 and represents 11% of all blood cancer [104]. Conventional chemoimmunotherapy, such as the combination of fludarabine, cyclophosphamide, and rituximab, is often effective at eliminating CLL cells from the peripheral blood. However, CLL cells in lymph nodes and bone marrow are usually resistant to such treatment due to signaling interactions with the lymph node microenvironment [105], which makes CLL incurable with chemotherapy [106]. This has focused much recent attention on the signaling pathways that mediate survival in CLL.
BCR signaling
B cell receptor (BCR) signaling pathway plays a crucial role in the pathogenesis of CLL [107]. In a normal B cell, the engagement of the BCR by antigen triggers a signaling pathway controlling proliferation, differentiation and antibody production. Activated BCR recruits kinases such as spleen tyrosine kinase (SYK) and the SRC kinase LYN that phosphorylate ITAM motifs on the cytoplasmic domains of the Ig co-receptors CD79a and CD79b. Such phosphorylation recruits and activates Bruton’s tyrosine kinase (BTK) and phosphoinositide 3-kinase (PI3K), subsequently activating many downstream targets including AKT/mTOR, NF-κB, and ERK. Interestingly, BCR activation can occur in a ligand-independent manner. The latter mechanism is especially important, as it is thought to be involved in B cell malignancies including CLL [108]. The major mouse model of dysregulated BCR signaling in CLL is the Emu-TCL1 transgenic mouse strain, which spontaneously develop clonal mature B-cell malignancies resembling CLL [109]. Based on better understanding of the molecular basis of BCR signaling pathways in CLL, extensive effort has been focused on targeting different components of these pathways [110]. Of particular interest are strategies targeting both the malignant CLL cell and the lymph node microenvironment [105].
SYK/BTK/PI3Kδ: from BCR signaling to the clinic
SYK is essential to normal B cell development, as lack of SYK induces severe B cell lymphopenia in mice [111]. SYK contributes to the initiation and amplification of BCR signaling upon BCR activation by antigen binding and also during ligand-independent activation [112]. In vitro and in vivo studies showed that SYK inhibition impaired BCR signaling, which decreased CLL cell migration, ERK activation and chemokine secretion [113]. These findings strongly implicate SYK as a valid target for therapy in B cell malignancies. A SYK inhibitor, fostamatinib, demonstrated significant activity against CLL in a phase I trial [114]. Secondgeneration, more selective SYK inhibitors have shown potent effects on CLL cell survival and migration in preclinical studies [115].
Bruton’s tyrosine kinase (BTK) was discovered as the product of a gene mutated in human X-linked agammaglobulinemia and in XID immunodeficient mice, both of which exhibit defects in B-cell development and signaling at the pro-B cell level. BTK is activated downstream of SYK/LYN following BCR stimulation, and in turn leads to activation of the PI3K/AKT and NF-κB pathways. The first clinical inhibitor of BTK is ibrutinib (formerly PCI-32765), a novel irreversible inhibitor of BTK that forms a covalent bond with a cysteine residue in the BTK catalytic domain [116]. Preclinical studies with ibrutinib showed inhibition of BTK phosphorylation and downstream pathways in CLL cells, concomitant with decreased proliferation, increased apoptosis, and blockade of stromal-mediated pro-survival signals [117, 118]. In a phase Ib/II clinical trial, ibrutinib was well-tolerated and showed clinical responses in the majority of CLL patients treated, including those with mutant p53 status [119].
Downstream of BTK, PI3K is critical for BCR signaling in both normal B cells and CLL cells [120]. The predominant PI3K catalytic p110 subunit isoform in hematopoietic cells and lymphocytes is the delta (δ) isoform. Mice lacking p110 have normal hematopoiesis except for the B-lymphoid lineage, where there is a reduction both in mature B cells and in BCR signaling [121]. CAL-101 (now GS-1101) is a reversible small molecule inhibitor of PI3Kδ [122]. It showed a potent ability to kill CLL cells in vitro while inhibiting chemokine-mediated chemotaxis and migration [123, 124]. In phase I trials, GS-1101 induced lymph node responses in 84% of CLL patients, independent of high-risk genetic features such as 11q deletions or p53 mutation [125]. Interestingly, both GS-1101 and ibrutinib appear to mobilize CLL cells from the lymphatic niches to the circulation, resulting in significant and sustained leukocytosis that probably reflects disruption of signaling that mediates adhesion of CLL cells to the microenvironment, including the CXCL12-CXCR4 axis.
