Abstract
Gamma-hydroxybutyric acid (GHB; sodium oxybate) is approved for narcolepsy symptom treatment, and it is also abused. This study compared the participant-rated, observer-rated effects, motor/cognitive, physiological, and reinforcing effects of GHB and ethanol in participants with histories of sedative (including alcohol) abuse. Fourteen participants lived on a residential unit for ~1 month. Sessions were conducted Monday through Friday. Measures were taken before, and repeatedly up to 24 hours after drug administration. Participants were administered GHB (1, 2, 4, 6, 8, and 10 g/70kg), ethanol (12, 24, 48, 72, 96, and 120 g/70kg), or placebo in a double-blind, within-subjects design. For safety, GHB and ethanol were administered in an ascending dose sequence, with placebos and both drugs intermixed across sessions. The sequence for each drug was stopped if significant impairment or intolerable effects occurred. Only 9 and 10 participants received the full dose range for GHB and ethanol, respectively. The highest doses of GHB and ethanol showed onset within 30 minutes, with peak effects at 60 minutes. GHB effects dissipated between 4 and 6 hours, while ethanol effects dissipated between 6 and 8 hours. Dose-related effects were observed for both drugs on a variety of measures assessing sedative drug effects, abuse liability, performance impairment, and physiological effects. Within-session measures of abuse liability were similar between the two drugs. However, post-session measures of abuse liability, including a direct preference test between the highest tolerated doses of each drug, suggested somewhat greater abuse liability for GHB, due most likely to the delayed aversive ethanol effects (e.g., headache).
Keywords: Gamma-hydroxybutyric acid, GHB, sodium oxybate, ethanol, alcohol
Gamma-hydroxybutyric acid (GHB; sodium oxybate) is a naturally occurring, biologically active metabolite of the neurotransmitter gamma-aminobutyric acid (GABA), with low affinity and efficacy for GABA-B receptors (Lingenhoehl et al., 1999; Mathivet, Bernasconi, De Barry, Marescaux, & Bittiger, 1997) and high affinity for the GHB receptor (Hechler, Gobaille, & Maitre, 1992). GHB is currently marketed in the U.S. for the treatment of cataplexy associated with narcolepsy and excessive daytime sleepiness associated with narcolepsy. In addition, GHB has been used as a recreational drug. GHB use has been associated with emergency department visits, with over 1,000 per year for the years 2004 – 2009 (Substance Abuse and Mental Health Services Administration, 2011). Epidemiology and case reports show that GHB is used as a recreational drug, with some users meeting DSM-IV criteria for dependence (Craig, Gomez, McManus, & Bania, 2000; Degenhardt, Darke, & Dillon, 2002; Galloway et al., 1997; McDaniel & Miotto, 2001).
Understanding the abuse liability relative to other drugs of abuse is critical given the importance of its medical application and substantial concerns over its abuse. As an example of the complexity in balancing these issues, in the U.S. GHB is controlled on a bifurcated schedule as a controlled substance. Under this framework, GHB is regulated as a schedule I drug, with the exception that the pharmaceutical product Xyrem®, which contains GHB as the active ingredient, is regulated as a schedule III drug. Xyrem® is approved for the treatment of cataplexy associated with narcolepsy and excessive daytime sleepiness associated with narcolepsy.
GHB is reportedly often consumed to increase sociability (Miotto et al., 2001; Stein et al., 2011; Sumnall, Woolfall, Edwards, Cole, & Beynon, 2008) with repeated administrations over the course of an evening to maintain a desired level of effect (Dean, Morgenthaler, & Fowkes, 1997). These use patterns, along with its liquid form and corresponding mode of administration (drinking), are remarkably similar to those of alcohol (i.e., ethanol), the most widely non-medically consumed sedative hypnotic in the US and world. Previous research suggests that the abuse liability of GHB may be somewhat less than barbiturates, and somewhat greater than benzodiazepines. This conclusion is drawn from a laboratory abuse liability study (Carter, Richards, Mintzer, & Griffiths, 2006) and a cross-study multidimensional review of sedative hypnotic abuse liability (Griffiths & Johnson, 2005). Limited information is provided by previous studies comparing GHB to ethanol due to lack of dose effect examination and use of relatively low doses (Abanades et al., 2007; Thai, Dyer, Benowitz, & Haller, 2006). A direct comparison of the abuse liability between GHB and ethanol is relevant because 1) the aforementioned similarities between the use of GHB and ethanol suggest potential drug substitutability, (Bickel, DeGrandpre, & Higgins, 1995; Johnson, Bickel, & Kirshenbaum, 2004), which may explain why some alcohol users may also become GHB users, and also may inform the possible efficacy of GHB in treatment of alcoholism (Addolorato, Leggio, Ferrulli, Caputo, & Gasbarrini, 2009; Caputo, Vignoli, Maremmani, Bernardi, & Zoli, 2009; Gallimberti, Spella, Soncini, & Gessa, 2000); 2) the abuse potential characteristics of alcohol are widely known to both the scientific community and general public, making alcohol a valuable comparator for GHB; 3) GHB and alcohol are often used concurrently, therefore comparison of their relative abuse liabilities at a wide range of doses may inform future work investigating their interactive effects. This laboratory study compared the behavioral, participant-rated, and observer-rated effects of GHB and ethanol under double blind conditions in participants with histories of sedative (including alcohol) abuse. In addition a choice procedure was utilized in which participants were re-administered the highest tolerated dose of both drugs, and choose which they preferred to receive once again on a final session.
Method
Participants
Fourteen (11 male and 3 female) community volunteers participated in this residential research study. Participants were recruited with posted notices and newspaper advertisements. Volunteers were screened by telephone to determine whether they met major inclusion/exclusion criteria, and thus whether they were eligible for an in-person screening session. Participants had a history of recreational nonmedical use of both ethanol and other sedative-hypnotics to the point of intoxication within the last year. Although participants had recent histories of use of these drugs, they were not physically dependent (i.e., showed no withdrawal signs or symptoms) as assessed by observation by nursing staff during the first several days of living on the residential research unit. Other inclusion criteria included being 21–50 years old, being within 20% of their ideal body weight according to Metropolitan Life height-weight tables, and being healthy as determined by screening for medical problems via a personal interview, a medical questionnaire, a physical examination, an electrocardiogram (ECG), and routine medical blood and urinalysis laboratory tests. Exclusion criteria included pregnancy (determined by urinalysis at screening and weekly throughout participation) or breastfeeding for females, a history of hypersensitivity/allergy or other contraindications to alcohol or other sedatives, or a history of current serious medical or psychiatric conditions, including heart disease, lung disease, diabetes, seizure disorders, significant gastrointestinal disturbances, narrow angle glaucoma, sleep apnea, schizophrenia, bipolar disorder, paranoia, multiple personality disorder. Participants were compensated ~$2,500 (85$ per day) for completing this study requiring living on a restricted residential research unit for ~1 month. The study was approved by a Johns Hopkins Medicine Institutional Review Board, and all volunteers signed written informed consent.
