Abstract
Background
Sleep apnea (SA) is associated with an increased risk of atrial fibrillation (AF). We sought to determine the effect of SA on cardiac structure in patients with AF, whether therapy for SA was associated with beneficial cardiac structural remodelling, and whether beneficial cardiac structural remodelling translated into a reduced risk of recurrence of AF after pulmonary venous isolation (PVI).
Methods and Results
A consecutive group of 720 patients underwent a cardiac magnetic resonance study before PVI. Patients with SA (n=142, 20%) were more likely to be male, diabetic, and hypertensive and have an increased pulmonary artery pressure, right ventricular volume, atrial dimensions, and left ventricular mass. Treated SA was defined as duration of continuous positive airway pressure therapy of >4 hours per night. Treated SA patients (n=71, 50%) were more likely to have paroxysmal AF, a lower blood pressure, lower ventricular mass, and smaller left atrium. During a follow‐up of 42 months, AF recurred in 245 patients. The cumulative incidence of AF recurrence was 51% in patients with SA, 30% in patients without SA, 68% in patients with untreated SA, and 35% in patients with treated SA. In a multivariable model, the presence of SA (hazard ratio 2.79, CI 1.97 to 3.94, P<0.0001) and untreated SA (hazard ratio 1.61, CI 1.35 to 1.92, P<0.0001) were highly associated with AF recurrence.
Conclusions
Patients with SA have an increased blood pressure, pulmonary artery pressure, right ventricular volume, left atrial size, and left ventricular mass. Therapy with continuous positive airway pressure is associated with lower blood pressure, atrial size, and ventricular mass, and a lower risk of AF recurrence after PVI.
Keywords: atrial fibrillation, cardiac magnetic resonance, sleep apnea
Introduction
There are strong epidemiological data linking sleep apnea (SA) and atrial fibrillation (AF).1–2 SA is a relatively common condition with a prevalence of almost 20% among adults in the United States, and the burden is increasing.3–4 Adverse cardiovascular outcomes are common in patients with SA1 and include hypertension,5 stroke,1 heart failure,6 and the occurrence of AF.2 AF is the most common cardiac rhythm disorder in SA,7 and the complex interplay between AF and SA has led to calls for further investigation.8–9 Approximately 50% of patients with AF are likely to have SA,2 and recurrence rates of AF are higher with either medical therapy or electrical cardioversion in patients with SA.10–11 Catheter ablation offers a viable alternative in symptomatic patients who are refractory to pharmacological therapy,12–13 and the use of catheter ablation is increasing.14 Data suggest that the presence of SA has an effect on the therapeutic success of pulmonary vein isolation (PVI) for AF.15–18 However, the mechanisms through which SA increases the risk of AF are unclear. Specifically, there are limited data testing the interplay among SA, adverse cardiac structural remodeling, and the risk for recurrence of AF. Furthermore, there are limited data testing whether therapy for SA is associated with a beneficial effect on cardiac structural remodeling among patients with AF. We hypothesized that SA would be associated with adverse cardiac structural remodeling, that therapy for SA would be associated with a beneficial effect on cardiac structure, and that the treatment of SA would reduce the incidence of AF recurrence after PVI.
Methods
We prospectively collected data on all consecutive patients from September 2005 through June 2011 who underwent a cardiac magnetic resonance (CMR) study before PVI at a single institution (Brigham and Women's Hospital). The study indication was specifically for identification of pulmonary vein anatomy before planned PVI for treatment of recurrent AF. Our institutional practice is for all patients with AF, without standard contraindications to the performance of a magnetic resonance study, to undergo a CMR study before PVI to identify pulmonary vein anatomy.19 Contraindications to a contrast CMR study include the presence of a permanent pacemaker, any metallic hazards, severe claustrophobia, and severe impairment of renal function (glomerular filtration rate <30 mL/min per 1.73 m2). Separation into paroxysmal and persistent AF was defined as consistent with guidelines.20 Heart failure was defined as a clinical history of heart failure or reduced ejection fraction (EF). Participants were defined as having a prior myocardial infarction when a history of myocardial infarction was supported by hospital records or when there was evidence by ECG criteria (Minnesota codes 1.1.1 to 1.2.8).21 All patients undergoing a first CMR study before PVI were included; this included patients who had a prior failed PVI at another institution or if they did not undergo a CMR study before the previous procedure. The presence or absence of SA was prospectively determined before PVI with the use of a standardized questionnaire administered to all patients. All patients diagnosed with SA had undergone formal sleep studies. The diagnosis of SA was established in accordance with the sleep study criteria recommended by the American Academy of Sleep Medicine.22 Data regarding the extent of use of continuous positive airway pressure (CPAP) were obtained from each patient from follow‐up phone interviews. The protocol was approved by the Human Subjects Research Review Committee.