Conclusions and future directions
The chronic leukemias have served as a paradigm for targeted therapies in cancer, as a better understanding of the cellular signaling pathways governing proliferation, survival, and selfrenewal has led directly to molecularly targeted drugs that have dramatically impacted the natural history of the disease. In CML, the current interest is focused on preventing disease progression in the vulnerable first year on ABL1 TKI therapy, and on strategies to eliminate CML stem cells and allow TKI discontinuation without relapse. In the Ph-negative MPNs, the challenge is to expand the clinical responses to JAK2 inhibitors to alleviate more symptoms and clinical features of these diseases, including MF, cytopenias, elimination of the malignant clone, normalization of hemostasis and thrombosis, and prolongation of survival. In CLL, the excitement generated by the early clinical results with BCR signaling inhibitors is tempered by the realization that these therapies alone will probably not cure patients. Collectively, these clinical needs should stimulate a renaissance of research into signaling in the chronic myeloid and lymphoid leukemias, where cell biological and mouse modeling studies can be expected to yield new approaches to treatment.
Acknowledgements
Supported in part by NIH grants T32 CA009429 (W.A.) and R01 HL089747 (R.A.V.).
Footnotes
Disclosure: W. Ahmed received grant from T32 CA009429; R. Van Etten received grant from R01 HL089747.
References
- 1.Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112:4808–4817. doi: 10.1182/blood-2008-07-077958. [DOI] [PubMed] [Google Scholar]
- 2.Hochhaus A, O’Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 3.Gambacorti-Passerini C, Antolini L, Mahon FX, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103:553–561. doi: 10.1093/jnci/djr060. [DOI] [PubMed] [Google Scholar]
- 4.Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 5.Okimoto RA, Van Etten RA. Navigating the road toward optimal initial therapy for chronic myeloid leukemia. Curr Opin Hematol. 2011;18:89–97. doi: 10.1097/MOH.0b013e32834399a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham SM, Jorgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 7.Corbin AS, Agarwal A, Loriaux M, et al. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 9.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 11.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 12.Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 13.Griffin JH, Leung J, Bruner RJ, et al. Discovery of a fusion kinase in EOL-1 cells and idiopathic hypereosinophilic syndrome. Proc Natl Acad Sci USA. 2003;100:7830–7835. doi: 10.1073/pnas.0932698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golub TR, Barker GF, Lovett M, et al. Fusion of the PDGF receptor b to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994;77:307–316. doi: 10.1016/0092-8674(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 15.Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355:2452–2466. doi: 10.1056/NEJMra063728. [DOI] [PubMed] [Google Scholar]
- 16.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tefferi A. Challenges facing JAK inhibitor therapy for myeloproliferative neoplasms. N Engl J Med. 2012;366:844–846. doi: 10.1056/NEJMe1115119. [DOI] [PubMed] [Google Scholar]
- 19.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 20.McWhirter JR, Galasso DL, Wang JYJ. A coiled-coil oligomerization domain of Bcr is essential for the transforming function of Bcr-Abl oncoproteins. Mol Cell Biol. 1993;13:7587–7595. doi: 10.1128/mcb.13.12.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith KM, Yacobi R, Van Etten RA. Autoinhibition of Bcr-Abl through its SH3 domain. Mol Cell. 2003;12:27–37. doi: 10.1016/S1097-2765(03)00274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pendergast AM, Quilliam LA, Cripe LD, et al. BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell. 1993;75:175–185. [PubMed] [Google Scholar]
- 23.Sattler M, Mohi MG, Pride YB, et al. Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell. 2002;1:479–492. doi: 10.1016/s1535-6108(02)00074-0. [DOI] [PubMed] [Google Scholar]
- 24.Wohrle FU, Halbach S, Aumann K, et al. Gab2 signaling in chronic myeloid leukemia cells confers resistance to multiple Bcr-Abl inhibitors. Leukemia. 2012 doi: 10.1038/leu.2012.222. [DOI] [PubMed] [Google Scholar]
