Abstract
Background
Dabigatran is a novel oral anticoagulant approved for thromboprophylaxis in atrial fibrillation. Adoption patterns of this new agent in community practice are unknown.
Methods and Results
We studied patterns of dabigatran use among patients enrolled in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF) Registry between June 2010 and August 2011 and followed for 12 months. Among 9974 atrial fibrillation patients included, 1217 (12%) were treated with dabigatran during the study. Overall, patients receiving dabigatran were younger (median age 72 versus 75 years, P<0.0001), more likely to be white (92% versus 89%, P=0.005), more likely to have private insurance (33% versus 25%, P<0.0001), and less likely to have prior cardiovascular disease (4% versus 33%, P<0.0001). They had more new‐onset atrial fibrillation (8.8% versus 4.1%, P<0.0001), lower CHADS2 scores (estimated risk based on the presence of congestive heart failure, hypertension, aged ≥75 years, diabetes mellitus, and prior stroke or transient ischemic attack; mean 2.0 versus 2.3, P<0.0001), and lower Anticoagulation and Risk Factors in Atrial Fibrillation scores (mean 2.4 versus 2.8, P<0.0001). More than half (n=14/25, 56%) of patients with severe kidney disease were not prescribed reduced dosing, whereas 10% (n=91/920) with preserved renal function received lower dosing. Among patients not on dabigatran at baseline, 8% had dabigatran initiated during follow‐up. Patient education was significantly associated with switching from warfarin to dabigatran (adjusted odds ratio for postgraduate 1.73, P=0.007), whereas antiarrhythmic drug use significantly correlated with de novo adoption of dabigatran (adjusted odds ratio 2.4, P<0.0001).
Conclusions
Patients receiving dabigatran were younger and at a lower risk of stroke and bleeding. Patients appeared to drive switching from warfarin, whereas clinical characteristics influenced de novo start of dabigatran. These data suggest cautious early uptake of dabigatran, and more careful attention to dosing adjustments is warranted.
Clinical Trial Registration
URL: Clinicaltrials.gov. Unique identifier: NCT01165710.
Keywords: anticoagulant, atrial fibrillation, dabigatran, dosing, pharmacoepidemiology
Introduction
Atrial fibrillation (AF) increases the risk of stroke or systemic embolism in patients by up to 5‐fold.1 Traditional therapy with vitamin K antagonism (ie, warfarin) has reduced that risk to ≈1% annually, depending on the population treated.2 However, warfarin has significant shortcomings, particularly its narrow therapeutic window, need for frequent monitoring, and numerous drug and food interactions. Alternatives to warfarin have been a long‐sought goal in the clinical care of patients with AF. The Randomized Evaluation of Long‐Term Anticoagulation Therapy (RE‐LY) trial demonstrated that a novel direct thrombin inhibitor, dabigatran etexilate, could reduce risks of ischemic and overall strokes while having no higher risk of bleeding relative to warfarin.3 Based on these data, dabigatran became the first alternative, novel oral anticoagulant (OAC) approved for thromboembolism prevention in nonvalvular AF.3–4
Many had predicted that the uptake of this alternative would be very rapid, given that it lacked many of warfarin's pitfalls. Patients no longer required routine monitoring, dietary intake did not alter anticoagulant effect, and dose adjustments were not required on a routine basis (eg, during temporary antibiotic therapy). However, there is limited knowledge regarding the uptake patterns of dabigatran for AF in contemporary US practice and available data are limited to physician surveys or administrative claims.5–6 It is not clear what proportion of patients with AF in the United States is treated with dabigatran and what drives selection of such patients. Last, dosing of dabigatran presented a challenge for regulatory authorities,4,7 and it is not clear how providers responded in routine practice, particularly for older patients and/or those with renal insufficiency. The objectives of the current analysis were to (1) describe early patterns of dabigatran use in community practice, (2) identify patient and/or provider factors associated with the use of dabigatran in patients with AF, and (3) describe dosing patterns of dabigatran.
Methods
The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF) is a nationwide registry of outpatients with AF treated by primary care physicians, cardiologists, and/or electrophysiologists. Sites were invited to participate based on achieving a nationally representative sample, through an adaptive design geared toward heterogeneity of practice‐type and geography. Site management and study coordination were performed by the Duke Clinical Research Institute. Each site enrolled consecutive patients, aged ≥18 years, with electrocardiographically documented AF that was not due to a reversible cause. They were expected to provide follow‐up every 6 months for ≥2 years and could not be included if life expectancy was <6 months. A web‐based case report form was used to gather data, primarily from the patient's medical record and treating physician. Data components included demographics, medical history, AF history (including symptoms), medical therapies, vital signs, laboratory and echocardiographic measures, and incident procedures and adverse events. Additional details of the ORBIT‐AF design and rationale have been previously described.8
The ORBIT‐AF Registry began before the availability of any novel OACs (June 2010), however, dabigatran was approved shortly thereafter (October 2010).9 The registry completed enrollment before approval of any other novel anticoagulants, and rivaroxaban and apixaban were subsequently approved during the follow‐up phase (Figure 1).
Figure 1.
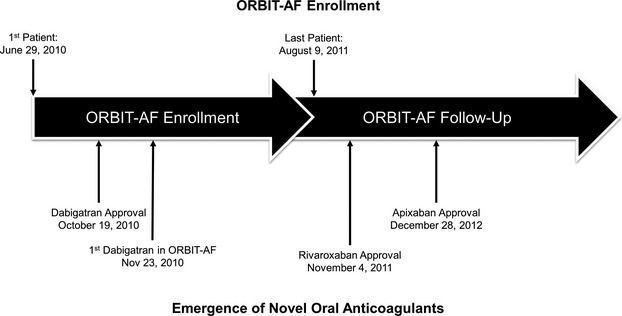
Timeline of ORBIT‐AF enrollment vs emergence of novel oral anticoagulants in the US. ORBIT‐AF indicates Outcomes Registry for Better Informed Treatment of Atrial Fibrillation.
Collection of data on medical therapies included historical anticoagulation use, current anticoagulants, dosing, monitoring of international normalized ratios (INRs, where appropriate), and reasons for any discontinuations or contraindications. Warfarin monitoring (INR) was reported throughout follow‐up and was requested at baseline (but subject to availability at enrollment). Dosing for dabigatran is recorded as total daily dosing, and sites are prompted to confirm each entry of anticoagulant dosing to ensure appropriate dose reporting. Individual twice‐daily dosing levels were calculated from the total daily dose. Sites were instructed to record the present medical therapy, as well as the reasons for any discontinuations in therapies, every 6 months. The present analysis includes patient follow‐up to 1 year.