Drugs
GHB (Xyrem®, 500 mg/ml GHB solution; Orphan Medical; Minnetonka, MN, currently known as Jazz Pharmaceuticals, Palo Alto, CA), ethanol (ethyl alcohol 95% USP, Warner Graham Co.; Cockeysville, MD), and placebo were delivered in separate sessions using the same vehicle solution, and consumed orally. Sodium citrate solution (389 mg/ml, providing an equimolar concentration of sodium relative to the GHB solution; also provided by Orphan Medical) was used to match sodium content across conditions. GHB drinks contained a 30 ml solution consisting of a combination of GHB solution (at the volume providing the intended dose) and sodium citrate solution, to which 370 ml deionized water and 600 ml of cranberry juice cocktail (Ocean Spray; Lakeville-Middleboro, MA) were added, bringing the total solution volume to 1000 ml. Ethanol drinks contained 30 ml sodium citrate solution, to which 370 ml was added consisting of a combination of ethanol (at the volume providing the intended dose) and deionized water. To this 600 ml of cranberry juice cocktail was added, bringing the total solution volume to 1000 ml. Placebo drinks contained 30 ml sodium citrate solution, 370 ml water, and 600 ml juice cocktail. The total solution was consumed over a targeted 15 min period, although at the higher ethanol sessions some participants took up to 1 h to consume all of the solution due to unpleasant taste.
Procedure
This was a double-blind study, conducted on a 14-bed residential research unit, which compared the behavioral pharmacology of GHB and ethanol. Participants were awoken by 0700 hours and were allowed to smoke cigarettes until drug/placebo dosing at approximately 0930 hours. Participants were maintained on a caffeine-free diet for the duration of the study and were not allowed to eat or drink caloric beverages after midnight before a session. Participants were allowed to smoke cigarettes and eat after 1215 hours or after the drug effect resolved, whichever occurred later. The experimental room contained a hospital bed, a chair, a desk, an Apple Macintosh computer (Apple Computer, Inc., Cupertino, CA), and an automated ECG and blood pressure monitor (Criticare Systems Inc., Waukesha, WI). A crash cart was available in the event of a medical emergency. When not performing experimental tasks, participants were allowed to engage in recreational activities (e.g., watch television or read).
GHB and ethanol were administered in separate sessions at a range of doses in an ascending dose design. Sessions were conducted once per day, and generally took place 5 days per week (Monday through Friday, except holidays). In some cases sessions were postponed if the participant did not feel well, including if there were any adverse effects resulting from the previous session’s drug effect (i.e., hangover). The study was comprised of two phases. Phase 1, which was a maximum of 17 sessions, was an ascending, dose-run-up in which GHB, ethanol, and placebos were given in separate sessions in an intermixed fashion. Phase 2 (3 sessions) utilized a choice procedure to assess the relative reinforcing effects of these two drugs. In both phases, participant-rated, observer-rated, motor/cognitive, drug reinforcement, and physiological measures were assessed.
A single administration of drug (or placebo) solution occurred in each session (see Drugs section). All drugs were administered orally as solutions. In order to reduce the possibility that odor cues were used to discriminate the presence of ethanol across the conditions, participants were required to wear a swimmer’s nose clip during consumption of each of the three drinks. Furthermore, approximately 0.2 ml of 95% ethanol was sprayed into the mouth of each participant immediately after consuming each of the three drinks to obscure the taste and scent of ethanol in the solution across dose conditions.
Phase 1 - Dose-run up of GHB and Ethanol
This phase consisted of a maximum of 17 sessions, occurring on separate days. GHB and ethanol were administered on a maximum of 6 sessions each, and placebo was administered on four sessions. During each session a single dose of drug (i.e., GHB, ethanol, or placebo) was administered. Outcome measures were collected before drug administration and throughout the day, and consisted of participant-rated, behavioral, motor/cognitive, drug reinforcement, and physiological measures. The final session (17th session for those receiving all doses of GHB and ethanol) of Phase 1 was a “Lottery” session, in which drug (or placebo) administration was determined by the Multiple Choice Procedure (MCP) (see below) administered during the preceding sessions of Phase 1. This session was conducted in an identical fashion to other sessions in this phase.
The sequence of dosing permitted administration of ascending doses of both drugs, while: 1) intermixing the order of GHB and ethanol, and 2) inserting the 4 placebo sessions in quasi-random locations in the dosing sequence. For purposes of randomizing the order of GHB and ethanol administration, doses of GHB and ethanol were grouped into six pairs, each consisting of a single dose each of GHB and ethanol. Doses were administered in an ascending order across pairs. Within each pair of doses, the order of GHB and ethanol were randomized for each participant. The following six pairs of doses of GHB and ethanol were administered: 1) 1 g/70kg GHB and 12 g/70kg ethanol, 2) 2 g/70kg GHB and 24 g/70kg ethanol, 3) 4 g/70kg GHB and 48 g/70kg ethanol, 4) 6 g/70kg GHB and 72 g/70kg ethanol, 5) 8 g/70kg GHB and 96 g/70kg ethanol, and 6) 10 g/70kg GHB and 120 g/70kg ethanol. These pairs of doses were selected to provide approximately equivalent levels of sedative drug effects based on previous research on GHB (Carter et al., 2006) and ethanol e.g., (Mintzer, Guarino, Kirk, Roache, & Griffiths, 1997). Also, ascending doses were selected that resulting in equivalent relative dose increases for both drugs. For every three successive active drug sessions, a placebo session was randomly placed before, after, or within the three active doses (i.e., four possible placement positions).
As the doses ascended throughout the study, no further doses of a given drug were administered if a participant reached a “stopping point”, defined by either: 1) a participant experiencing significant behavioral impairment, or 2) the participant experienced intolerable vomiting. Significant behavioral impairment was defined as a failure to complete both the Circular Lights task and the Subjective Effects Questionnaire at any single time point during a session. When a stopping point was reached, subsequent doses of that drug were eliminated from the dosing schedule and doses of the other drug and placebos were moved earlier in the sequence and the relative order of these sessions remained unchanged.
Phase 2 – Direct comparison of GHB and ethanol reinforcement
This phase involved three sessions and consisted of the GHB versus ethanol choice procedure. The within-session procedures for these sessions were identical to those of Phase 1, and the outcome measures of Phase 1 (see Outcome Measures section below) were collected in an identical fashion in Phase 2. In the first session, the participant received one letter-coded drug (either GHB or ethanol, identified to the participant as Drug A) and in the next session, the participant received a different letter-coded drug (e.g., Drug B). On the morning of the third session in the phase, the participant chose which letter-coded drug he/she would receive (e.g., choice between Drug A and B), and provided a brief written narrative describing her or his reasons for the choice. Sequence of exposure to GHB and ethanol conditions was mixed across participants. The dose of each of the two drugs used for this comparison (i.e., the drug comparison dose) was defined as one dose lower than the dose causing a stopping point (as defined in the Phase 1 section). For example, if a participant experienced significant behavioral impairment at a GHB dose of 10 g/70kg, then the drug comparison dose of GHB would be 8 g/70kg. If a participant tolerated the maximum dose of a drug (e.g., 10 g/70 kg GHB or 120 g/70 kg ethanol) without achieving a stopping point then that dose would be defined as the maximum tolerated dose for comparison.
Outcome Measures
Four types of outcome measures were assessed: participant-rated, observer-rated, motor/cognitive, and physiological. On drug sessions days measures were assessed before drug administration and 0.5, 1, 2, 3, 4, 6, 8, 12 and 24 h after drug administration, except where noted below for specific measures. Assessment times are relative to the beginning of drug administration, even in cases with longer administration duration. In order to give participants experience with these tasks, practice trials were given before the first session. Unless otherwise stated, questionnaires regarding participant-rated and observer-rated measures were administered on a desk-top computer. The participant or staff member used a computer mouse to point to and select one of the various response options displayed on the screen.
