Abstract
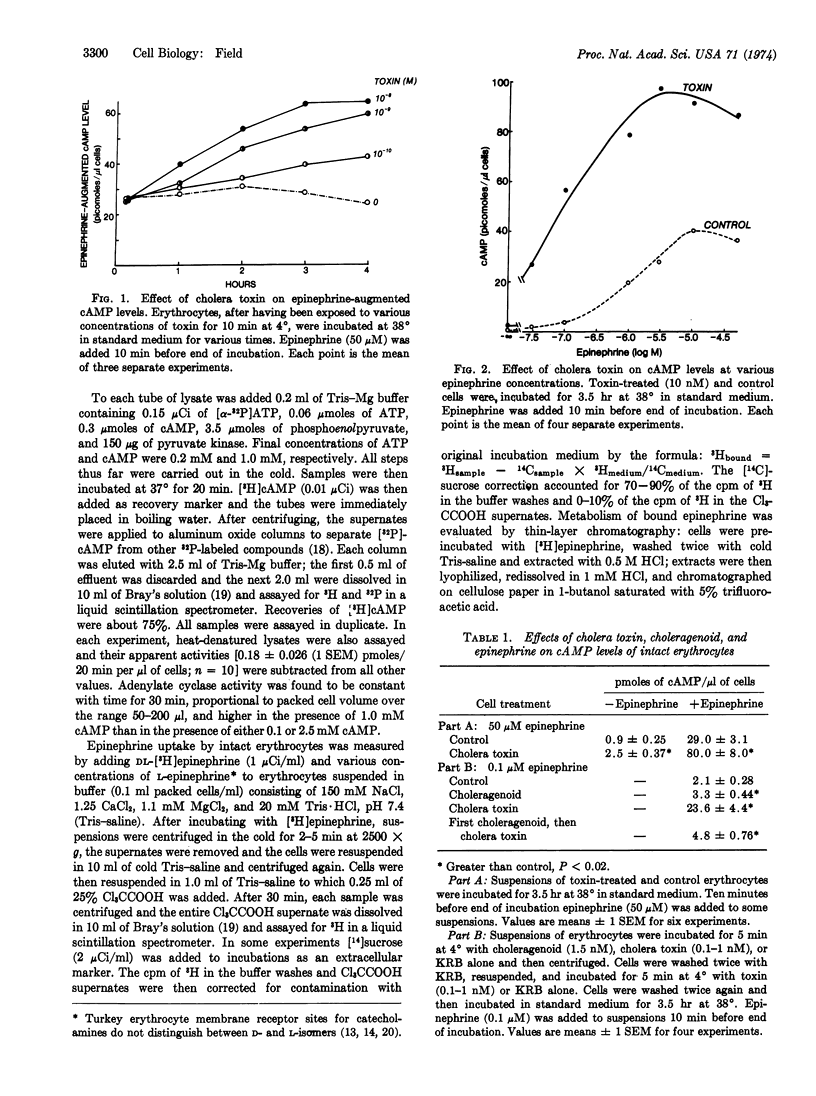
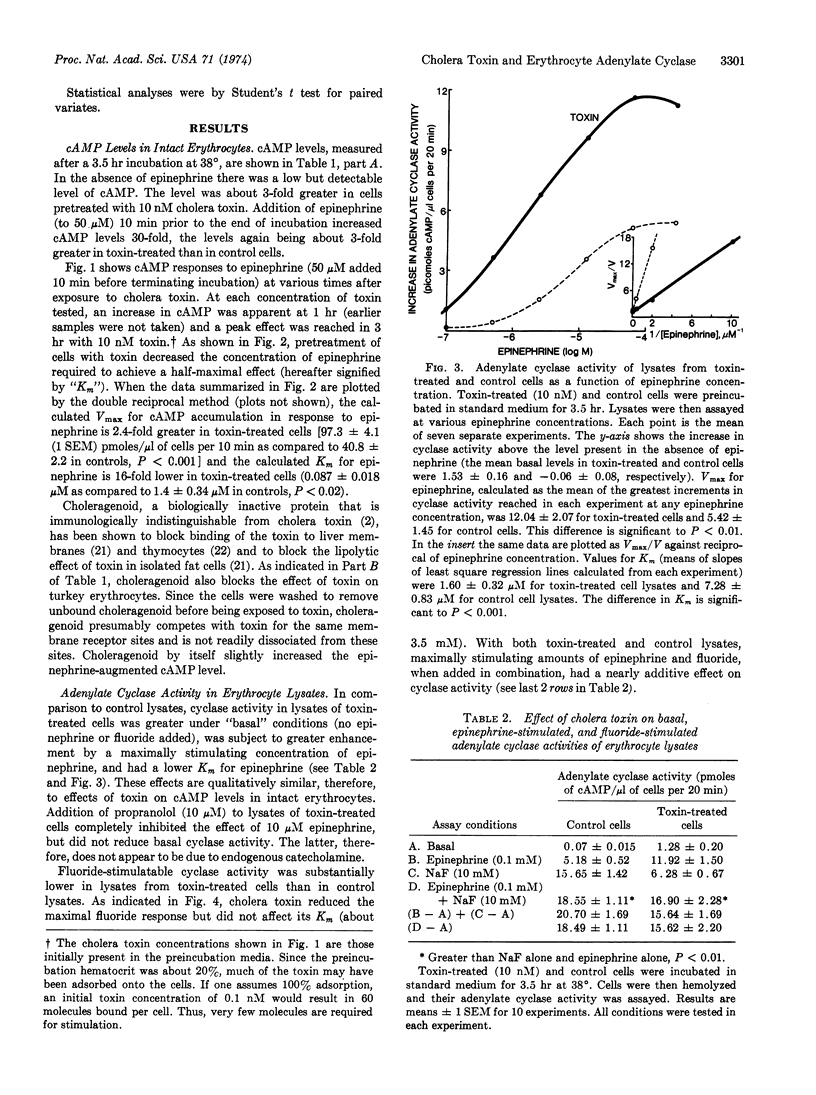
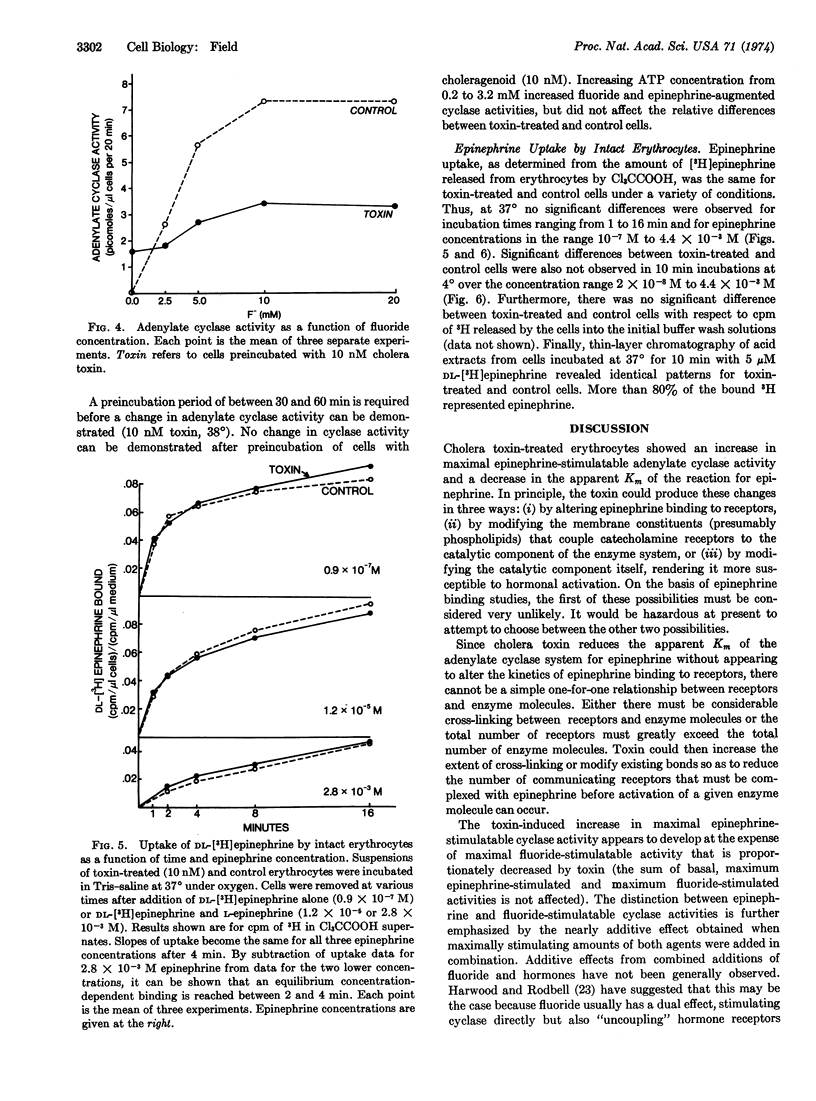
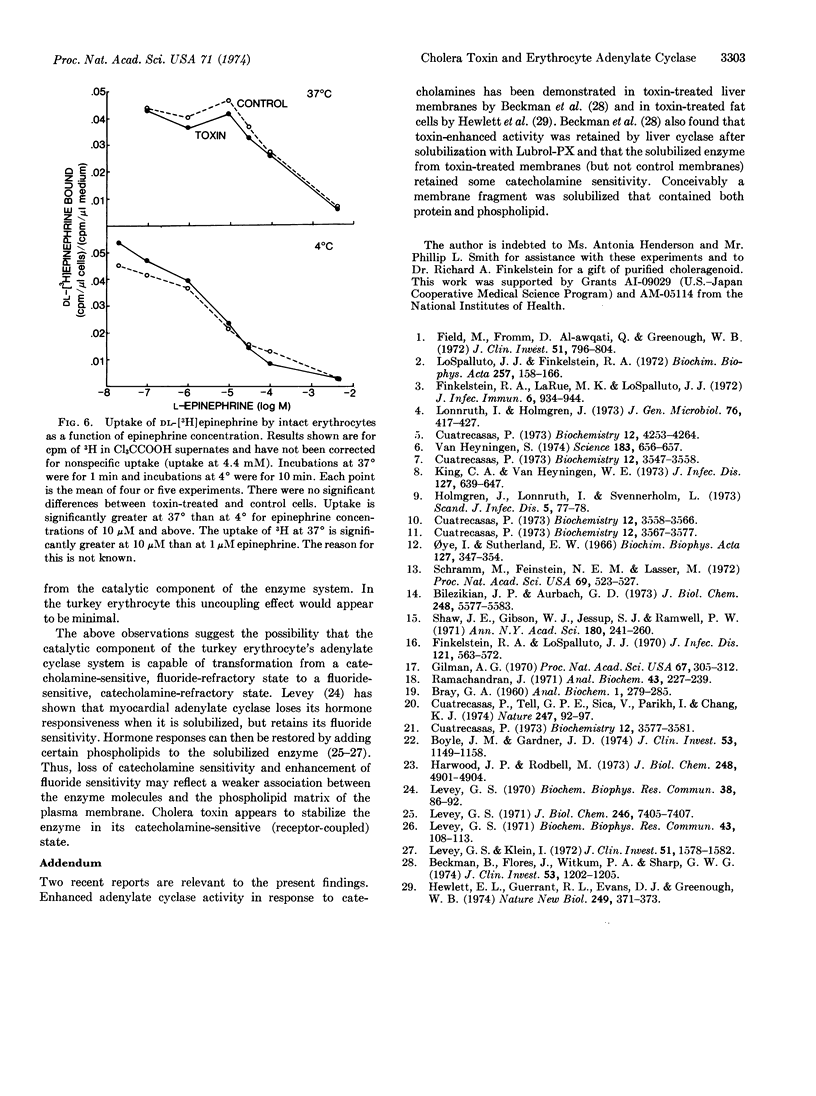
Preincubating turkey erythrocytes with cholera toxin alters their adenylate cyclase (EC 4.6.1.1) system: basal activity, maximal epinephrine-stimulatable activity, and affinity of the enzyme reaction for epinephrine are all increased. Pretreatment of erythrocytes with choleragenoid prevents these changes. Cholera toxin does not alter [3H]epinephrine uptake by intact erythrocytes. The increase in epinephrine-stimulatable cyclase activity appears to occur at the expense of fluoride-stimulatable activity, which is decreased by the toxin. In lysates from both toxin-treated and control cells, maximally stimulating amounts of epinephrine and fluoride, when added in combination, have a nearly additive effect on cyclase activity. These observations suggest that the adenylate cyclase system of the turkey erythrocyte may exist in two interconvertible forms, one that is catecholamine-responsive but fluoride-insensitive, and another that is fluoride-sensitive but not coupled to catecholamine receptors. Cholera toxin appears to stabilize the enzyme in its hormone receptor-coupled form.