Study Population
The overall study population included all patients in the registry who had ≥1 visit (baseline or follow‐up) on or after the first reported use of dabigatran in the registry and thus were eligible for treatment with dabigatran. First, we assessed temporal uptake of dabigatran chronologically. Next, patients who were treated with dabigatran during the study period were compared with patients who did not receive dabigatran. Additionally, we described dabigatran dosing patterns overall and by age and renal function.
Patients Adopting Dabigatran During Follow‐up
To identify specific factors associated with the initiation of dabigatran, the population of dabigatran users was subsequently limited to 2 populations: (1) patients receiving warfarin at baseline and (2) patients without OAC at baseline (antiplatelet agents may have been used). To assess baseline and follow‐up characteristics that may have influenced dabigatran adoption, we purposefully did not include patients already taking dabigatran at baseline (n=501; no other OACs were used at baseline). Patients without follow‐up visits at 6 or 12 months (n=670) and those missing data on dabigatran at follow‐up (n=9) were also excluded (Figure 2). Each of these groups was queried for rates of dabigatran use at subsequent follow‐up (6 or 12 months), and comparison was made between patients who did not adopt dabigatran and those who did. Multivariable models were used to identify factors associated with dabigatran adoption at follow‐up.
Figure 2.
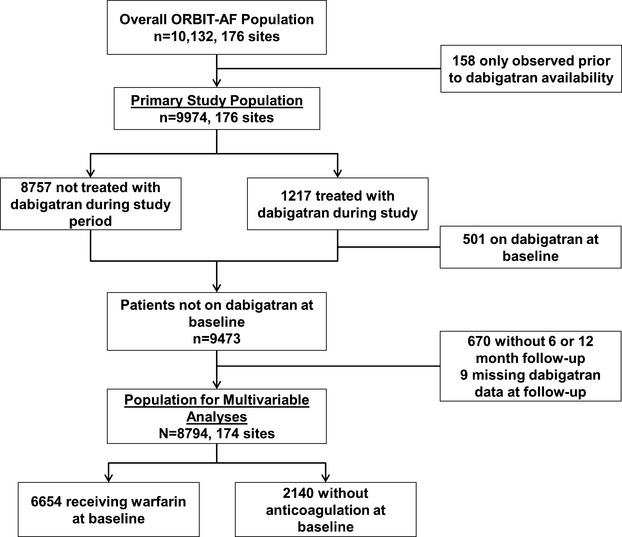
Patient inclusion and exclusion in the current analysis. ORBIT‐AF indicates Outcomes Registry for Better Informed Treatment of Atrial Fibrillation.
Statistical Methods
All baseline characteristics and univariate data are presented as frequencies and percentages for categorical variables and medians (IQR) or means (SD) for continuous variables. The baseline characteristics were compared using the χ2 for categorical variables and the Wilcoxon rank sum test for continuous variables.
We identified factors associated with initiating dabigatran in 2 distinct populations: (1) those taking warfarin at baseline (switched to dabigatran versus those not) and (2) those without OACs at baseline (started dabigatran versus those not). Dabigatran use was captured in discrete time intervals at 6 and 12 months, rather than specific dates. We therefore used a proportional odds model for discrete time to identify factors related to starting dabigatran at either time interval. This method essentially fit a logistic regression model for the binary occurrence of event, at each discrete time point, and combined the results to provide a single odds ratio (OR) for the effect of covariates. The method can also be viewed as a discrete time Cox model for time‐to‐starting dabigatran. As with time‐to‐event analyses, individuals contributed all available follow‐up information and were censored (removed from the risk set) when the patient was lost to follow‐up. Thus, these models included patients with ≥1 follow‐up visit but not necessarily full follow‐up.10–11
Candidate variables included demographics, medical history, vital signs, laboratory data, AF status, pharmacotherapy, contraindication to OAC, functional status, and provider specialty. All continuous variables were evaluated for nonlinearity with the outcome, and nonlinear relationships were addressed using linear splines.
Missing data were multiply imputed, and final estimates and standard errors reflect the combined analysis over 5 imputed datasets (missingness was <5% for all the candidate variables except serum creatinine [7%], hematocrit [11%], and left ventricular ejection fraction [11%]). Model selection using backward selection with a stay criterion of 0.05 using the first imputed dataset was used to obtain a model in which each factor was independently associated with switching to dabigatran within 1 year. The model was fit using logistic generalized estimating equations with exchangeable working correlation matrix to account for within‐site clustering because patients at the same site are more likely to have similar responses relative to patients at other sites (ie, within‐center correlation for responses). We used empirical standard errors, robust to mis‐specification of the correlation structure. Backward selection with an inclusion criterion of 0.05 was used to build the models. Adjusted associations for outcomes were displayed as ORs with 95% CIs.
Two separate sensitivity analyses were performed. In the first, the time‐in‐therapeutic range (TTR) of baseline INR data was calculated using a modification of the Rosendaal method12 and was included as a predictor in the multivariable model for switching to dabigatran (among patients receiving warfarin at baseline). We imputed daily INR values between the first and last measured INR among INR values obtained before baseline. This analysis was performed only for patients receiving warfarin for ≥60 days before baseline, with ≥2 INR values measured before baseline. Overall, 5315 patients (89% of those on warfarin at baseline for ≥60 days) had ≥2 INR values available at baseline and TTR was calculated using these values. For the remaining 11%, TTR was imputed using multiple imputation for the sensitivity analysis. The second sensitivity analysis was performed to evaluate the contribution of post baseline events into the models for switching. In both patient populations (warfarin and no OAC at baseline), separate, time‐dependent covariates for cause‐specific hospitalizations were added to the baseline models for switching to dabigatran. Cause‐specific events were classified by the investigator and included cardiovascular, bleeding, or noncardiovascular, nonbleeding and hospitalization. If a cardiovascular event occurred before 6 months, the time‐dependent covariate would take a value of 1 at both the 6‐month and 12‐month intervals. To the extent that events preceded switching, these associations are predictive. It is also possible that switching preceded events but was not measured until a later interval.
All analyses of the aggregate, deidentified data were performed at the Duke Clinical Research Institute using SAS software (version 9.3, SAS Institute).
Results
The overall ORBIT‐AF population included 10 132 patients from 176 sites from June 29, 2010, through August 9, 2011 (Figure 2). Dabigatran use was first reported in the registry on November 23, 2010. After excluding 158 patients who were not observed after that date, there was a study population of 9974 patients from 176 sites. Of these, 1217 (12%) were treated with dabigatran during the study period. Temporal use of dabigatran is shown in Figure 3.
Figure 3.