Participant-Rated and Observer-Rated Measures
Subject-Rated Drug-Effect Questionnaire (Rush, Frey, & Griffiths, 1999)
This questionnaire consisted of two parts. The first question asked participants to rate their present level of alertness or sleepiness on a visual analog scale. The second part of this questionnaire consisted of 34 subjective effect questions that were rated on a five-point scale.
Pharmacological-Class Questionnaire (Rush et al., 1999)
This questionnaire required participants to categorize the drug effect as being most similar to one of 14 classes of psychoactive drugs. Participants completed this task the morning following their session.
Hangover rating
At 12 hours post-administration, participants responded to this question: “Since the end of the drug effect, have you felt hangover effects from this morning’s drug?” Response options were “no hangover at all,” “possible mild hangover but not sure,” “definite mild hangover,” “moderate strong hangover,” and “very strong hangover,” with corresponding numerical values ranging from 0 – 4, respectively.
Drug-Effect Questionnaire (DEQ) (Mumford, Rush, & Griffiths, 1995)
This questionnaire consisted of two parts: drug strength and drug liking. Participants rated the strength of drug effect on five-point scale. Participants also rated their liking (or disliking) of the drug on a bidirectional nine-point scale.
Addiction Research Center Inventory (ARCI) (Jasinski, 1977; Martin, Sloan, Sapira, & Jasinski, 1971)
The short form of the ARCI consisted of 49 true/false questions and contained five major subscales: morphine-benzedrine group (MBG, a measure of euphoria), pentobarbital, chlorpromazine, alcohol group (PCAG, a measure of sedation), lysergic acid diethylamide (LSD, a measure of dysphoria), benzedrine group (BG) and amphetamine (A) scales (empirically derived amphetamine-sensitive scales).
Next-Day Questionnaire (NDQ) (Rush et al., 1999)
Approximately 24 hours after study drug administration, participants completed this questionnaire rating the overall effect of yesterday’s drug. The questionnaire consisted of seven items. Participants were asked to: (1) rate the overall strength of the drug effect, (2) rate their overall liking of the drug effect, (3) rate the overall good effects of the drug, (4) rate the overall bad effects of the drug, (5) rate the degree to which they would like to take the drug again, (6) estimate the amount of money the drug would be worth on the street, and (7) estimate the amount of money they personally would be willing to pay for the drug on the street. In addition, participants completed a Pharmacological Class Questionnaire identical to that described above, but which required participants to choose the single drug category that best characterized the overall drug effect they experienced the day before.
Drug vs. Money Multiple Choice Procedure (MCP) (Griffiths, Troisi, Silverman, & Mumford, 1993; Griffiths, Rush, & Puhala, 1996)
This procedure provided a contingency-based assessment of the monetary value of each drug condition. Twenty-four hours after drug administration, the participant made a series of 70 choices between receiving various amounts of money and receiving the drug condition again. The monetary values range from $0.25 to $25. The data from this form are expressed as the maximum dollar amount at which the participant chose drug over money (i.e., the “crossover point”). During the reinforcement session at the end of the study, the participant actually received the consequence of one of his or her choices.
Performance Estimates (Roache & Griffiths, 1985)
Immediately before each assessment of the digit-symbol-substitution test (DSST) and the circular lights task, participants estimated their anticipated performance relative to “normal” using a computerized assessment. Higher scores indicated greater underestimation of performance relative to her/his actual performance at that time point.
Observer-Rated Questionnaire
This questionnaire was completed by a staff member who rated the participant on sedation/sleepiness, muscle relaxation (locomotor and non-locomotor), impaired posture, impaired speech, confusion/disorientation, stimulation/arousal, and drug strength on a five-point scale (Rush et al., 1999). The observer recorded the duration of time the participant spent sleeping in the past hour. In addition staff rated participants’ level of alertness on a five-point scale (Carter et al., 2006).
Motor/Cognitive Performance Measures
Digit-Symbol-Substitution Test (DSST) (McLeod, Griffiths, Bigelow, & Yingling, 1982)
This was a computerized task in which the participant used a numeric keypad to enter a geometric pattern associated with one of nine digits displayed on a video screen. Dependent measures were trials completed and trials correct.
Digit-Enter and Recall (Roache & Griffiths, 1987)
In this task participants used a numeric keypad to reproduce randomly selected eight-digit numbers that were displayed on the computer screen one at a time. The task consisted of two components, an enter component in which participants copied (entered) the eight-digit number while it was displayed on the screen, and a recall component in which the participant recalled the eight-digit number from memory after it disappeared from the screen. The dependent measure was the total number of eight-digit numbers correctly reproduced in the second (recall) component out of a possible 10.
Balance (Carter et al., 2006)
This task assessed the participant’s ability to stand upright on one foot with his or her eyes closed and arms extended to the side at shoulder height.
Circular Lights (Griffiths, Bigelow, & Liebson, 1983)
This task involved rapid hand-eye coordinated movements in which participants pressed a series of 16 buttons (circularly-arranged around a 54 cm diameter) as rapidly as possible in response to the randomly-sequenced illumination of their associated lights. The dependent measure was the number of correct button presses during the 60 s trial.
Word recall/recognition (explicit memory) (Mintzer & Griffiths, 1998)
Approximately 1 hr after drug administration, participants were presented with one subset of 16 words, which appeared on the computer screen one at a time. Approximately 6 hr after drug administration, participants’ memory for the words presented in the pleasantness rating task was tested using both recall and recognition tests. The score in the recall test was the number of words (out of 16) correctly recalled. Scores in the recognition task were calculated as the percentage of words (out of 16) correctly recognized (hit rate) and the percentage of distracter words (out of 16) incorrectly recognized as old (false alarm rate); these scores were used to derive signal detection measures of the participant’s ability to discriminate between old and new items (d′) and his or her response bias (C) (Green & Swets, 1966; Snodgrass & Corwin, 1988).
Physiological measures
Blood pressure and heart rate
Blood pressure (systolic and diastolic pressure using oscillometric method with the blood-pressure cuff placed on the arm) and heart rate were monitored using a Non-Invasive Patient Monitor Model 507E (Criticare Systems, Inc., Waukesha, WI).
Statistical Analysis
Although four placebo sessions were conducted, analyses included only a single placebo condition, with the data comprised of the mean of values from the four placebo sessions for each volunteer. Motor/cognitive performance measures were analyzed as percent of pre-drug score, with the exception of the Word Recall/Recognition task, which was only measured at a single time point. Participant and observer rated scores, as well as the Word Recall/Recognition task items, were analyzed as absolute scores (i.e., not percent of pre-drug). Peak effects data were determined for each multiple-time-point measure for each participant. For each measure, peak effects were defined as either the maximal or minimal score from 0.5 to 12 h after drug administration, depending on whether a majority of individual drug conditions showed greater absolute increases (maximal values) or decreases (minimal values) from baseline. Maximal values were used for DEQ, Observer-rated, and most SEQ items, as well as systolic blood pressure and pulse. Minimal values were used for all other measures, including SEQ items Comfortable, Relaxed, and Energetic. Subjective-Effect Questionnaire and Next-Day Questionnaire item “Drug Liking,” was used to derive two measures: liking and disliking. Raw scores from 0 to 4 served as measures of liking, while raw scores from 0 to −4 served as measures of disliking. If the raw score indicated liking (i.e., 1 to 4), the disliking score was defined as 0. Likewise, if the raw score indicated disliking (i.e., −1 to −4), the liking score was defined as 0. Disliking scores were converted to positives. Performance estimates were transformed relative to actual performance using methods described previously (Roache and Griffiths, 1985).