- 25.Ghaffari S, Jagani Z, Kitidis C, et al. Cytokines and BCR-ABL mediate suppression of TRAIL-induced apoptosis through inhibition of forkhead FOXO3a transcription factor. Proc Natl Acad Sci USA. 2003;100:6523–6528. doi: 10.1073/pnas.0731871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jagani Z, Singh A, Khosravi-Far R. FoxO tumor suppressors and BCR-ABL-induced leukemia: a matter of evasion of apoptosis. Biochim Biophys Acta. 2008;1785:63–84. doi: 10.1016/j.bbcan.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naka K, Hoshii T, Muraguchi T, et al. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–680. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- 28.Pellicano F, Cilloni D, Helgason GV, et al. FOXO transcription factor activity is partially retained in quiescent CML stem cells and induced by tyrosine kinase inhibitors in CML progenitor cells. Blood. 2009 doi: 10.1182/blood-2009-06-226621. [DOI] [PubMed] [Google Scholar]
- 29.Hurtz C, Hatzi K, Cerchietti L, et al. BCL6-mediated repression of p53 is critical for leukemia stem cell survival in chronic myeloid leukemia. J Exp Med. 2011;208:2163–2174. doi: 10.1084/jem.20110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayerhofer M, Aichberger KJ, Florian S, et al. Identification of mTOR as a novel bifunctional target in chronic myeloid leukemia: dissection of growth-inhibitory and VEGF-suppressive effects of rapamycin in leukemic cells. FASEB J. 2005;19:960–962. doi: 10.1096/fj.04-1973fje. [DOI] [PubMed] [Google Scholar]
- 31.Mohi MG, Boulton C, Gu TL, et al. Combination of rapamycin and protein tyrosine kinase (PTK) inhibitors for the treatment of leukemias caused by oncogenic PTKs. Proc Natl Acad Sci USA. 2004;101:3130–3135. doi: 10.1073/pnas.0400063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janes MR, Limon JJ, So L, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–213. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carayol N, Vakana E, Sassano A, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci USA. 2010;107:12469–12474. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vakana E, Platanias LC. AMPK in BCR-ABL expressing leukemias. Regulatory effects and therapeutic implications. Oncotarget. 2011;2:1322–1328. doi: 10.18632/oncotarget.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoki K, Li Y, Xu T, et al. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puissant A, Robert G, Fenouille N, et al. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70:1042–1052. doi: 10.1158/0008-5472.CAN-09-3537. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Wang L, Li L, et al. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012;21:266–281. doi: 10.1016/j.ccr.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan H, Wang Z, Li L, et al. Activation of stress response gene SIRT1 by BCR-ABL promotes leukemogenesis. Blood. 2012;119:1904–1914. doi: 10.1182/blood-2011-06-361691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puil L, Liu J, Gish G, et al. Bcr-Abl oncoproteins bind directly to activators of the Ras signalling pathway. EMBO J. 1994;13:764–773. doi: 10.1002/j.1460-2075.1994.tb06319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- 42.Pellicano F, Simara P, Sinclair A, et al. The MEK inhibitor PD184352 enhances BMS-214662-induced apoptosis in CD34+ CML stem/progenitor cells. Leukemia. 2011;25:1159–1167. doi: 10.1038/leu.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancini M, Veljkovic N, Corradi V, et al. 14-3-3 ligand prevents nuclear import of c-ABL protein in chronic myeloid leukemia. Traffic (Copenhagen, Denmark) 2009;10:637–647. doi: 10.1111/j.1600-0854.2009.00897.x. [DOI] [PubMed] [Google Scholar]
- 44.Hess P, Pihan G, Sawyers CL, et al. Survival signaling mediated by c-Jun NH(2)-terminal kinase in transformed B lymphoblasts. Nat Genet. 2002;32:201–205. doi: 10.1038/ng946. [DOI] [PubMed] [Google Scholar]
- 45.Ilaria RL, Van Etten RA. P210 and P190BCR/ABL induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem. 1996;271:31704–31710. doi: 10.1074/jbc.271.49.31704. [DOI] [PubMed] [Google Scholar]
- 46.Moriggl R, Sexl V, Kenner L, et al. Stat5 tetramer formation is associated with leukemogenesis. Cancer Cell. 2005;7:87–99. doi: 10.1016/j.ccr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Sillaber C, Gesbert F, Frank DA, et al. STAT5 activation contributes to growth and viability in Bcr/Abl-transformed cells. Blood. 2000;95:2118–2125. [PubMed] [Google Scholar]