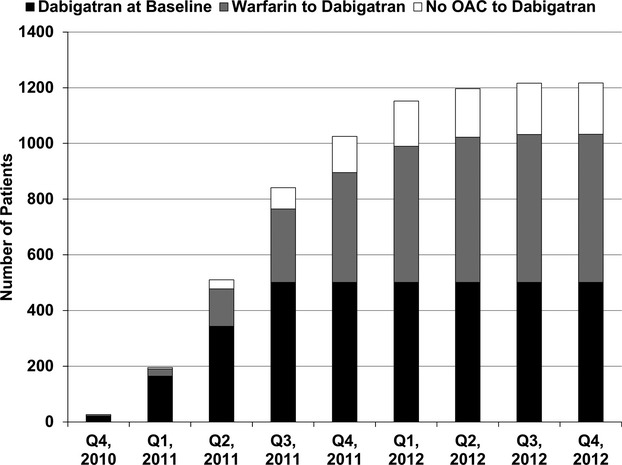
Temporal adoption of dabigatran in ORBIT‐AF. OAC indicates oral anticoagulation; ORBIT‐AF, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation.
Patients treated with dabigatran were younger (median age 72 versus 75 years, P<0.0001), more likely to be white (92% versus 89%, P=0.005), more likely to have private insurance (33% versus 25%, P<0.0001), and had higher calculated creatinine clearance (CrCl, median 78 versus 69 mL/min per 1.73 m2, P<0.0001) compared with patients who did not receive dabigatran (Table 1).
Table 1.
Demographics, Past Medical History, and Laboratory Studies of Study Population
| Total (N=9974) | Dabigatran Treatment (n=1217) | No Dabigatran Treatment (n=8757) | P Value | |
|---|---|---|---|---|
| Age, y | 75 (67 to 82) | 72 (64 to 80) | 75 (67 to 82) | <0.0001* |
| Female sex | 42 | 41 | 43 | 0.3 |
| Race | ||||
| White | 89 | 92 | 89 | 0.005 |
| Black or African American | 4.9 | 3.5 | 5.1 | |
| Hispanic | 4.3 | 2.9 | 4.4 | |
| Other | 1.4 | 1.6 | 1.4 | |
| Health insurance status | ||||
| Medicare or Medicaid | 70 | 63 | 71 | <0.0001* |
| Private | 26 | 33 | 25 | |
| Other | 4.9 | 4.7 | 4.9 | |
| Hypertension | 83 | 82 | 83 | 0.6 |
| Hyperlipidemia | 72 | 70 | 72 | 0.1 |
| Diabetes | 29 | 26 | 30 | 0.004* |
| COPD | 16 | 13 | 17 | 0.002* |
| Osteoporosis | 13 | 12 | 13 | 0.1 |
| Prior gastrointestinal bleeding | 9.0 | 7.2 | 9.3 | 0.02* |
| Cognitive impairment or dementia | 3.1 | 3.1 | 3.1 | 0.9 |
| Frailty | 5.7 | 3.5 | 6.0 | 0.0005* |
| BMI, kg/m2 | 29 (25 to 34) | 30 (26 to 35) | 29 (25 to 34) | <0.0001* |
| Hemoglobin, g/dL | 13.5 (12.3 to 14.6) | 13.7 (12.6 to 14.9) | 13.5 (12.2 to 14.6) | <0.0001* |
| Calculated creatinine clearance*, mL/min per 1.73 m2 | 70 (50 to 97) | 78 (57 to 105) | 69 (49 to 95) | <0.0001* |
Values are presented as % or median (interquartile range), unless noted otherwise. BMI indicates body mass index; COPD, chronic obstructive pulmonary disease.
As calculated by the Cockcroft‐Gault formula.
Those receiving dabigatran were less likely to have any form of cardiovascular disease (Table 2), including peripheral vascular disease (11% versus 14%, P=0.002), coronary artery disease (24% versus 33%, P<0.0001), and cerebrovascular disease (13% versus 16%, P=0.001). Left ventricular ejection fraction was higher in patients treated with dabigatran (median 58% versus 55, P<0.0001).
Table 2.
Cardiovascular History
| Total (N=9974) | Dabigatran Treatment (n=1217) | No Dabigatran Treatment (n=8757) | P Value | |
|---|---|---|---|---|
| Peripheral vascular disease | 13 | 11 | 14 | 0.002 |
| Coronary artery disease | 32 | 24 | 33 | <0.0001 |
| Prior MI | 16 | 10 | 17 | <0.0001 |
| Prior CABG | 15 | 10 | 15 | <0.0001 |
| Prior PCI | 17 | 13 | 18 | <0.0001 |
| Heart failure | 32 | 25 | 33 | <0.0001 |
| Implanted cardiac device | 27 | 20 | 28 | <0.0001 |
| Significant valve disease | 25 | 18 | 26 | <0.0001 |
| Moderate/severe mitral stenosis | 1.4 | 0.6 | 1.5 | 0.009 |
| Prior valve replacement | 8.1 | 3.2 | 8.8 | <0.0001 |
| Mechanical valve | 3.1 | 0.5 | 3.5 | <0.0001 |
| Prior cerebrovascular events | 16 | 13 | 16 | 0.001 |
| Stroke (all‐cause) | 8.8 | 6.7 | 9.1 | 0.005 |
| Stroke—nonhemorrhagic | 7.9 | 6.0 | 8.2 | 0.009 |
| Stroke—hemorrhagic | 0.8 | 0.6 | 0.8 | 0.4 |
| Other intracranial bleeding | 0.9 | 1.0 | 0.9 | 0.8 |
| TIA | 8.1 | 6.9 | 8.2 | 0.1 |
| Cardiac medications | ||||
| β‐Blocker | 64 | 62 | 64 | 0.10 |
| Nondihydropyridine calcium channel blocker | 17 | 18 | 16 | 0.3 |
| ACEI or ARB | 51 | 52 | 51 | 0.6 |
| Statin | 55 | 54 | 55 | 0.4 |
| LVEF, % | 55 (50 to 61) | 58 (52 to 65) | 55 (50 to 60) | <0.0001 |
| LA diameter, cm | 4.4 (3.9 to 5.0) | 4.4 (3.9 to 4.9) | 4.4 (3.9 to 5.0) | 0.0498 |
Values are presented as % or median (IQR). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass graft surgery; LA, left atrium; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Historical AF data and anticoagulation history are presented in Table 3. Compared with patients not treated with dabigatran, those receiving dabigatran were more likely to have new‐onset AF at baseline (8.8% versus 4.1%) and had lower CHADS2 scores (estimated risk based on the presence of congestive heart failure, hypertension, aged ≥75 years, diabetes mellitus, and prior stroke or transient ischemic attack; mean 2.0 versus 2.3, P<0.0001) and Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) scores (mean 2.4 versus 2.8, P<0.0001). They were more likely to be managed with a rhythm control strategy (38% versus 31%, P<0.0001), including prior cardioversion (35% versus 29%, P<0.0001), prior antiarrhythmic therapy (50% versus 45%, P=0.0001), and prior catheter ablation for AF (8.7% versus 5.1%, P<0.0001). Management by an electrophysiology provider was slightly more common in patients receiving dabigatran (19% versus 17%, P=0.03).