Because some participants reached a stopping point before receiving the maximum dose of a particular drug, not all participants were exposed to all drug conditions. Therefore, data were analyzed using repeated measures regression models in SAS PROC MIXED (SAS Institute Inc., Cary, NC, USA) which take into account the covariance structure of the repeated measures, and handle missing data better than traditional ANOVAs (Wolfinger and Chang, 1995). The primary analyses of outcome measures consisted of repeated measures regression using drug condition as a factor and completion status (i.e., whether or not a participant completed all drug conditions in Phase 1) as a covariate. These analyses were performed on Next-Day Questionnaire items, Word Recall/Recognition task measures, the Multiple Choice Procedure cross over point, and peak effects for other measures. Planned comparison tests were used to compare each of the drug conditions to placebo, and to compare to the highest dose of the two drugs with each other. In order to compare the magnitude of drug effects between the higher doses of both drugs, planned contrasts were conducted comparing the least-squares adjusted means between the top 3 doses of GHB and the top 3 doses of ethanol. Time-course analyses were performed for measures collected at multiple times during a session, using repeated measures regression with time and condition as factors and using completion status as a covariate. Planned comparisons were performed to compare each active drug condition to placebo at each time point. For all statistical tests p ≤ .05 was considered significant.
Results
Phase 1 - Dose-run up of GHB and Ethanol
Drug tolerability
In this ascending-dose study of both GHB and ethanol, all 14 participants received the four lowest doses of each drug (i.e., 1, 2, 4, and 6 g/70 kg GHB, and 12, 24, 48, and 72 g/7kg ethanol). Because participants reached stopping points due to significant behavioral impairment or nausea/vomiting, only 13 and 9 participants received 8 and 10 g/70 kg GHB, respectively, and only 13 and 10 participants received 96 and 120 g/70 kg ethanol, respectively.
Timecourse of Drug Effects
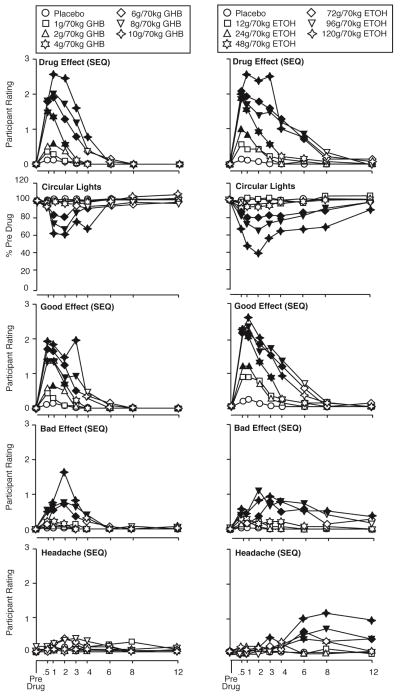
Both GHB and ethanol produced dose- and time-related participant-rated, observer-rated, and behavioral effects. Figure 1 shows the timecourse of GHB and ethanol on ratings of drug effect, circular lights performance, participant ratings of “good effects.” “bad effects,” and “headache.” Drug effect ratings peaked at approximately 1 hour for GHB and 2 hours for ethanol, with high dose effects resolving between 4 and 6 hours for GHB and between 8 and 12 hours for ethanol. The timecourse of circular lights effects are shown as being generally representative of motor/cognitive performance effects, which mirrored participant-rated drug effect in that GHB effects generally peaked at approximately 1 hour post-administration, and ethanol effects generally peaked at approximately 2 hours. Although this general timecouse observed for “drug effect” and motor/cognitive effects for each drug was approximately similar to the timecourse of ratings for “good effect,” ratings for “bad effect” and “headache” showed an important difference across drugs. That is, for GHB ratings for “bad effect” and “headache” followed a timecourse consistent with acute intoxication (i.e., similar to “good effects” and circular lights task), for ethanol the ratings for “bad effect” and “headache” followed a delayed timecourse, with plateau effect for “bad effects” and peak effect for “headache” at the 12 hour time point, at which point “good effects” and circular lights effects had resolved.
Figure 1.
Time course of effects of GHB (left panels) and ethanol (right panels) on DEQ participant ratings of drug effect, circular lights score, SEQ participant ratings of good effect and bad effects, and SEQ participant ratings of headache. Ordinates show participant ratings, with the exception of circular lights, for which the ordinates show score. Abscissas show time after drug administration in hours. Data points show least-squares adjusted means. Filled symbols indicate values that are significantly different from the corresponding placebo value at the same time point (planned comparisons).
Level of Alertness
GHB appeared to cause greater decreases in level of alertness than ethanol (Table 1). Half or more of the participants were rated as “awake and alert” at all ethanol doses, and a minority of participants reached a minimal rating of “drowsy or asleep, responding to verbal and light tactile stimulation” at the highest 3 doses. Ethanol did not result in lower level of alertness ratings than this for any participant at any dose. GHB showed greater decreases in level of alertness across doses, with the highest dose resulting in a rating of “awake and alert” for only 1 participant. Even at the lowest GHB dose, 1 participant was rated as “Drowsy or asleep, responding to verbal and light tactile stimulation.” One participants reached a minimal rating of “asleep, responding to pain only” at the 6 g/70 kg dose of GHB, and another participant reached this rating at the highest dose of GHB.
Table 1.
Minimal Value Observer-Rated Level of Alertness
| Level of alertness rating1 | Pl n=14 |
Ethanol (g/70kg) | GHB (g/70kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 12 n=14 |
24 n=14 |
48 n=14 |
72 n=14 |
96 n=13 |
120 n=10 |
1 n=14 |
2 n=14 |
4 n=14 |
6 n=14 |
8 n=13 |
10 n=9 |
||
| Awake and alert | 100 | 93 | 93 | 93 | 64 | 46 | 50 | 93 | 93 | 71 | 64 | 23 | 11 |
| Drowsy, but easily aroused with verbal commands | 0 | 7 | 7 | 7 | 7 | 15 | 20 | 0 | 7 | 21 | 0 | 15 | 33 |
| Drowsy or asleep, responding to verbal and light tactile stimulation | 0 | 0 | 0 | 0 | 29 | 38 | 30 | 7 | 0 | 7 | 29 | 62 | 44 |
| Drowsy or asleep, not responding to loud voice, responding to rougher tactile stimulation only | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Asleep, responding to pain only | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 11 |
Data show the percentage of participants that were assessed as reaching the indicated level of alertness rating in each dose condition.
Participant- and Observer-rated Effects
Both drugs showed significant effects for a variety of measures (Table 2). Among the participant- and observer-rated measures, both drugs (at a minimum of one dose each) significantly increased (or decreases where specified) ratings for items related to general drug effect (SEQ drug effect, DEQ drug effect, NDQ drug effect, observer drug effect), items related to abuse liability (SEQ like, SEQ good effect, DEQ liking, NDQ liking, NDQ good effect), and items related to sedative drug effects (e.g., SEQ alertness/sleepiness VAS toward sleepiness, SEQ depressant, SEQ mentally slow, SEQ tired, ARCI PCAG, ARCI sedation, observer relaxed, observer sleep time, observer total sleep, observer level of alertness (decrease)).