Keywords: cyclic AMP, epinephrine, hormone receptor, fluoride
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman B., Flores J., Witkum P. A., Sharp G. W. Studies on the mode of action of cholera toxin. Effects on solubilized adenylate cyclase. J Clin Invest. 1974 Apr;53(4):1202–1205. doi: 10.1172/JCI107660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikian J. P., Aurbach G. D. A beta-adrenergic receptor of the turkey erythrocyte. I. Binding of catecholamine and relationship to adenylate cyclase activity. J Biol Chem. 1973 Aug 25;248(16):5577–5583. [PubMed] [Google Scholar]
- Boyle J. M., Gardner J. D. Sequence of events mediating the effect of cholera toxin on rat thymocytes. J Clin Invest. 1974 Apr;53(4):1149–1158. doi: 10.1172/JCI107653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Cholera toxin-fat cell interaction and the mechanism of activation of the lipolytic response. Biochemistry. 1973 Aug 28;12(18):3567–3577. doi: 10.1021/bi00742a033. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I., Hollenberg M. D. Affinity chromatography and structural analysis of Vibrio cholerae enterotoxin-ganglioside agarose and the biological effects of ganglioside-containing soluble polymers. Biochemistry. 1973 Oct 9;12(21):4253–4264. doi: 10.1021/bi00745a033. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Tell G. P., Sica V., Parikh I., Chang K. J. Noradrenaline binding and the search for catecholamine receptors. Nature. 1974 Jan 11;247(5436):92–97. doi: 10.1038/247092a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Vibrio cholerae choleragenoid. Mechanism of inhibition of cholera toxin action. Biochemistry. 1973 Aug 28;12(18):3577–3581. doi: 10.1021/bi00742a034. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., al-Awqati Q., Greenough W. B., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972 Apr;51(4):796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., LaRue M. K., LoSpalluto J. J. Properties of the cholera exo-enterotoxin: effects of dispersing agents and reducing agents in gel filtration and electrophoresis. Infect Immun. 1972 Dec;6(6):934–944. doi: 10.1128/iai.6.6.934-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. P., Rodbell M. Inhibition by fluoride ion of hormonal activation of fat cell adenylate cyclase. J Biol Chem. 1973 Jul 25;248(14):4901–4904. [PubMed] [Google Scholar]
- Hewlett E. L., Guerrant R. L., Evans D. J., Jr, Greenough W. B., 3rd Toxins of Vibrio cholerae and Escherichia coli stimulate adenyl cyclase in rat fat cells. Nature. 1974 May 24;249(455):371–373. doi: 10.1038/249371a0. [DOI] [PubMed] [Google Scholar]
- Heyningen S Van Cholera toxin: interaction of subunits with ganglioside GM1. Science. 1974 Feb 15;183(4125):656–657. doi: 10.1126/science.183.4125.656. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Fixation and inactivation of cholera toxin by GM1 ganglioside. Scand J Infect Dis. 1973;5(1):77–78. doi: 10.3109/inf.1973.5.issue-1.15. [DOI] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- Levey G. S., Klein I. Solubilized myocardial adenylate cyclase. Restoration of histamine responsiveness by phosphatidylserine. J Clin Invest. 1972 Jun;51(6):1578–1582. doi: 10.1172/JCI106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey G. S. Restoration of glucagon responsiveness of solubilized myocardial adenyl cyclase by phosphatidylserine. Biochem Biophys Res Commun. 1971 Apr 2;43(1):108–113. doi: 10.1016/s0006-291x(71)80093-1. [DOI] [PubMed] [Google Scholar]
- Levey G. S. Restoration of norepinephrine responsiveness of solubilized myocardial adenylate cyclase by phosphatidylinositol. J Biol Chem. 1971 Dec 10;246(23):7405–7407. [PubMed] [Google Scholar]
- Levey G. S. Solubilization of myocardial adenyl cyclase. Biochem Biophys Res Commun. 1970 Jan 6;38(1):86–92. doi: 10.1016/0006-291x(70)91087-9. [DOI] [PubMed] [Google Scholar]
- Lospalluto J. J., Finkelstein R. A. Chemical and physical properties of cholera exo-enterotoxin (choleragen) and its spontaneously formed toxoid (choleragenoid). Biochim Biophys Acta. 1972 Jan 26;257(1):158–166. doi: 10.1016/0005-2795(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Lönnroth I., Holmgren J. Subunit structure of cholera toxin. J Gen Microbiol. 1973 Jun;76(2):417–427. doi: 10.1099/00221287-76-2-417. [DOI] [PubMed] [Google Scholar]
- Oye I., Sutherland E. W. The effect of epinephrine and other agents on adenyl cyclase in the cell membrane of avian erythrocytes. Biochim Biophys Acta. 1966 Oct 31;127(2):347–354. doi: 10.1016/0304-4165(66)90389-8. [DOI] [PubMed] [Google Scholar]
- Ramachandran J. A new simple method for separation of adenosine 3',5'-cyclic monophosphate from other nucleotides and its use in the assay of adenyl cyclase. Anal Biochem. 1971 Sep;43(1):227–239. doi: 10.1016/0003-2697(71)90128-x. [DOI] [PubMed] [Google Scholar]
- Schramm M., Feinstein H., Naim E., Lang E., Lasser M. Epinephrine binding to the catecholamine receptor and activation of the adenylate cyclase in erythrocyte membranes (hormone receptor- -adrenergic receptor-cyclic AMP-turkey). Proc Natl Acad Sci U S A. 1972 Feb;69(2):523–527. doi: 10.1073/pnas.69.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J., Gibson W., Jessup S., Ramwell P. The effect of PGE-1 on cyclic AMP and ion movements in turkey erythrocytes. Ann N Y Acad Sci. 1971 Apr 30;180:241–260. doi: 10.1111/j.1749-6632.1971.tb53195.x. [DOI] [PubMed] [Google Scholar]