Table 3.
Atrial Fibrillation and Anticoagulation History
| Total (N=9974) | Dabigatran Treatment (n=1217) | No Dabigatran Treatment (n=8757) | P Value | |
|---|---|---|---|---|
| AF type at baseline | ||||
| New onset | 4.7 | 8.8 | 4.1 | <0.0001* |
| Paroxysmal | 51 | 49 | 51 | |
| Persistent | 17 | 18 | 17 | |
| Longstanding persistent | 28 | 23 | 29 | |
| Time from AF diagnosis >12 mo | 81 | 70 | 83 | <0.0001* |
| Rhythm control treatment strategy reported | 32 | 38 | 31 | <0.0001* |
| CHADS2 score, mean (SD) | 2.3 (1.3) | 2.0 (1.2) | 2.3 (1.3) | <0.0001* |
| CHADS2 score groups | ||||
| 0 | 6.6 | 7.6 | 6.4 | <0.0001* |
| 1 | 22 | 30 | 21 | |
| ≥2 | 71 | 62 | 72 | |
| ATRIA score, mean (SD) | 2.8 (2.0) | 2.4 (1.8) | 2.8 (2.0) | <0.0001* |
| Prior cardioversion | 30 | 35 | 29 | <0.0001* |
| Prior catheter ablation for AF | 5.5 | 8.7 | 5.1 | <0.0001* |
| Prior antiarrhythmic therapy | 45 | 50 | 45 | 0.0001* |
| Current antiarrhythmic therapy | 29 | 36 | 28 | <0.0001* |
| Amiodarone | 10.0 | 9.5 | 10.0 | 0.5 |
| Sotalol | 6.1 | 8.1 | 5.9 | 0.002* |
| Dronedarone | 4.6 | 7.6 | 4.2 | <0.0001* |
| Flecainide | 2.9 | 4.0 | 2.8 | 0.02* |
| Propafenone | 2.4 | 2.7 | 2.3 | 0.4 |
| Dofetilide | 1.9 | 2.6 | 1.8 | 0.08 |
| Baseline antiplatelet therapy | ||||
| Aspirin | 44 | 39 | 45 | 0.0002* |
| Clopidogrel | 7.0 | 4.2 | 7.4 | <0.0001* |
| Anticoagulation clinic management at baseline | 43 | 36 | 44 | 0.0003* |
| Relative or absolute contraindication to anticoagulation | 13 | 11 | 13 | 0.049* |
| Treating provider specialty* | ||||
| Primary care provider | 67 | 65 | 68 | 0.06 |
| Cardiologist | 80 | 81 | 80 | 0.4 |
| Electrophysiologist | 17 | 19 | 17 | 0.03* |
| Neurologist | 2.1 | 1.5 | 2.2 | 0.1 |
Values are presented as% or median (IQR), unless noted otherwise. AF indicates atrial fibrillation; ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; CHADS2, estimated risk based on the presence of congestive heart failure, hypertension, aged ≥75 years, diabetes mellitus, and prior stroke or transient ischemic attack.
Provider specialty is not mutually exclusive; each patient may have ≥1 specialists involved in the care of AF patients.
Dabigatran Dosing
Dosing strategies of dabigatran, stratified by age and CrCl,13 are shown in Figure 4. The use of 150 mg twice daily was the prevailing dosing strategy, across subgroups, except in patients with CrCl 15 to 30 mL/min per 1.73 m2 (56% received 150 mg twice daily). Of patients aged ≥80 years with CrCl >30 mL/min per 1.73 m2 (n=256), 14% were prescribed 75 mg twice daily. Ten percent of patients under the age of 80, with preserved renal function, were prescribed 75 mg twice daily. P‐glycoprotein inhibitors were used in a minority of these patients with preserved renal function receiving the lower dabigatran dose (10.8% received dronedarone, 17% received nondihydropyridine calcium channel blockers, 6.7% received amiodarone, and 0.8% received quinidine).
Figure 4.
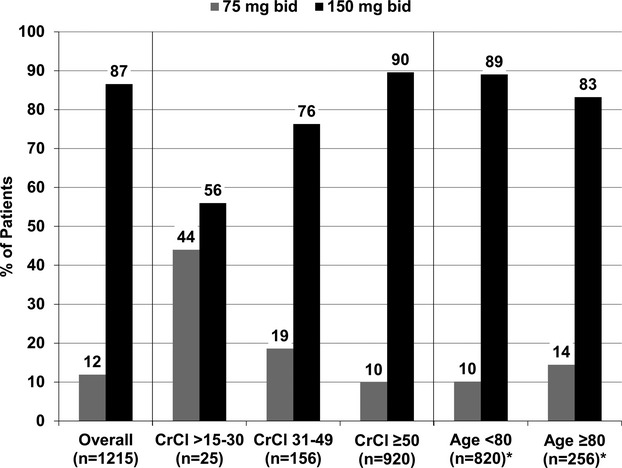
Distribution of dabigatran dosing overall and in high‐risk subgroups. Numbers may not sum to 100% due to reporting of other dosing regimens. *Excludes patients with CrCl <30 mL/min per 1.73 m2. CrCl indicates creatinine clearance calculated by the Cockcroft‐Gault formula.13
Adoption of Dabigatran During Follow‐up
Among 6654 patients receiving warfarin at baseline, 532 (8.0%) were switched to dabigatran at 6‐ or 12‐month follow‐up. As described by the site investigator, major reasons for the discontinuation of warfarin in these patients included (reasons are not mutually exclusive) physician preference (n=213, 40%), patient preference (n=171, 32%), inability to adhere to and/or monitor warfarin (n=32, 6.0%), high bleeding risk (n=10, 1.9%), incident bleeding event (n=4, 0.8%), and “other” (n=96, 18%). Warfarin discontinuation reason was not available for 182 (34%) of patients. Of 2140 patients not receiving OAC at baseline, 184 (8.6%) adopted dabigatran at follow‐up. Demographics (Table 4), cardiovascular history (Table 5), and AF history (Table 6) are shown for patients who did and those who did not adopt dabigatran during follow‐up.
Table 4.