Table 2.
All participant and observer rated, motor/cognitive, and physiological outcome measures (peak measures for those for which timecourse was assessed) for which either 1) a significant planned comparison effect was found for at least 1 dose of either drug compared to placebo, or 2) a significant effect was found in the planned contrast comparing the least-squares adjusted mean of the highest 3 doses between each drug.
| Measure | Drug vs placebo a | Drug vs drug b | |
|---|---|---|---|
|
| |||
| GHB vs PL | ETOH vs PL | GHB vs ETOH | |
| Participant & Observer Rated | |||
| Alertness/sleepiness VAS (SEQ) | + | + | NS |
| Drug Effect (SEQ) | + | + | NS |
| Arousing (SEQ) | + | + | NS |
| Depressant (SEQ) | + | + | NS |
| Like (SEQ) | + | + | NS |
| Good Effect (SEQ) | + | + | NS |
| Bad Effect (SEQ) | + | + | NS |
| Headache (SEQ) | NS | + | ETOH > GHB |
| Confused (SEQ) | + | + | NS |
| Sleepy (SEQ) | + | + | NS |
| Blurred Vision (SEQ) | + | + | GHB > ETOH |
| Limp (SEQ) | + | + | NS |
| Lightheaded (SEQ) | + | + | GHB > ETOH |
| Queasy (SEQ) | + | + | NS |
| Fatigued (SEQ) | + | + | NS |
| Unsteady (SEQ) | + | + | NS |
| Hot (SEQ) | + | + | NS |
| Diff. Concentrating (SEQ) | + | + | NS |
| Slurred Speech (SEQ) | + | + | NS |
| Mentally slow (SEQ) | + | + | NS |
| Heavy limbs (SEQ) | + | + | NS |
| Tired (SEQ) | + | + | NS |
| Easy going (SEQ) | + | + | NS |
| Forgetful (SEQ) | + | + | NS |
| Talkative (SEQ) | + | + | NS |
| Excited (SEQ) | NS | + | NS |
| Comfortable (SEQ) | + | NS | GHB > ETOH |
| Relaxed (SEQ) | + | NS | NS |
| Nervous (SEQ) | + | + | NS |
| Numbness (SEQ) | + | + | NS |
| Restless (SEQ) | NS | + | NS |
| Dry Mouth (SEQ) | + | + | NS |
| Shaky (SEQ) | + | + | NS |
| Irritable (SEQ) | NS | + | NS |
| Drug Effect (DEQ) | + | + | NS |
| Liking (DEQ) | + | + | NS |
| PEQ (DEQ) | + | + | NS |
| Disliking (DEQ) | NS | + | NS |
| PCAG (ARCI) | + | + | GHB > ETOH |
| Benzedrine (ARCI) | − | − | GHB > ETOH |
| Amphetamine (ARCI) | NS | + | NS |
| MBG (ARCI) | NS | + | NS |
| LSD (ARCI) | + | + | GHB > ETOH |
| Euphoria (ARCI) | + | + | NS |
| Sedation (ARCI) | + | + | GHB > ETOH |
| Drug effect (NDQ) | + | + | NS |
| Liking (NDQ) | + | + | GHB > ETOH |
| Disliking (NDQ) | NS | + | NS |
| Good effects (NDQ) | + | + | GHB > ETOH |
| Bad effects (NDQ) | + | + | NS |
| Take again (NDQ) | + | + | GHB > ETOH |
| DE worth (NDQ) | + | + | NS |
| Willing to pay (NDQ) | + | + | NS |
| Hangover rating | NS | + | ETOH > GHB |
| Crossover point (MCP) | NS | − | GHB > ETOH |
| DSST Performance Estimate | NS | − | NS |
| Cir Lights Performance Estimate | NS | − | NS |
| Word Recall Performance Estimate | − | − | NS |
| Level of Alertness (Observer) | − | − | GHB > ETOH |
| Relaxed (Observer) | + | + | NS |
| Speech (Observer) | + | + | NS |
| Confused (Observer) | + | + | NS |
| Stimulated (Observer) | + | + | NS |
| Drug effect (Observer) | + | + | NS |
| Posture (Observer) | + | + | NS |
| Sleep Time (Observer) | + | + | NS |
| Total Sleep Time (Observer) | + | + | NS |
| Motor/Cognitive | |||
| Immediate trials (Digit recall) | − | − | NS |
| Word Recall | NS | − | NS |
| Word Recognition d′ | − | − | ETOH > GHB |
| DSST | − | − | NS |
| Balance | − | − | NS |
| Circular lights | − | − | NS |
| Physiological | |||
| Systolic | + | + | NS |
| Diastolic | − | − | NS |
| Pulse | + | + | ETOH > GHB |
These two columns show the results of planned comparisons of placebo with 12, 24, 48, and 120 g/70 kg ETOH and 1, 2, 4, 6, 8, and 10 g/70 kg GHB. Symbol (+ or −) indicates that at least one dose of the drug was significantly different from placebo (p ≤ .05); symbol (+ or −) also indicates the direction of the drug effect relative to placebo. For the alertness/sleepiness VAS, higher scores denote sleepiness. For performance estimates, + indicates that participants underestimated performance relative to actual performance more so for the drug than placebo. NS indicates no dose of that drug was different from placebo.
This column shows the results of a planned contrast comparing the mean of the top 3 doses of GHB (6, 8, 10 g/70kg) to the mean of the top 3 doses of ETOH (72, 96, 120 g/70kg). The drug to the left of the symbol (>) produced a significantly greater effect. NS indicates that the effects produced by the doses of the two drugs were not significantly different.
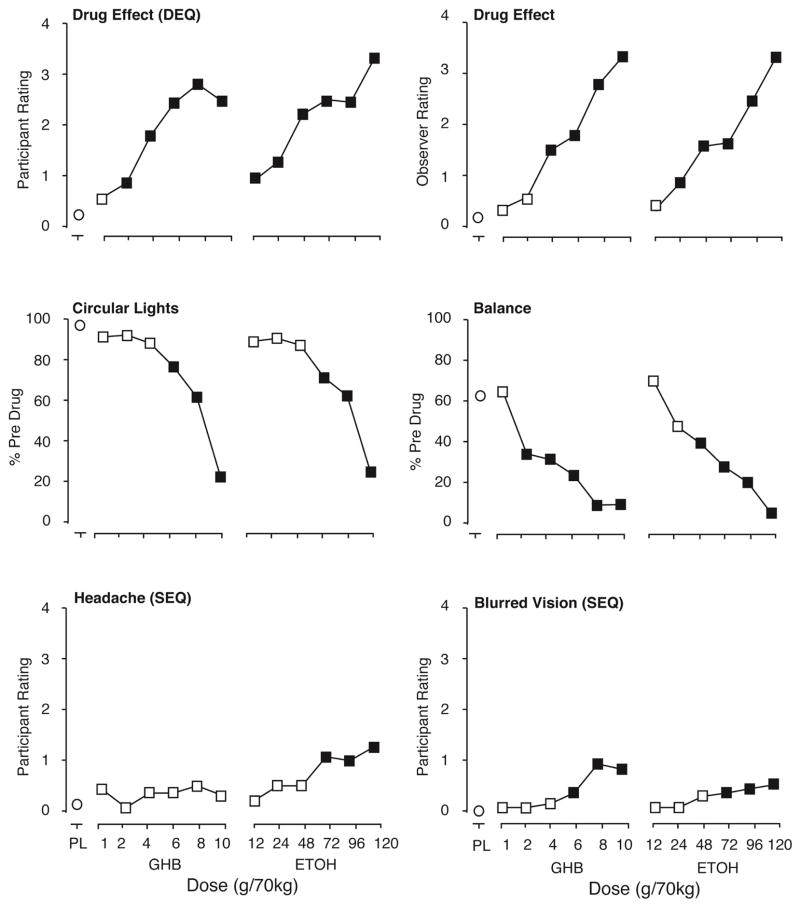
These similarities notwithstanding, the data also showed difference in the effects of the two drugs (Table 2). In some cases one drug but not the other significantly increased ratings in these domains (e.g., ARCI euphoria was increased by ethanol but not GHB, and SEQ sleepy was increased by GHB but not ethanol). In comparing the highest 3 doses of each drug, GHB showed greater effects than ethanol for SEQ blurred vision, SEQ lightheaded, SEQ comfortable, ARCI PCAG, ARCI Benzedrine (decrease), ARCI LSD, ARCI sedation, NDQ liking, NDQ good effects, NDQ take again, and MCP crossover point, and observer level of alertness. In contrast, ethanol showed greater effects than GHB for SEQ headache, and hangover rating. The top 2 panels of Fig. 2 show peak effects for participant-rated (DEQ) and observer-rated drug effect. The bottom 2 panels of Fig. 2 shows peak effects for headache, for which ethanol had a greater effect, and blurred vision, for which GHB had a greater effect.