CMR Protocol
All images were acquired with ECG gating, breath‐holding, and the patient in a supine position. Subjects were imaged on either a 1.5‐ or 3.0‐T CMR system (Signa CV/I HDXt platform, General Electric Healthcare, and Tim Trio, Siemens, respectively). The CMR protocol consisted of cine steady‐state free precession imaging for cardiac function and late gadolinium enhancement (LGE) imaging as previously described.23 A single bolus of 0.15 mmol/kg gadolinium‐diethylenetriamine pentacetate was used. LGE was quantified by a semiautomatic detection method using a previously validated method (Mass Research, Leiden University Medical Center). The mass of left ventricular (LV) LGE is measured in grams, which is then expressed as a percentage of total LV mass. LGE was quantified using regions defined as >50% of maximal signal intensity of the enhanced area (full‐width at half‐maximum).24 The distribution of LGE was characterized as subendocardial, transmural, mid‐wall, epicardial, or focal/involving the insertion points.
PVI Protocol
For patients with paroxysmal AF, PVI consisted of point‐by‐point radiofrequency ablation to encircle the left and right pulmonary veins or by the use of a cryo‐ballon catheter (Artic Front, Medtronic Inc). In all cases, PVI was confirmed by recording within the veins using a circular multipolar catheter to confirm entrance block into the veins. For patients with persistent AF, additional linear left atrial (LA) ablations were performed in addition to PVI. Often, this consisted of linear ablations to create conduction block across the LA roof, and along the region between the lateral mitral annulus and left inferior pulmonary vein.25 Areas of complex fractionated electrograms during AF were also targeted for ablation. If sinus rhythm could not be restored with ablation alone, administration of ibutilide or external cardioversion was performed to restore sinus rhythm.
Methods of Clinical Follow‐up
The outcome of interest was recurrence of AF, which was defined as AF occurring >3 months after PVI and confirmed by either ECG or prolonged cardiac monitoring. Patients underwent ECG testing at all clinical visits; routine prolonged cardiac monitoring was performed in the patients reporting symptom consistent with AF. Patients were followed post procedure monthly for the first 3 months and then at 3‐ to 6‐month intervals for the first 2 years, all via clinic visits. The duration of follow‐up was determined from the CMR study date to the occurrence of an end point. If a recurrence of AF did not occur, patients were censored at the date of last clinical follow‐up.
Statistical Analysis
Continuous data are presented as mean±SD. Continuous data were compared using an unpaired Student t test or Mann–Whitney nonparametric test as appropriate. Nominal data are presented as number and percentages and were compared with a χ2 test. The hazard ratio for the prediction of AF was calculated using a Cox regression model. The Cox regression model consisted of the following clinical variables (age, sex, a history of diabetes, heart failure, and hypertension, LA size, body mass index, LVEF, and the use of a statin or angiotensin‐converting enzyme inhibitor) and a variable that indicated status of SA treatment. To confirm that the proportional hazards assumption was met, we tested the proportional hazards assumption of the relationship between SA and recurrent AF over time by creating an interaction term in our model for time. The effect estimate for the interaction term was not statistically significant (P=0.14). Patients with SA were separated into 2 groups based on the median time of treatment with CPAP. Nontreated SA was defined as CPAP duration of less than this median duration of therapy of 4 hours. Event curves were determined according to the Kaplan–Meier method, and comparisons of cumulative event rates were performed by using the log‐rank test. A 2‐tailed P value of <0.05 was considered significant for all other analyses. SAS was used for statistical analysis (SAS Institute Inc).