Demographics, Past Medical History, and Laboratory Studies
| Use of Warfarin at Baseline | No OAC at Baseline | |||||
|---|---|---|---|---|---|---|
| Not Switched to Dabigatran (n=6122) | Switched to Dabigatran (n=532) | P Value | Not Switched to Dabigatran (n=1956) | Switched to Dabigatran (n=184) | P Value | |
| Age, y | 76 (68 to 82) | 73 (64 to 80) | <0.0001 | 74 (64 to 82) | 68 (62 to 80) | 0.005 |
| Female | 43 | 41 | 0.4 | 43 | 41 | 0.5 |
| Race | ||||||
| White | 89 | 94 | 0.002 | 89 | 90 | 0.9 |
| Black or African American | 4.7 | 2.8 | 5.1 | 4.4 | ||
| Hispanic | 4.5 | 1.7 | 3.6 | 3.8 | ||
| Other | 1.3 | 1.9 | 1.7 | 1.1 | ||
| Health insurance status | ||||||
| Medicare or Medicaid | 73 | 66 | 0.001 | 65 | 55 | 0.01 |
| Private | 22 | 29 | 30 | 40 | ||
| Other | 4.5 | 5.1 | 5.3 | 4.9 | ||
| Hypertension | 85 | 84 | 0.4 | 78 | 79 | 0.7 |
| Hyperlipidemia | 74 | 72 | 0.3 | 69 | 65 | 0.3 |
| Diabetes | 31 | 24 | 0.002 | 26 | 25 | 0.8 |
| COPD | 17 | 14 | 0.1 | 17 | 13 | 0.2 |
| Osteoporosis | 14 | 13 | 0.5 | 14 | 10 | 0.2 |
| Prior gastrointestinal bleeding | 8.2 | 7.3 | 0.5 | 13 | 7.1 | 0.01 |
| Cognitive impairment or dementia | 2.5 | 3.8 | 0.09 | 3.7 | 1.7 | 0.1 |
| Frailty | 5.2 | 4.1 | 0.3 | 8.2 | 2.7 | 0.007 |
| BMI, kg/m2 | 29 (26 to 34) | 29 (26 to 35) | 0.3 | 28 (25 to 33) | 30 (26 to 36) | 0.0002 |
| Hemoglobin, g/dL | 13.5 (12.3 to 14.6) | 13.7 (12.6 to 14.8) | 0.004 | 13.4 (12.1 to 14.5) | 13.7 (12.5 to 14.9) | 0.03 |
| Calculated creatinine clearance*, mL/min per 1.73 m2 | 69 (50 to 94) | 77 (55 to 101) | <0.0001 | 69 (48 to 99) | 77 (59 to 107) | 0.003 |
Values are presented as % or median (IQR). BMI indicates body mass index; COPD, chronic obstructive pulmonary disease; OAC, oral anticoagulant.
As calculated by the Cockcroft‐Gault formula.
Table 5.
Cardiovascular History
| Use of Warfarin at Baseline | No OAC at Baseline | |||||
|---|---|---|---|---|---|---|
| Not Switched to Dabigatran (n=6122) | Switched to Dabigatran (n=532) | P Value | Not Switched to Dabigatran (n=1956) | Switched to Dabigatran (n=184) | P Value | |
| Peripheral vascular disease | 14 | 10.0 | 0.01 | 13 | 14 | 0.6 |
| Coronary artery disease | 34 | 22 | <0.0001 | 33 | 26 | 0.03 |
| Prior MI | 17 | 8.5 | <0.0001 | 18 | 13 | 0.08 |
| Prior CABG | 16 | 9.6 | 0.0001 | 15 | 10 | 0.1 |
| Prior PCI | 18 | 12 | 0.001 | 18 | 16 | 0.4 |
| Heart Failure | 35 | 26 | <0.0001 | 27 | 18 | 0.01 |
| Implanted cardiac device | 30 | 22 | 0.0002 | 25 | 17 | 0.02 |
| Significant valve disease | 29 | 19 | <0.0001 | 20 | 16 | 0.2 |
| Moderate/severe mitral stenosis | 1.9 | 0.6 | 0.03 | 0.7 | 0.5 | 0.8 |
| Prior valve replacement | 10.3 | 4.1 | <0.0001 | 5.0 | 1.1 | 0.02 |
| Mechanical Valve | 4.4 | 0.6 | <0.0001 | 1.0 | 0 | 0.2 |
| Prior cerebrovascular events | 17 | 14 | 0.06 | 13 | 9.2 | 0.1 |
| Stroke (all cause) | 9.7 | 7.9 | 0.2 | 7.0 | 4.9 | 0.3 |
| Stroke—nonhemorrhagic | 9.0 | 7.3 | 0.2 | 5.6 | 3.8 | 0.3 |
| Stroke—hemorrhagic | 0.6 | 0.6 | 0.98 | 1.3 | 1.1 | 0.8 |
| Other intracranial bleeding | 0.6 | 0.9 | 0.4 | 1.8 | 1.6 | 0.8 |
| TIA | 9.2 | 7.9 | 0.3 | 5.7 | 3.3 | 0.2 |
| Cardiac medications | ||||||
| β‐Blocker | 67 | 61 | 0.006 | 58 | 62 | 0.2 |
| Nondihydropyridine calcium channel blocker | 17 | 21 | 0.053 | 14 | 16 | 0.5 |
| ACEI or ARB | 54 | 56 | 0.2 | 45 | 41 | 0.3 |
| Statin | 57 | 55 | 0.3 | 51 | 52 | 0.8 |
| LVEF, % | 55 (50 to 60) | 57 (50 to 64) | 0.003 | 60 (53 to 64) | 59 (53 to 65) | 0.7 |
| LA diameter, cm | 4.5 (4.0 to 5.1) | 4.5 (3.9 to 5.0) | 0.09 | 4.2 (3.7 to 4.7) | 4.3 (3.9 to 4.9) | 0.09 |
Values are presented as % or median (interquartile range). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass graft surgery; LA, left atrium; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OAC, oral anticoagulant; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Table 6.