Figure 2.
Participant rated, observer-rated, and motor/cognitive performance effects of GHB and Ethanol. Ordinates show score or rating expressed as peak effect (peak effects for performance measures are the minimum values and peak effects for the observer-rated measures are the maximum values through 12 hours post administration). Abscissas show dose. PL designates placebo. Data points show least-squares adjusted means. Filled symbols indicate values that are significantly different from placebo (planned comparisons).
On the pharmacological class questionnaire, participants identified placebo as a “blank or placebo” in 87% of cases. Rates of identification as placebo generally decreased with increasing doses. At doses from 1 to 4 g/70 kg GHB, the most common responses were “blank or placebo” and “benzodiazepines.” Higher doses were associated with increasing identification of GHB as an opiate. At 6 g/70 kg GHB, out of 14 participants, 6 identified it as other, 4 participants identified GHB as an opiate, 2 identified it as a benzodiazepine, 1 identified it as alcohol, and 1 identified it as a stimulant. At 8 g/70 kg GHB, out of 13 participants, 6 participants identified GHB as other, 3 identified it as an opiate, 3 identified it as a benzodiazepine, and 1 identified it as alcohol. At 10 g/70 kg GHB, out of 9 participants, 6 participants (67%) identified GHB as an opiate, 2 identified it as other, and 1 identified it as a benzodiazepine. Although ethanol was identified as something other than alcohol at the 12 and 24 g/70 kg doses (most commonly as “benzodiazepine” or “blank or placebo”), it was generally identified correctly as alcohol at higher doses, with 75% of response identifying it as alcohol in the dose range from 48 to 120 g/70 kg.
Motor/Cognitive Effects
Both GHB and ethanol significantly decreased all measures of motor and cognitive performance, with the exception that ethanol but not GHB significantly decreased word recall performance (Table 2). The only outcome that significantly differed between the 3 highest doses of each drug was that ethanol showed greater decreases than GHB on word recognition accuracy (Table 2). Fig. 2 shows peak performance effects on circular lights and balance as representative examples of performance effects.
Physiological
Both GHB and ethanol significantly increased systolic blood pressure and pulse, and decreased diastolic blood pressure. The only significant difference between the highest 3 doses of the drugs was that increases in pulse were greater for ethanol than GHB. It should be noted that the elevations in physiological variables were relatively modest. For systolic blood pressure, the largest mean (across participants) peak increase for GHB was 15.6 mm Hg more than placebo (observed at the 8 g/70 kg dose). For ethanol the largest mean increase in peak effects was 8.2 mm Hg (observed at 120 g/70 kg). For diastolic blood pressure, the largest mean (across participants) peak decrease in blood pressure for any dose of GHB was 7.7 mm Hg (observed at the 10 g/70 kg dose). For ethanol the largest mean decrease in peak effects was 13.7 mm Hg (observed at 120 g/70 kg). For pulse, the largest mean (across participants) peak increase in blood pressure for any dose of GHB was 10.0 bpm (observed at the 8 g/70 kg dose). For ethanol the largest mean increase in peak effects was 19.0 bpm (observed at 96 g/70 kg).
Phase 2 – Direct comparison of GHB and ethanol reinforcement
Three participants were discharged from the study before completing the choice phase, resulting in 11 participants who completed this phase. For these 11 participants, table 3 shows the maximum tolerated doses of GHB and ethanol (which were re-administered in the choice phase), and which drug (GHB or ethanol) the participant chose to receive on the final session. Four participants chose ethanol, and 7 participants chose GHB. A review of participant narratives describing reasons for the choice revealed that while participants generally described positive effects for both drugs, decisions were largely based on negative effects of the non-chosen drug (e.g., hangover/headache for ethanol, and uncontrollable sleep for GHB).
Table 3.
Drug choice data.
| Volunteer* | Highest tolerated GHB dose (g/70 kg) | Highest tolerated ETOH dose (g/70 kg) | Choice |
|---|---|---|---|
| 1 | 8 | 120 | ETOH |
| 2 | 8 | 72 | ETOH |
| 8 | 8 | 96 | ETOH |
| 10 | 10 | 120 | ETOH |
| 3 | 8 | 120 | GHB |
| 4 | 8 | 120 | GHB |
| 5 | 6 | 96 | GHB |
| 6 | 10 | 120 | GHB |
| 7 | 10 | 72 | GHB |
| 9 | 4 | 72 | GHB |
| 11 | 6 | 72 | GHB |
The two female participants were volunteers 8 and 9.
Discussion
This comparative study of GHB and ethanol in sedative abusing participants resulted in several general conclusions. GHB and ethanol both showed remarkable similarity in occasioning strong, dose-and time-related sedative type effects, with significant increases in several participant- and observer-rated measures of general drug effects and sedative effects, and significant decreases in performance on all cognitive/motor tasks. Both drugs showed changes in pulse and blood pressure. Ethanol was often identified as a sedative type drug. GHB was also often identified as a sedative. However, surprisingly, at higher doses GHB was identified as an opiate, with almost 70% identifying it as an opiate at 10 g/70 kg. This is a higher percent than in previous findings (Carter et al., 2006) in which 8 g/70 kg GHB was identified as “other” by 3 of 6 participants, as “opiate” by 2 participants, and “benzodiazepine or barbiturate” by only 1 of 6 participants. There is no apparent reason for the higher percent of opiate identification in the present study. The attribution of opiate-like effects to GHB is intriguing and merits investigation in future research. In addition to the similarities between the drugs on participant-rated, observer-rated, and cognitive/motor effects, another similarity is that both drugs resulted in aversive effects at high doses which prompted discontinuation of subsequent administration of higher doses in this ascending dose run up design. Only 9 of 14 participants received the highest dose of GHB, and only 10 received the highest dose of ethanol, with 7 individuals receiving the highest dose of both drugs, showing similar tolerability at the ranges of doses studied.