Results
Study Cohort Characteristics
In total, 720 consecutive patients were referred for a CMR in preparation for PVI. Cohort characteristics for the entire cohort and separated according to the presence or absence of SA are presented in Table 1. In brief, in the entire cohort, there were 531 men (74%) with an average age of 56±11 years (range 24 to 85 years). Patients presented a median of 49 months after the first symptomatic onset of AF (range 12 months to 12 years); 472 (65%) patients had persistent AF, 250 (35%) had paroxysmal AF, and 459 (65%) patients were in sinus rhythm at the time of the study. Of the total cohort, 142 (20%) were identified as having SA. Other comorbidities included diabetes in 106 (15%) patients and heart failure in 186 (26%). In total, 497 patients (69%) were receiving a class 1 or class 3 antiarrhythmic. SA was diagnosed a median of 26 months (IQR 18 to 31 months) before the CMR study, and median CPAP use per night was 4 hours (range 0 to 8 hours). Nonimaging variables were separated according to the presence or absence of SA (Table 1). Patients with SA were more likely male and to have a history of hypertension and diabetes mellitus. Patients with SA were more likely to be receiving an angiotensin‐converting enzyme inhibitor or an angiotensin II receptor blocker and a diuretic.
Table 1.
Patient Characteristics Stratified by SA
| Variable | Entire Cohort (720) | SA Positive (142) | SA Negative (578) | P Value |
|---|---|---|---|---|
| Age, y | 56 (11) | 57 (10) | 56 (11) | 0.57 |
| Male, n (%) | 531 (74) | 115 (81) | 416 (72) | 0.03 |
| Duration of AF, median (IQR) | 49 (29 to 82) | 50 (27 to 80) | 50 (29 to 84) | 0.86 |
| Paroxysmal atrial fibrillation, n (%) | 249 (35) | 51 (36) | 198 (34) | 0.77 |
| Persistent atrial fibrillation, n (%) | 471 (65) | 91 (64) | 380 (66) | 0.69 |
| Prior AF ablation, n (%) | 173 (24) | 30 (21) | 143 (25) | 0.38 |
| Cardiovascular risk factors, n (%) | ||||
| Diabetes | 106 (15) | 33 (23) | 73 (13) | 0.002 |
| Hypertension | 365 (51) | 90 (63) | 275 (48) | 0.0007 |
| Prior myocardial infarction | 56 (8) | 12 (8) | 44 (8) | 1.00 |
| Heart failure | 186 (26) | 42 (30) | 144 (25) | 0.29 |
| Sleep apnea | 142 (20) | 142 (100) | 0 (0) | — |
| Valvular heart disease | 77 (11) | 6 (4) | 71 (12) | 0.004 |
| Hyperthyroidism | 34 (5) | 5 (4) | 29 (5) | 0.66 |
| Hypercholesterolemia | 240 (33) | 50 (35) | 190 (33) | 0.62 |
| Alcohol excess | 59 (8) | 14 (10) | 45 (8) | 0.65 |
| Family history AF | 88 (12) | 12 (8) | 76 (13) | 0.15 |
| Medication, n (%) | ||||
| Aspirin | 325 (45) | 67 (47) | 258 (45) | 0.64 |
| β‐Blocker | 491 (68) | 98 (69) | 393 (68) | 0.84 |
| Calcium channel blocker | 164 (23) | 39 (27) | 125 (22) | 0.15 |
| ACE/ARB | 261 (36) | 64 (45) | 197 (34) | 0.02 |
| Class 1 antiarrhythmic | 162 (23) | 25 (18) | 137 (34) | 0.14 |
| Class 3 antiarrhythmic | 335 (47) | 77 (54) | 258 (45) | 0.05 |
| Digoxin | 64 (9) | 14 (10) | 50 (9) | 0.39 |
| Spironolactone | 19 (3) | 4 (3) | 15 (3) | 0.78 |
| Diuretics | 126 (18) | 35 (25) | 91 (16) | 0.02 |
| Statin | 222 (31) | 44 (31) | 178 (31) | 0.61 |
| BMI, kg/m2 | 29.5 (5) | 32.5 (6) | 29 (5) | <0.0001 |
| Systolic blood pressure, mm Hg | 127 (17) | 127 (19) | 127 (17) | 0.81 |
| Diastolic blood pressure, mm Hg | 75 (12) | 77 (11) | 75 (12) | 0.06 |
| Heart rate, beats/min | 73 (17) | 73 (17) | 72 (17) | 0.77 |
| ECG parameters | ||||
| Sinus rhythm at presentation | 459 (65) | 85 (60) | 374 (65) | 0.29 |
| AV delay, ms | 172 (31) | 177 (32) | 171 (31) | 0.18 |
| QRS duration, ms | 96 (15) | 96 (13) | 96 (16) | 0.77 |
| QTc duration, ms | 442 (33) | 442 (31) | 441 (33) | 0.61 |
| LVH by ECG (Sokolov criteria) | 56 (8) | 16 (11) | 40 (7) | 0.11 |
| GFR, mL/min per 1.73 m2 | 83 (17) | 83 (16) | 83 (17) | 0.77 |
All data are number (percentage) or mean (SD) unless otherwise indicated. ACE/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker; AF, atrial fibrillation; AV, atrioventricular; BMI, body mass index; GFR, glomerular filtration rate using the Modification of Diet in Renal Disease formula done at the time of the CMR; LVH, left ventricular hypertrophy; SA, sleep apnea.