- 48.Scherr M, Chaturvedi A, Battmer K, et al. Enhanced sensitivity to inhibition of SHP2, STAT5, and Gab2 expression in chronic myeloid leukemia (CML) Blood. 2006;107:3279–3287. doi: 10.1182/blood-2005-08-3087. [DOI] [PubMed] [Google Scholar]
- 49.Hoelbl A, Schuster C, Kovacic B, et al. Stat5 is indispensable for the maintenance of bcr/abl-positive leukaemia. EMBO Mol Med. 2010;2:98–110. doi: 10.1002/emmm.201000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walz C, Ahmed W, Lazarides K, et al. Essential role for Stat5a/b in myeloproliferative neoplasms induced by BCR-ABL1 and Jak2V617F in mice. Blood. 2012;119:3550–3560. doi: 10.1182/blood-2011-12-397554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie S, Wang Y, Liu J, et al. Involvement of Jak2 tyrosine phosphorylation in Bcr-Abl transformation. Oncogene. 2001;20:6188–6195. doi: 10.1038/sj.onc.1204834. [DOI] [PubMed] [Google Scholar]
- 52.Samanta AK, Lin H, Sun T, et al. Janus kinase 2: a critical target in chronic myelogenous leukemia. Cancer Res. 2006;66:6468–6472. doi: 10.1158/0008-5472.CAN-06-0025. [DOI] [PubMed] [Google Scholar]
- 53.Samanta A, Perazzona B, Chakraborty S, et al. Janus kinase 2 regulates Bcr-Abl signaling in chronic myeloid leukemia. Leukemia. 2011;25:463–472. doi: 10.1038/leu.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wertheim JA, Perera SA, Hammer DA, et al. Localization of BCR-ABL to F-actin regulates cell adhesion but does not attenuate CML development. Blood. 2003;102:2220–2228. doi: 10.1182/blood-2003-01-0062. [DOI] [PubMed] [Google Scholar]
- 55.Hantschel O, Warsch W, Eckelhart E, et al. BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia. Nat Chem Biol. 2012;8:285–293. doi: 10.1038/nchembio.775. [DOI] [PubMed] [Google Scholar]
- 56.Traer E, Mackenzie R, Snead J, et al. Blockade of JAK2-mediated extrinsic survival signals restores sensitivity of CML cells to ABL inhibitors. Leukemia. 2011 doi: 10.1038/leu.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beachy PA, Karhadkar SS, Berman DM. Mending and malignancy. Nature. 2004;431:402. doi: 10.1038/431402a. [DOI] [PubMed] [Google Scholar]
- 58.Regl G, Kasper M, Schnidar H, et al. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004;64:7724–7731. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- 59.Jagani Z, Dorsch M, Warmuth M. Hedgehog pathway activation in chronic myeloid leukemia. Cell Cycle. 2010;9:3449–3456. doi: 10.4161/cc.9.17.12945. [DOI] [PubMed] [Google Scholar]
- 60.Dierks C, Beigi R, Guo GR, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Zhang B, Irvine D, Ho YW, et al. Inhibition of chronic myeloid leukemia stem cells by the combination of the Hedgehog pathway inhibitor LDE225 with nilotinib. Blood. 2010;116:514a. [Google Scholar]
- 62.Shih A, Schairer A, Barrett CL, et al. Cycling Toward Leukemia Stem Cell Elimination Wtih a Selective Sonic Hedgehog Antagonist. Blood. 2011;118:3776a. [Google Scholar]
- 63.Jamieson CHM, Ailles LE, Dylla SJ, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 64.Radich JP, Dai H, Mao M, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci USA. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao C, Blum J, Chen A, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu Y, Chen Y, Douglas L, et al. beta-Catenin is essential for survival of leukemic stem cells insensitive to kinase inhibition in mice with BCR-ABL-induced chronic myeloid leukemia. Leukemia. 2009;23:109–116. doi: 10.1038/leu.2008.262. [DOI] [PubMed] [Google Scholar]
- 67.Heidel FH, Bullinger L, Feng Z, et al. Genetic and pharmacologic inhibition of beta-catenin targets imatinib-resistant leukemia stem cells in CML. Cell stem cell. 2012;10:412–424. doi: 10.1016/j.stem.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Etten RA, Shannon KM. Focus on myeloproliferative diseases and myelodysplastic syndromes. Cancer Cell. 2004;6:547–552. doi: 10.1016/j.ccr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Zhao R, Xing S, Li Z, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112:2190–2198. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abdel-Wahab O, Pardanani A, Bernard OA, et al. Unraveling the genetic underpinnings of myeloproliferative neoplasms and understanding their effect on disease course and response to therapy: Proceedings from the 6th international post-ASH symposium. Am J Hematol. 2012;87:562–568. doi: 10.1002/ajh.23169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Busque L, Patel JP, Figueroa ME, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44:1179–1181. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ungureanu D, Wu J, Pekkala T, et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat Struct Mol Biol. 2011;18:971–976. doi: 10.1038/nsmb.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones AV, Kreil S, Zoi K, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–2168. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 75.Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pikman Y, Lee BH, Mercher T, et al. MPLW515L Is a Novel Somatic Activating Mutation in Myelofibrosis with Myeloid Metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wernig G, Mercher T, Okabe R, et al. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–4281. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lacout C, Pisani DF, Tulliez M, et al. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108:1652–1660. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 79.Zaleskas VM, Krause DS, Lazarides K, et al. Molecular Pathogenesis and Therapy of Polycythemia Induced in Mice by JAK2 V617F. PLoS ONE. 2006;1:e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bumm TG, Elsea C, Corbin AS, et al. Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 2006;66:11156–11165. doi: 10.1158/0008-5472.CAN-06-2210. [DOI] [PubMed] [Google Scholar]
- 81.Pecquet C, Staerk J, Chaligne R, et al. Induction of myeloproliferative disorder and myelofibrosis by thrombopoietin receptor W515 mutants is mediated by cytosolic tyrosine 112 of the receptor. Blood. 2010;115:1037–1048. doi: 10.1182/blood-2008-10-183558. [DOI] [PubMed] [Google Scholar]
- 82.Tiedt R, Hao-Shen H, Looser R, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–3940. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- 83.Xing S, Wanting TH, Zhao W, et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111:5109–5117. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shide K, Shimoda HK, Kumano T, et al. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia. 2008;22:87–95. doi: 10.1038/sj.leu.2405043. [DOI] [PubMed] [Google Scholar]
- 85.Akada H, Yan D, Zou H, et al. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood. 2010;115:3589–3597. doi: 10.1182/blood-2009-04-215848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marty C, Lacout C, Martin A, et al. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood. 2010;116:783–787. doi: 10.1182/blood-2009-12-257063. [DOI] [PubMed] [Google Scholar]
- 87.Li J, Spensberger D, Ahn JS, et al. JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood. 2010;116:1528–1538. doi: 10.1182/blood-2009-12-259747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mullally A, Lane SW, Ball B, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010;17:584–596. doi: 10.1016/j.ccr.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li J, Kent DG, Chen E, et al. Mouse models of myeloproliferative neoplasms: JAK of all grades. Dis Model Mech. 2011;4:311–317. doi: 10.1242/dmm.006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scott LM, Scott MA, Campbell PJ, et al. Progenitors homozygous for the V617F mutation occur in most patients with polycythemia vera, but not essential thrombocythemia. Blood. 2006;108:2435–2437. doi: 10.1182/blood-2006-04-018259. [DOI] [PubMed] [Google Scholar]
- 91.Godfrey AL, Chen E, Pagano F, et al. JAK2V617F homozygosity arises commonly and recurrently in PV and ET, but PV is characterized by expansion of a dominant homozygous subclone. Blood. 2012;120:2704–2707. doi: 10.1182/blood-2012-05-431791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anand S, Stedham F, Beer P, et al. Effects of the JAK2 mutation on the hematopoietic stem and progenitor compartment in human myeloproliferative neoplasms. Blood. 2011;118:177–181. doi: 10.1182/blood-2010-12-327593. [DOI] [PubMed] [Google Scholar]
- 93.Yan D, Hutchison RE, Mohi G. Critical requirement for Stat5 in a mouse model of polycythemia vera. Blood. 2012;119:3539–3549. doi: 10.1182/blood-2011-03-345215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reilly JT. Idiopathic myelofibrosis: pathogenesis to treatment. Hematol Oncol Clin North Am. 2006;24:56–63. doi: 10.1002/hon.771. [DOI] [PubMed] [Google Scholar]
- 95.Martyre MC, Romquin N, Le Bousse-Kerdiles MC, et al. Transforming growth factor-beta and megakaryocytes in the pathogenesis of idiopathic myelofibrosis. Br J Haematol. 1994;88:9–16. doi: 10.1111/j.1365-2141.1994.tb04970.x. [DOI] [PubMed] [Google Scholar]