Despite these similarities, the two drugs showed some clinically important differences. Specifically, GHB had a shorter timecourse than ethanol, GHB was more likely to cause sleep than ethanol, and ethanol produced greater increases in ratings of headache and hangover than GHB. One caveat to the finding of shorter timecourse for GHB is that some participants took up to an hour to drink ethanol at higher doses, which could have partially contributed to the longer timecourse of ethanol. However, the extended administration period for ethanol was not sufficient to fully account for the longer timecourse for ethanol.
A very intriguing distinction between the drugs relates to the contrast between abuse-liability related measures assessed during the active acute effects (same-day measures) and retrospective (post-session measures) of drug effect. Specifically, the same-day measures SEQ liking, SEQ good effect, and DEQ liking showed similar increases for both drugs with no significant difference between the drugs. However, the post-session measures NDQ liking, NDQ good effects, NDQ take again, and MCP crossover value showed significantly greater ratings or values for GHB than ethanol. Although preference was not reliable across participants, 7 of 11 participants chose to receive again the highest tolerated dose of GHB rather than ethanol during the phase II direct drug preference procedure. Interestingly, written participant narratives attributed positive qualities to both drugs, and indicated that decisions were generally based upon consideration of the aversive effects of the non-chosen drug (e.g., headache from ethanol; involuntary sleep for GHB). Consistent with this, ratings of aversive effects of ethanol were generally higher at later times (e.g., significant increases in the hangover rating assessed 12 h post-administration; timecourse of “bad effects” and “headache” as shown in Fig. 1). Therefore, it appears that these aversive effects were responsible for the lower apparent abuse liability of ethanol on post-session day measures. The delayed aversive effects of ethanol are consistent with the metabolism of ethanol to acetaldehyde which is associated with toxic effects, whereas GHB is metabolized to the relatively non-toxic compounds carbon dioxide and water (Dean et al., 1997; Walkenstein, Wiser, Gudmundsen, & Kimmel, 1964). Collectively, the data suggest that under active drug intoxication the two drugs appear to have similar abuse liability, however, the delayed aversive effects of ethanol may play a role in limiting its abuse liability in certain individuals relative to GHB over longer scales of time (i.e., across days and weeks).
Another interesting distinction between the two drugs is that GHB had less severe memory (word recognition) impairing effects than ethanol, although both drugs caused significant impairment on this measure. These data are consistent with one previous study in our laboratory in sedative abusers showing that triazolam and pentobarbital had greater memory impairing effects than GHB (Carter et al., 2006), and another report from our laboratory in which volunteers who did not abuse sedatives showed greater memory impairment for triazolam than GHB (Carter, Griffiths, & Mintzer, 2009). These data are interesting given that clinical reports claiming that memory impairing effects of GHB have contributed to its use in sexual assaults (Schwartz, Milteer, & LeBeau, 2000; Varela, Nogue, Oros, & Miro, 2004). The present data, along with previous reports (Carter et al., 2006; Carter et al., 2009; Grove-White & Kelman, 1971; Metcalf, Emde, & Stripe, 1966) suggest that memory impairment may not be the driving mechanism in anecdotal reports of GHB being involved in sexual assault. Rather, as suggested by the present study and previous research (Carter et al., 2006), it may be that the strong sleep-inducing effects of GHB, rather than memory-impairing effects, maybe be involved in such cases of sexual assault (Carter et al., 2006).
In conclusion, the present within-subject comparison of GHB and ethanol showed time- and dose-related sedative effects that were largely similar between the two drugs. However, clinically important differences between the two drugs were also noted, including a shorter timecourse of GHB than ethanol, greater indication of abuse liability of GHB than ethanol on retrospective, but not same-day, measures of abuse liability, and greater memory impairing effects for ethanol relative to GHB. Given these data, interesting areas for future research would be determining the interactive effects of GHB and ethanol at multiple doses, examining the substitutability of the two drugs in a self-administration research, and examining the substitutability of the two drugs in a drug discrimination procedure.
Acknowledgments
Role of the funding source
This work was supported by the National Institute on Drug Abuse (NIDA) through R01DA003889.
The authors would like to thank Eric C. Strain, M.D., Annie Umbricht, M.D., and the medical staff at the Behavioral Pharmacology Research Unit for medical screening and medical coverage, Benjamin McKay and Margaret Klinedinst for collecting and organizing the data. GHB and sodium citrate (GHB placebo) solutions were kindly provided by Orphan Medical; Minnetonka, MN, currently known as Jazz Pharmaceuticals, Palo Alto, CA.
Footnotes
Contributors
Drs. Johnson and Griffiths designed the study and wrote the protocol and contributed to interpretation of results. Dr. Johnson conducted experimental sessions and conducted statistical analyses of the data. Dr. Johnson wrote the first draft of the manuscript. Both authors contributed to and have approved the final manuscript.
Conflict of interest
Matthew Johnson has consulted for Eli Lilly on issues related to drug abuse liability. During the past 3 years, on issues related to drug abuse liability, Roland Griffiths has been a consultant to or has received contracts or grants from: Alexza Pharmaceuticals, Bristol-Myers Squibb, Hoffman-La Roche Inc., Jazz Pharmaceuticals Inc., Merck & Co, Sanofi-Aventis, Transcept Pharmaceuticals Inc., and Vanda Pharmaceuticals.