Baseline Imaging Characteristics Separated According to the Presence of SA
Imaging characteristics are presented in Table 2. On echocardiogram, the mean LVEF was 55±10 and estimated pulmonary artery systolic pressure was 29±8 mm Hg among the entire cohort (Table 2). On CMR, the mean LV end‐diastolic volume was 167±42 mL, EF was 56±10%, LV mass indexed to body surface area was 71±12 g, right ventricular (RV) end‐diastolic volume was 163±42 mL, and RVEF was 52±8% (Table 2). Late gadolinium enhancement was present in 108 patients (15%, Table 2). The LGE pattern was mid‐myocardial in 34 (31%), subendocardial in 48 (44%), insertion point in 12 (11%) patients, epicardial in 1 (1%), and transmural in 15 (13%). The average extent of LGE was 6.4±4%. Imaging characteristics of patients were grouped according to the presence or absence of SA (Table 2). Patients with SA had an increased indexed LV mass, higher estimated pulmonary artery systolic pressure, larger LA size, and reduced RVEF compared with patients without SA. Patients with SA had an increased incidence of LGE compared with patients without SA. Patients with SA were more likely to have insertion‐point LGE than were patients without SA; otherwise, there was no difference in the location or extent of LGE between patients with and without SA.
Table 2.
Imaging Characteristics Stratified by SA
| Variable | Total Cohort (750) | SA Positive (142) | SA Negative (578) | P Value |
|---|---|---|---|---|
| Echocardiographic parameters | ||||
| LVEF, % | 55 (10) | 56 (9) | 55 (11) | 0.38 |
| LV diastolic dimension, mm | 49 (5) | 50 (5) | 49 (5) | 0.09 |
| Estimated PASP, mm Hg | 29 (8) | 31 (7) | 28 (8) | 0.0002 |
| Left atrial dimension, mm | 41 (6) | 43 (6) | 41 (6) | 0.004 |
| Cardiac magnetic resonance | ||||
| LVEDV, mL | 167 (42) | 174 (43) | 165 (42) | 0.05 |
| LVEDV index, mL/m2 | 80 (19) | 80 (20) | 80 (19) | 0.90 |
| LVESV, mL | 74 (28) | 77 (26) | 74 (28) | 0.19 |
| LVESV index, mL/m2 | 36 (13) | 36 (12) | 36 (13) | 0.89 |
| LVEF, % | 56 (10) | 56 (10) | 56 (10) | 0.99 |
| LV mass, g | 148 (34) | 164 (34) | 144 (31) | 0.006 |
| LV mass index, g/m2 | 71 (12) | 75 (14) | 70 (12) | 0.001 |
| RVEDV, mL | 163 (42) | 171 (44) | 161 (41) | 0.01 |
| RVEDV index, mL/m2 | 78 (18) | 78 (19) | 78 (18) | 0.66 |
| RVESV, mL | 80 (26) | 87 (27) | 78 (26) | 0.0009 |
| RVESV index, mL/m2 | 39 (12) | 40 (11) | 38 (12) | 0.19 |
| RVEF, % | 52 (8) | 51 (8) | 53 (8) | 0.03 |
| Left atrial dimension, mm | 41 (7) | 44 (8) | 40 (7) | <0.0001 |
| LV LGE, n (%) | 108 (15) | 30 (21) | 78 (13) | 0.03 |
| LV LGE FWHM, % of LV mass | 6.4 (4) | 6.6 (3) | 6.3 (4) | 0.50 |
| LV LGE location | ||||
| Subendocardial | 48 (44) | 12 (44) | 36 (44) | 0.63 |
| Transmural | 15 (13) | 3 (11) | 12 (15) | 0.76 |
| Epicardial | 1 (1) | 0 (0) | 1 (1) | 0.20 |
| Mid‐myocardial | 34 (31) | 7 (26) | 27 (33) | 0.63 |
| Insertion points | 12 (11) | 8 (6) | 4 (1) | 0.0005 |
All data are number (percentage) or mean (SD) unless otherwise indicated. LGE FWHM indicates late gadolinium enhancement using full‐width half‐maximum method; LV, left ventricular; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; PASP, pulmonary artery systolic pressure; RVEDV, right ventricular end‐diastolic volume; RVESV, right ventricular end‐systolic volume; RVEF, right ventricular ejection fraction; SA, sleep apnea.