Atrial Fibrillation and Anticoagulation History
| Use of Warfarin at Baseline | No OAC at Baseline | |||||
|---|---|---|---|---|---|---|
| Not Switched to Dabigatran (n=6122) | Switched to Dabigatran (n=532) | P Value | Not Switched to Dabigatran (n=1956) | Switched to Dabigatran (n=184) | P Value | |
| AF type at baseline | ||||||
| New onset | 3.0 | 5.1 | 0.04 | 6.1 | 10 | 0.003 |
| Paroxysmal | 46 | 48 | 66 | 53 | ||
| Persistent | 18 | 17 | 13 | 16 | ||
| Longstanding persistent | 33 | 30 | 14 | 20 | ||
| Time from AF diagnosis >12 mo | 85 | 78 | <0.0001 | 78 | 76 | 0.5 |
| Rhythm control treatment strategy reported | 28 | 32 | 0.04 | 41 | 46 | 0.2 |
| CHADS2 score, mean (SD) | 2.4 (1.3) | 2.1 (1.2) | <0.0001 | 2.0 (1.4) | 1.8 (1.1) | 0.02 |
| CHADS2 score groups | ||||||
| 0 | 4.3 | 5.6 | <0.0001 | 13 | 11 | 0.2 |
| 1 | 19 | 29 | 26 | 34 | ||
| ≥2 | 77 | 65 | 61 | 54 | ||
| ATRIA Score, mean (SD) | 2.8 (1.9) | 2.5 (1.8) | 0.0001 | 2.8 (2.1) | 2.5 (1.9) | 0.3 |
| Prior cardioversion | 32 | 35 | 0.2 | 22 | 30 | 0.02 |
| Prior catheter ablation for AF | 5.0 | 7.7 | 0.006 | 5.7 | 7.6 | 0.3 |
| Prior antiarrhythmic therapy | 44 | 51 | 0.004 | 48 | 54 | 0.1 |
| Current antiarrhythmic therapy | 26 | 31 | 0.01 | 35 | 43 | 0.03 |
| Amiodarone | 10.0 | 8.1 | 0.2 | 11 | 6.5 | 0.08 |
| Dronedarone | 3.8 | 7.0 | 0.0004 | 5.8 | 8.7 | 0.1 |
| Sotalol | 5.3 | 7.5 | 0.03 | 7.4 | 9.2 | 0.4 |
| Flecainide | 2.1 | 2.4 | 0.6 | 5.0 | 5.4 | 0.8 |
| Propafenone | 1.9 | 2.1 | 0.7 | 3.8 | 7.1 | 0.03 |
| Dofetilide | 1.9 | 3.0 | 0.08 | 1.8 | 1.6 | 0.8 |
| Baseline antiplatelet therapy | ||||||
| Aspirin | 36 | 37 | 0.6 | 74 | 67 | 0.046 |
| Clopidogrel | 4.8 | 3.6 | 0.2 | 16 | 10 | 0.04 |
| Anticoagulation clinic management at baseline | 45 | 36 | <0.0001 | — | — | — |
| Relative or absolute contraindication to anticoagulation | 4.7 | 3.4 | 0.2 | 40 | 26 | 0.0001 |
| Treating provider specialty* | ||||||
| Primary care provider | 69 | 66 | 0.1 | 65 | 71 | 0.1 |
| Cardiologist | 81 | 82 | 0.6 | 77 | 82 | 0.1 |
| Electrophysiologist | 17 | 19 | 0.3 | 16 | 16 | 0.9 |
| Neurologist | 2.5 | 1.7 | 0.2 | 1.2 | 0 | 0.1 |
Values are presented as %, unless noted otherwise. AF indicates atrial fibrillation; ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; CHADS2, estimated risk based on the presence of congestive heart failure, hypertension, aged ≥75 years, diabetes mellitus, and prior stroke or transient ischemic attack; OAC, oral anticoagulation.
Provider specialty is not mutually exclusive; each patient may have ≥1 specialists involved in the care of AF patients.
Multivariable models of factors associated with adoption of dabigatran during follow‐up are shown in Figure 5. They differed for patients receiving warfarin at baseline versus those receiving no OAC at baseline. In patients receiving warfarin, advanced education (adjusted OR for postgraduate 1.73, 95% CI 1.16 to 2.57, P=0.007) and cognitive impairment (adjusted OR 1.92, 95% CI 1.20 to 3.07, P=0.007) were associated with adoption of dabigatran. Among patients not receiving OAC at baseline, current antiarrhythmic use (adjusted OR 2.37, 95% CI 1.69 to 3.33, P<0.0001) was significantly associated with dabigatran initiation.
Figure 5.
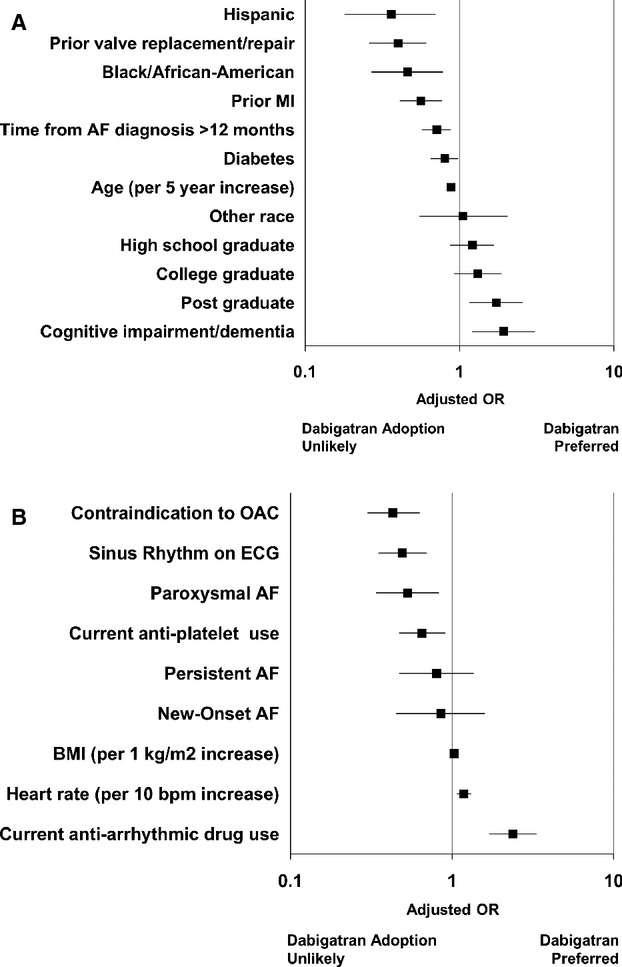
Factors significantly associated with adoption of dabigatran at follow‐up in patients receiving warfarin at baseline (A, c‐index=0.65) and in patients not using anticoagulation at baseline (B, c‐index=0.71). Reference groups: Race (vs white), AF type (vs long‐standing persistent), Education level (vs some school). AF indicates atrial fibrillation; BMI, body mass index; ECG, electrocardiogram; MI, myocardial infarction; OAC, oral anticoagulation.
Sensitivity Analyses
In patients receiving warfarin at baseline, median TTR at baseline among patients switched to dabigatran was 55% (IQR 38 to 73) versus 60% among patients not switched (IQR 42 to 75). Addition of TTR at baseline contributed minimally to the overall model (c‐index from 0.65 to 0.66, adjusted OR for dabigatran adoption per 5% increase in TTR=0.99, 95% CI 0.97 to 1.01, P=0.2). Addition of interim cause‐specific hospitalization during follow‐up (as defined by the site investigator) to patients receiving warfarin at baseline model also contributed minimally to model discrimination (c‐index from 0.65 to 0.66). Interim cardiovascular hospitalization (adjusted OR 1.32, 95% CI 1.04 to 1.68, P=0.02) and noncardiovascular, nonbleeding hospitalization (adjusted OR 1.42, 95% CI 1.09 to 1.85, P=0.01) were both significantly associated with dabigatran adoption, whereas bleeding hospitalization did not have a significant association (adjusted OR 0.84, 95% CI 0.41 to 1.73, P=0.6).