References
- Abanades S, Farré M, Barral D, Torrens M, Closas N, Langohr K, de la Torre R. Relative abuse liability of gamma-hydroxybutyric acid, flunitrazepam, and ethanol in club drug users. Journal of Clinical Psychopharmacology. 2007;27:625–38. doi: 10.1097/jcp.0b013e31815a2542. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, Caputo F, Gasbarrini A. The therapeutic potential of gamma-hydroxybutyric acid for alcohol dependence: Balancing the risks and benefits. A focus on clinical data. Expert Opinion on Investigational Drugs. 2009;18(5):675–686. doi: 10.1517/13543780902905855. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST. The behavioral economics of concurrent drug reinforcers: A review and reanalysis of drug self-administration research. Psychopharmacology (Berl) 1995;118(3):250–9. doi: 10.1007/BF02245952. [DOI] [PubMed] [Google Scholar]
- Caputo F, Vignoli T, Maremmani I, Bernardi M, Zoli G. Gamma hydroxybutyric acid (GHB) for the treatment of alcohol dependence: A review. International Journal of Environmental Research and Public Health. 2009;6(6):1917–1929. doi: 10.3390/ijerph6061917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR, Mintzer MZ. Cognitive, psychomotor, and subjective effects of sodium oxybate and triazolam in healthy volunteers. Psychopharmacology. 2009;206(1):141–154. doi: 10.1007/s00213-009-1589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR. Relative abuse liability of GHB in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology. 2006;31:2537–2551. doi: 10.1038/sj.npp.1301146. [DOI] [PubMed] [Google Scholar]
- Craig K, Gomez HF, McManus JL, Bania TC. Severe gamma-hydroxybutyrate withdrawal: A case report and literature review. The Journal of Emergency Medicine. 2000;18(1):65–70. doi: 10.1016/s0736-4679(99)00163-8. [DOI] [PubMed] [Google Scholar]
- Dean W, Morgenthaler J, Fowkes SW. GHB: The natural mood enhancer : The authoritative guide to its responsible use. Petaluma, CA: Smart Publications; 1997. [Google Scholar]
- Degenhardt L, Darke S, Dillon P. GHB use among australians: Characteristics, use patterns and associated harm. Drug and Alcohol Dependence. 2002;67(1):89–94. doi: 10.1016/s0376-8716(02)00017-0. S0376871602000170 [pii] [DOI] [PubMed] [Google Scholar]
- Gallimberti L, Spella MR, Soncini CA, Gessa GL. Gamma-hydroxybutyric acid in the treatment of alcohol and heroin dependence. Alcohol (Fayetteville, NY) 2000;20(3):257–262. doi: 10.1016/s0741-8329(99)00089-0. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Frederick SL, Staggers FE, Jr, Gonzales M, Stalcup SA, Smith DE. Gamma-hydroxybutyrate: An emerging drug of abuse that causes physical dependence. Addiction (Abingdon, England) 1997;92(1):89–96. [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson I. Differential effects of diazepam and pentobarbitol on mood and behavior. Archives of General Psychiatry. 1983;40:865–873. doi: 10.1001/archpsyc.1983.01790070055007. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: A conceptual framework and algorithm for differentiating among compounds. Journal of Clinical Psychiatry. 2005;66:S31–S41. [PubMed] [Google Scholar]
- Griffiths RR, Rush CR, Puhala KA. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Exp Clin Psychopharmacol. 1996;4(1):97–106. [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple-choice procedure: An efficient approach for investigating drug reinforcement in humans. Behavioral Pharmacology. 1993;4:3–13. [PubMed] [Google Scholar]
- Grove-White IG, Kelman GR. Effect of methohexitone, diazepam and sodium 4-hydroxybutyrate on short-term memory. British Journal of Anaesthesia. 1971;43(2):113–116. doi: 10.1093/bja/43.2.113. [DOI] [PubMed] [Google Scholar]
- Hechler V, Gobaille S, Maitre M. Selective distribution pattern of gamma-hydroxybutyrate receptors in the rat forebrain and midbrain as revealed by quantitative autoradiography. Brain Research. 1992;572(1–2):345–348. doi: 10.1016/0006-8993(92)90498-x. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. Assessment of the abuse potentiality of morphine-like drugs (methods used in man) In: Martin WR, editor. Drug addiction I. New York: Springer-Verlag; 1977. pp. 197–258. [Google Scholar]
- Johnson MW, Bickel WK, Kirshenbaum AP. Substitutes for tobacco smoking: A behavioral economic analysis of nicotine gum, denicotinized cigarettes, and nicotine-containing cigarettes. Drug Alcohol Depend. 2004;74(3):253–64. doi: 10.1016/j.drugalcdep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Lingenhoehl K, Brom R, Heid J, Beck P, Froestl W, Kaupmann K, Mosbacher J. Gamma-hydroxybutyrate is a weak agonist at recombinant GABA(B) receptors. Neuropharmacology. 1999;38(11):1667–1673. doi: 10.1016/s0028-3908(99)00131-8. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12(2):245–58. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Mathivet P, Bernasconi R, De Barry J, Marescaux C, Bittiger H. Binding characteristics of gamma-hydroxybutyric acid as a weak but selective GABAB receptor agonist. European Journal of Pharmacology. 1997;321(1):67–75. doi: 10.1016/s0014-2999(96)00916-8. [DOI] [PubMed] [Google Scholar]
- McDaniel CH, Miotto KA. Gamma hydroxybutyrate (GHB) and gamma butyrolactone (GBL) withdrawal: Five case studies. J Psychoactive Drugs. 2001;33(2):143–9. doi: 10.1080/02791072.2001.10400479. [DOI] [PubMed] [Google Scholar]
- McLeod D, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instrum. 1982;14:463–6. [Google Scholar]
- Metcalf DR, Emde RN, Stripe JT. An EEG-behavioral study of sodium hydroxybutyrate in humans. Electroencephalography and Clinical Neurophysiology. 1966;20(5):506–512. doi: 10.1016/0013-4694(66)90107-6. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Flunitrazepam and triazolam: A comparison of behavioral effects and abuse liability. Drug and Alcohol Dependence. 1998;53:49–66. doi: 10.1016/s0376-8716(98)00110-0. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Guarino J, Kirk T, Roache JD, Griffiths RR. Ethanol and pentobarbital: Comparison of behavioral and subjective effects in sedative drug abusers. Experimental and Clinical Psychopharmacology. 1997;5:203–215. doi: 10.1037//1064-1297.5.3.203. [DOI] [PubMed] [Google Scholar]
- Miotto K, Darakjian J, Basch J, Murray S, Zogg J, Rawson R. Gamma-hydroxybutyric acid: Patterns of use, effects and withdrawal. Am J Addict. 2001;10(3):232–41. doi: 10.1080/105504901750532111. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Rush CR, Griffiths RR. Abecarnil and alprazolam in humans: Behavioral, subjective and reinforcing effects. J Pharmacol Exp Ther. 1995;272(2):570–80. [PubMed] [Google Scholar]
- Roache JD, Griffiths RR. Comparison of triazolam and pentobarbital: Performance impairment, subjective effects and abuse liability. J Pharmacol Exp Ther. 1985;234(1):120–33. [PubMed] [Google Scholar]
- Roache JD, Griffiths RR. Lorazepam and meprobamate dose effects in humans: Behavioral effects and abuse liability. J Pharmacol Exp Ther. 1987;243(3):978–88. [PubMed] [Google Scholar]
- Rush CR, Frey JM, Griffiths RR. Zaleplon and triazolam in humans: Acute behavioral effects and abuse potential. Psychopharmacology (Berl) 1999;145(1):39–51. doi: 10.1007/s002130051030. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Milteer R, LeBeau MA. Drug-facilitated sexual assault (‘date rape’) Southern Medical Journal. 2000;93(6):558–561. [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology General. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Stein LA, Lebeau R, Clair M, Martin R, Bryant M, Storti S, Monti P. A web-based study of gamma hydroxybutyrate (GHB): Patterns, experiences, and functions of use. The American Journal on Addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2011;20(1):30–39. doi: 10.1111/j.1521-0391.2010.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Drug abuse warning network, 2009: National estimates of drug-related emergency department visits. HHS publication no. (SMA) 11-4659, DAWN series D-35. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. [Google Scholar]
- Sumnall HR, Woolfall K, Edwards S, Cole JC, Beynon CM. Use, function, and subjective experiences of gamma-hydroxybutyrate (GHB) Drug and Alcohol Dependence. 2008;92(1–3):286–290. doi: 10.1016/j.drugalcdep.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Thai D, Dyer JE, Benowitz NL, Haller CA. Gamma-hydroxybutyrate and ethanol effects and interactions in humans. Journal of Clinical Psychopharmacology. 2006;26:524–9. doi: 10.1097/01.jcp.0000237944.57893.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela M, Nogue S, Oros M, Miro O. Gamma hydroxybutirate use for sexual assault. Emergency Medicine Journal : EMJ. 2004;21(2):255–256. doi: 10.1136/emj.2002.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkenstein SS, Wiser R, Gudmundsen C, Kimmel H. Metabolism of gamma-hydroxybutyric acid. Biochimica Et Biophysica Acta. 1964;86:640–642. doi: 10.1016/0304-4165(64)90107-2. [DOI] [PubMed] [Google Scholar]
- Wolfinger R, Chang M. Comparing the SAS GLM and MIXED procedures for repeated measurements analysis. SAS User Group International (SUGI) Proceedings 1995. 1995 Retrieved from: http://support.sas.com/rnd/app/stat/papers/mixedglm.pdf.