Imaging Characteristics Separated According to Therapy of SA
Patients with SA were further divided according to the median CPAP treatment of 4 hours per night (Tables 3 and 4). There were differences in patients' characteristics and imaging variables between SA patients above and below the median duration of CPAP therapy. Patients who received less CPAP therapy were more likely to have persistent than paroxysmal AF and had a higher systolic blood pressure, an increased LV mass, larger LA dimensions, and a lower incidence of subendocardial LGE.
Table 3.
Characteristics of Participants With SA and Stratified by Treatment Group
| Variable | Total SA Cohort (142) | SA Treated Group (71) | SA Untreated Group (71) | P Value |
|---|---|---|---|---|
| Age, y | 57 (10) | 56 (9) | 57 (10) | 0.60 |
| Male, n (%) | 115 (81) | 60 (85) | 55 (77) | 0.39 |
| Duration of AF, median (IQR) | 50 (27 to 80) | 49 (35 to 84) | 52 (28 to 73) | 0.75 |
| Paroxysmal atrial fibrillation, n (%) | 51 (36) | 32 (45) | 19 (27) | 0.04 |
| Persistent atrial fibrillation, n (%) | 91 (64) | 39 (55) | 52 (73) | 0.04 |
| Prior AF ablation, n (%) | 30 (21) | 11 (15) | 19 (27) | 0.15 |
| Cardiovascular risk factors, n (%) | ||||
| Diabetes | 33 (23) | 16 (23) | 17 (24) | 1.00 |
| Hypertension | 90 (63) | 47 (66) | 43 (61) | 0.61 |
| Prior myocardial infarction | 12 (8) | 6 (8) | 6 (8) | 1.00 |
| Heart failure | 42 (30) | 18 (25) | 24 (34) | 0.36 |
| Valvular heart disease | 6 (4) | 2 (3) | 4 (6) | 0.68 |
| Hyperthyroidism | 5 (4) | 3 (4) | 2 (3) | 1.00 |
| Hypercholesterolemia | 50 (35) | 25 (35) | 25 (35) | 1.00 |
| Alcohol excess | 14 (10) | 9 (13) | 5 (7) | 0.40 |
| Family history AF | 12 (8) | 6 (8) | 6 (8) | 1.00 |
| Medication, n (%) | ||||
| Aspirin | 67 (47) | 37 (53) | 30 (49) | 0.31 |
| β‐Blocker | 98 (69) | 48 (68) | 50 (70) | 0.86 |
| Calcium channel blocker | 39 (27) | 20 (28) | 19 (27) | 1.00 |
| ACE/ARB | 64 (45) | 29 (41) | 35 (49) | 0.40 |
| Class 1 antiarrhythmic | 25 (18) | 14 (20) | 11 (15) | 0.66 |
| Class 3 antiarrhythmic | 77 (54) | 36 (51) | 41 (58) | 0.51 |
| Digoxin | 14 (10) | 7 (10) | 7 (10) | 1.00 |
| Spironolactone | 4 (3) | 2 (3) | 2 (3) | 1.00 |
| Diuretics | 35 (25) | 13 (18) | 22 (31) | 0.12 |
| Statin | 44 (31) | 21 (30) | 23 (32) | 0.86 |
| BMI, kg/m2 | 32.5 (6) | 32.3 (6) | 32.7 (5) | 0.62 |
| Systolic blood pressure, mm Hg | 127 (19) | 124 (17) | 131 (18) | 0.03 |
| Diastolic blood pressure, mm Hg | 77 (11) | 77 (11) | 76 (9) | 0.23 |
| Heart rate, beats/min | 73 (17) | 72 (17) | 74 (18) | 0.66 |
| ECG parameters | ||||
| Sinus rhythm at presentation | 85 (60) | 44 (62) | 41 (58) | 0.73 |