- 96.Yan XQ, Lacey D, Fletcher F, et al. Chronic exposure to retroviral vector encoded MGDF (mpl-ligand) induces lineage-specific growth and differentiation of megakaryocytes in mice. Blood. 1995;86:4025–4033. [PubMed] [Google Scholar]
- 97.Villeval JL, Cohen-Solal K, Tulliez M, et al. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood. 1997;90:4369–4383. [PubMed] [Google Scholar]
- 98.Yan XQ, Lacey D, Hill D, et al. A model of myelofibrosis and osteosclerosis in mice induced by overexpressing thrombopoietin (mpl ligand): reversal of disease by bone marrow transplantation. Blood. 1996;88:402–409. [PubMed] [Google Scholar]
- 99.Chagraoui H, Komura E, Tulliez M, et al. Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood. 2002;100:3495–3503. doi: 10.1182/blood-2002-04-1133. [DOI] [PubMed] [Google Scholar]
- 100.Tyner JW, Bumm TG, Deininger J, et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood. 2010;115:5232–5240. doi: 10.1182/blood-2009-05-223727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zaleskas VM, Chan WW, Evangelista P, et al. A Selective and Potent Oral Inhibitor of the JAK2 Tyrosine Kinase Reverses Polycythemia and Leukocytosis Induced by JAK2 V617F in a Mouse Model. Blood. 2007;110:557a. [Google Scholar]
- 102.Koppikar P, Abdel-Wahab O, Hedvat C, et al. Efficacy of the JAK2 inhibitor INCB16562 in a murine model of MPLW515L-induced thrombocytosis and myelofibrosis. Blood. 2010;115:2919–2927. doi: 10.1182/blood-2009-04-218842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koppikar P, Bhagwat N, Kilpivaara O, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489:155–159. doi: 10.1038/nature11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 105.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burger JA, Ghia P, Rosenwald A, et al. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–3375. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120:1175–1184. doi: 10.1182/blood-2012-02-362624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duhren-von Minden M, Ubelhart R, Schneider D, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012;489:309–312. doi: 10.1038/nature11309. [DOI] [PubMed] [Google Scholar]
- 109.Bichi R, Shinton SA, Martin ES, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci USA. 2002;99:6955–6960. doi: 10.1073/pnas.102181599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wiestner A. Emerging role of kinase targeted strategies in chronic lymphocytic leukemia. Blood. 2012 doi: 10.1182/blood-2012-05-423194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Turner M, Mee PJ, Costello PS, et al. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 112.Chen L, Monti S, Juszczynski P, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood. 2008;111:2230–2237. doi: 10.1182/blood-2007-07-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Buchner M, Baer C, Prinz G, et al. Spleen tyrosine kinase inhibition prevents chemokine-and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood. 2010;115:4497–4506. doi: 10.1182/blood-2009-07-233692. [DOI] [PubMed] [Google Scholar]
- 114.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoellenriegel J, Coffey GP, Sinha U, et al. Selective, novel spleen tyrosine kinase (Syk) inhibitors suppress chronic lymphocytic leukemia B-cell activation and migration. Leukemia. 2012;26:1576–1583. doi: 10.1038/leu.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.O’Brien S, Burger J, Blum KA, et al. The Bruton’s Tyrosine Kinase (BTK) Inhibitor PCI-32765 Induces Durable Responses in Relapsed or Refractory (R/R) Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL): Follow-up of a Phase Ib/II Study. Blood. 2011;118:983a. [Google Scholar]
- 120.Ramadani F, Bolland DJ, Garcon F, et al. The PI3K isoforms p110alpha and p110delta are essential for pre-B cell receptor signaling and B cell development. Sci Signal. 2010;3:ra60. doi: 10.1126/scisignal.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jou ST, Carpino N, Takahashi Y, et al. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol Cell Biol. 2002;22:8580–8591. doi: 10.1128/MCB.22.24.8580-8591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Coutre S, Byrd JC, Furman RR, et al. Phase I study of CAL-101, an isoform-selective inhibitor of phosphatidylinositol 3- kinase P110d, in patients with previously treated chronic lymphocytic leukemia. J Clin Oncol. 2011;29:6631a. [Google Scholar]