In patients not receiving OAC at baseline, addition of interim hospitalization data modestly improved the discriminatory power of the model (c‐index from 0.71 to 0.73). Cardiovascular hospitalization (adjusted OR 2.72, 95% CI 1.89 to 3.93, P<0.0001) was significantly associated with dabigatran adoption, but bleeding (adjusted OR 1.09, 95% CI 0.3 to 5.65, P=0.9) or noncardiovascular, nonbleeding (adjusted OR 1.31, 95% CI 0.78 to 2.2, P=0.3) hospitalizations were not.
Discussion
The development of dabigatran heralded a new era in the use of OACs, and this analysis is among the first to provide the details of its uptake in the clinical care of US patients with AF. Use of dabigatran was modest in this population (12% overall) and appeared to plateau in late 2012. Patients receiving dabigatran were generally younger, more likely to have private health insurance, and less likely to have comorbid cardiovascular disease. A significant proportion (56%) of patients with severe kidney disease did not receive adjusted‐dose dabigatran, whereas 10% of patients with normal renal function received reduced dosing.
An alternative to warfarin has been a long‐sought goal and highly anticipated therapeutic option. yet a minority of patients in clinical practice received dabigatran during the study period. Furthermore, despite robust data demonstrating lower rates of stroke in patients receiving dabigatran compared with those receiving warfarin,3 patients treated with dabigatran in our study were at lower risk of stroke, according to CHADS2 scores. They were also at lower risk of bleeding, as represented by prior gastrointestinal bleeding rates and ATRIA bleeding score. These data suggest a conservative adoption strategy by many providers, transitioning patients to dabigatran who are least likely to experience an adverse event. It is possible that physicians were influenced by early case reports of fatal bleeding, coupled with the caveat in the package insert of increased nonintracranial bleeding among individuals aged ≥75 years, compared with warfarin. As more methodologically rigorous data have emerged,14 the rates of dabigatran use may increase. It is noteworthy that patient preference triggered the switch from warfarin for one‐third of the patients, reinforcing the importance of patient engagement in treatment decisions. This is also evidenced in the multivariable analysis, demonstrating patient‐related characteristics, such as education level and age, closely related to the switch from warfarin to dabigatran. In contrast, characteristics of AF disease (eg, AF persistence, antiarrhythmic use) more closely correlated with de novo initiation of dabigatran.
Our data might seem to contrast those from Kirley et al, who used broad US administrative claims data to show a significant increase in use of dabigatran, for both AF and other indications.6 They demonstrated an overall increase in dabigatran treatment from 3% to 19% of anticoagulation visits, but they also noted that in the last period of follow‐up (late 2011), only 63% of these dabigatran prescriptions were for AF. Furthermore, it is not clear what proportion of those patients had new or recent diagnoses of AF (a minority of our cohort). Our cohort more specifically addresses the question of implementing dabigatran in a population of AF patients with a previously established care plan for the prevention of thromboembolism. While some providers advocate uniformly transitioning patients from warfarin to new anticoagulants, others are more hesitant and the prevailing strategy had been unclear. These results from ORBIT‐AF demonstrate that most providers and patients have not been aggressive about adopting this new therapy but seem to reserve it for specific situations.
The appropriate level of penetrance for dabigatran use in AF patients is not clear. The early selection of lower‐risk younger patients for this breakthrough therapy may reflect physician reaction to isolated case reports of serious hemorrhage and concerns regarding prescription of the higher dose for older patients. It may also reflect overall conservatism in new drug adoption respectful of the years of experience with warfarin and the significant toxicity that emerged in postmarketing surveillance with the previous generation of oral, direct‐thrombin inhibitors.15–16
Initial descriptions of dabigatran uptake from administrative data in Denmark are consistent with ours. Sorensen et al demonstrated modest early use of dabigatran in patients with AF (5%), as well as significant deviations from recommended dosing practices.17 Furthermore, they demonstrated the preferred use of dabigatran in younger patients, with less comorbidity. The risk‐treatment paradox observed in our study and the Danish population highlights the reticence of providers to expose patients to the potential risk of a new anticoagulant, despite proved safety and efficacy. Of note, outcomes in Danish patients receiving dabigatran compared favorably with those of matched controls receiving warfarin.18
Older patients with AF represent a significant challenge in the management of stroke prevention, as the risks of both ischemic stroke and major hemorrhage (including intracranial hemorrhage) are increased.19–22 One strategy proposed to mitigate this risk treatment paradox is the use of modified dosing of novel anticoagulants in older patients. Guidelines in both Canada and Europe suggest the use of the 110‐mg dose of dabigatran for older individuals (≥80 years), even in the absence of renal dysfunction. In the United States, the Food and Drug Administration did not approve this dose, as it found no patient subgroup in which the benefit outweighed the risk.4,7 However, a 75‐mg twice‐daily dose was approved for individuals with severe renal impairment (CrCl 15 to 30 mL/min per 1.73 m2). Our data demonstrate that for 14% of patients aged ≥80 years (with preserved renal function), physicians are opting for the 75‐mg twice‐daily dose, possibly to offset bleeding risk. However, the sequelae of this dosing strategy are unknown. Notably, the prescribing information for the newest anticoagulant, apixaban, provides alternative dosing for elderly patients of low weight (with or without renal function impairment), based on the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial.23–24 While preliminary data on dabigatran have failed to show a significant increased risk of bleeding with the US dosing regimen,14 more detailed correlations among dose, age, and outcomes are needed to guide management.
Limitations
These data are derived from an observational cohort of patients in clinical practice participating in a voluntary registry and thus subject to the limitations inherent of such methods. Specifically, sampling and/or reporting bias may influence the results of dabigatran uptake. Data were acquired via chart review, and their accuracy is therefore dependent on completeness of initial documentation and thoroughness of subsequent abstraction. Additionally, factors associated with adoption of dabigatran cannot be interpreted as causal relationships for switching therapies, and residual measured and unmeasured confounding may account for some or all of these findings. Similarly, precise timing of dabigatran initiation, relative to interim events such as hospitalization, cannot be precisely ascertained; this also limits any causal inferences that can be made from these data. Last, the collection period of the registry overlapped with the approval of dabigatran in October 2010, thus capturing an early phase of adoption following approval. This could have a significant impact on the rate of uptake observed in our study.
Conclusions
A modest number of US patients with AF have adopted the use of dabigatran. A significant proportion of these transitions appear to be driven by the patients. Patients receiving dabigatran were younger, had less comorbidity, and were at lower risk of stroke and bleeding compared with those not treated with dabigatran. They are often prescribed doses of dabigatran that are not consistent with their renal function. These findings of modest update of dabigatran coupled with selection of lower‐risk AF patients suggest that there has been an initially conservative approach to the use of this new therapy in clinical practice.
Sources of Funding
The ORBIT‐AF registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ. Dr Steinberg was funded by National Institutes of Health T‐32 training grant 5T32HL7101‐37.
Disclosures
Dr Steinberg, Ms Holmes, Dr Gersh, and Dr Thomas report no disclosures. Dr Ansell reports modest consultant/advisory board support from Bristol Myers Squibb, Pfizer, Janssen, Daiichi, Boehringer Ingelheim, and Alere. Dr Mahaffey reports significant research grant support from Johnson and Johnson and significant consultant/advisory board support from Johnson and Johnson. Dr Singer reports significant research grant support from Johnson and Johnson; modest consultant/advisory board support from Bayer HealthCare, Boehringer Ingelheim, Bristol‐Myers Squibb, Johnson and Johnson, and Pfizer; and significant consultant/advisory board support from Daiichi Sankyo. Dr Ezekowitz reports significant speakers bureau support from Boehringer Ingelheim; modest consultant/advisory board support from Pozen Inc, Eisai, and Astra Zeneca; and significant consultant/advisory board support from Boehringer Ingelheim, ARYx Therapeutics, Pfizer, Sanofi, Bristol Myers Squibb, Portola, Daiichi Sanko, Medtronic, Merck, Gilead, and Janssen Scientific Affairs. Dr Fonarow reports modest consultant/advisory board support from Ortho McNeil. Dr Kowey reports modest consultant/advisory board support from Boehringer Ingelheim, Bristol Myers Squibb, Johnson & Johnson, Portola, Merck, Sanofi, and Daiichi Sankyo. Dr Chang reports significant employment with Johnson & Johnson. Dr Piccini reports significant research grant support from Johnson & Johnson/Janssen Pharmaceuticals; significant other research support from Bayer HealthCare Pharmaceuticals Inc (formerly Berlex Labs), Boston Scientific Corporation, and Johnson & Johnson Pharmaceutical Research & Development; modest consultant/advisory board support from Forest Laboratories, Inc and Medtronic, Inc; and significant consultant/advisory board support from Johnson & Johnson/Janssen Pharmaceuticals. Dr Peterson reports significant research grant support from Eli Lilly & Company, Janssen Pharmaceuticals, Inc, and the American Heart Association; and modest consultant/advisory board support from Boehringer Ingelheim, Bristol‐Myers Squibb, Janssen Pharmaceuticals, Inc, Pfizer, and Genentech Inc. Dr Hylek reports modest speakers bureau support form Boehringer‐Ingelheim and Bayer and modest consultant/advisory board support from Johnson & Johnson, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Pfizer, and Ortho‐McNeil‐Janssen.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991; 22:983-988 [DOI] [PubMed] [Google Scholar]
- 2.Ezekowitz MD, Levine JA. Preventing stroke in patients with atrial fibrillation. JAMA. 1999; 281:1830-1835 [DOI] [PubMed] [Google Scholar]
- 3.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139-1151 [DOI] [PubMed] [Google Scholar]
- 4.Beasley BN, Unger EF, Temple R. Anticoagulant options—why the FDA approved a higher but not a lower dose of dabigatran. N Engl J Med. 2011; 364:1788-1790 [DOI] [PubMed] [Google Scholar]
- 5.Huang C, Siu M, Vu L, Wong S, Shin J. Factors influencing doctors' selection of dabigatran in non‐valvular atrial fibrillation. J Eval Clin Pract. 2013; 19:938-943 [DOI] [PubMed] [Google Scholar]
- 6.Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012; 5:615-621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kowey PR, Naccarelli GV. The food and drug administration decision not to approve the 110 mg dose of dabigatran: give us a way out. Am J Med. 2012; 125:732. [DOI] [PubMed] [Google Scholar]
- 8.Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Kong MH, Lopes RD, Mills RM, Peterson ED. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT‐AF. Am Heart J. 2011; 162:606-612 [DOI] [PubMed] [Google Scholar]
- 9.Boehringer‐Ingelheim Dabigatran prescribing information 2010. Package insert; 2011 [Google Scholar]
- 10.Allison PD. Discrete‐time methods for the analysis of event histories. Sociol Methodol. 1982; 13:61-98 [Google Scholar]
- 11.D'Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med. 1990; 9:1501-1515 [DOI] [PubMed] [Google Scholar]
- 12.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993; 69:236-239 [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976; 16:31-41 [DOI] [PubMed] [Google Scholar]
- 14.Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med. 2013; 368:1272-1274 [DOI] [PubMed] [Google Scholar]
- 15.Agnelli G, Eriksson BI, Cohen AT, Bergqvist D, Dahl OE, Lassen MR, Mouret P, Rosencher N, Andersson M, Bylock A, Jensen E, Boberg B. Safety assessment of new antithrombotic agents: lessons from the EXTEND study on ximelagatran. Thromb Res. 2009; 123:488-497 [DOI] [PubMed] [Google Scholar]
- 16.Albers GW, Diener HC, Frison L, Grind M, Nevinson M, Partridge S, Halperin JL, Horrow J, Olsson SB, Petersen P, Vahanian A. Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA. 2005; 293:690-698 [DOI] [PubMed] [Google Scholar]
- 17.Sorensen R, Gislason G, Torp‐Pedersen C, Olesen JB, Fosbol EL, Hvidtfeldt MW, Karasoy D, Lamberts M, Charlot M, Kober L, Weeke P, Lip GY, Hansen ML. Dabigatran use in Danish atrial fibrillation patients in 2011: a nationwide study. BMJ Open. 2013; 310.1136/bmjopen‐2013‐002758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen TB, Rasmussen LH, Skjoth F, Due KM, Callreus T, Rosenzweig M, Lip GY. Efficacy and safety of dabigatran etexilate and warfarin in “real‐world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013; 61:2264-2273 [DOI] [PubMed] [Google Scholar]
- 19.Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, Reilly PA, Ezekowitz MD, Yusuf S. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE‐LY Trial. Stroke. 2012; 43:1511-1517 [DOI] [PubMed] [Google Scholar]
- 20.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. Thirty‐day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke. 2012; 43:1795-1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the national registry of atrial fibrillation (NRAF). Am Heart J. 2006; 151:713-719 [DOI] [PubMed] [Google Scholar]
- 22.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010; 137:263-272 [DOI] [PubMed] [Google Scholar]
- 23.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981-992 [DOI] [PubMed] [Google Scholar]
- 24.Squibb B‐M. Apixaban prescribing information 2012. Package insert; 2013 [Google Scholar]