| AV delay, ms | 177 (32) | 174 (33) | 180 (31) | 0.39 |
| QRS duration, ms | 96 (13) | 96 (12) | 97 (15) | 0.85 |
| QTc duration, ms | 442 (31) | 440 (30) | 445 (32) | 0.42 |
| LVH by ECG (Sokolov criteria) | 16 (11) | 6 (8) | 10 (14) | 0.43 |
| GFR, mL/min per 1.73 m2 | 83 (16) | 81 (16) | 85 (17) | 0.13 |
All data are number (percentage) or mean (SD) unless otherwise indicated. ACE/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker; AF, atrial fibrillation; BMI, body mass index; GFR, glomerular filtration rate using the Modification of Diet in Renal Disease formula done at the time of the CMR; LVH, left ventricular hypertrophy; SA, sleep apnea.
Table 4.
Imaging Characteristics of Participants With SA and Startified by Treatment Group
| Variable | SA Positive (142) | SA Treated (71) | SA Untreated (71) | P Value |
|---|---|---|---|---|
| Echocardiographic parameters | ||||
| LVEF, % | 56 (9) | 56 (9) | 55 (10) | 0.27 |
| LV diastolic dimension, mm | 50 (5) | 50 (5) | 49 (6) | 0.62 |
| Estimated PASP, mm Hg | 31 (7) | 30 (7) | 32 (8) | 0.31 |
| Left atrial dimension, mm | 43 (6) | 41 (6) | 44 (6) | 0.002 |
| Cardiac magnetic resonance | ||||
| LVEDV, mL | 174 (43) | 169 (40) | 179 (45) | 0.18 |
| LVEDV index, mL/m2 | 80 (20) | 78 (19) | 82 (22) | 0.30 |
| LVESV, mL | 77 (26) | 77 (25) | 77 (25) | 0.88 |
| LVESV index, mL/m2 | 36 (12) | 36 (12) | 35 (12) | 0.74 |
| LVEF, % | 56 (10) | 55 (10) | 57 (10) | 0.88 |
| LV mass, g | 164 (34) | 154 (29) | 174 (36) | 0.005 |
| LV mass index, g/m2 | 75 (14) | 71 (11) | 80 (14) | 0.0002 |
| RVEDV, mL | 171 (44) | 167 (37) | 176 (51) | 0.27 |
| RVEDV index, mL/m2 | 78 (19) | 77 (16) | 80 (21) | 0.47 |
| RVESV, mL | 87 (27) | 86 (24) | 86 (29) | 0.97 |
| RVESV index, mL/m2 | 40 (11) | 40 (11) | 39 (12) | 0.67 |
| RVEF, % | 51 (8) | 50 (7) | 51 (8) | 0.24 |
| Left atrial dimension, mm | 44 (8) | 41 (8) | 46 (8) | 0.0008 |
| LV LGE, n (%) | 27 (19) | 12 (18) | 15 (20) | 0.67 |
| LV LGE FWHM, % of LV mass | 6.0 (3) | 5.4 (3) | 6.6 (3) | 0.29 |
| LV LGE location | ||||
| Subendocardial | 12 (8) | 2 (3) | 10 (14) | 0.03 |
| Transmural | 3 (2) | 2 (3) | 1 (1) | 1.00 |
| Epicardial | 0 (0) | — | — | — |
| Mid‐myocardial | 7 (5) | 6 (8) | 3 (4) | 0.49 |
| Insertion points | 8 (6) | 3 (4) | 5 (7) | 0.72 |
All data are number (percentage) or mean (SD) unless otherwise indicated. LGE FWHM indicates late gadolinium enhancement using full‐width half‐maximum method; LV, left ventricular; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; PASP, pulmonary artery systolic pressure; RVEDV, right ventricular end‐diastolic volume; RVESV, right ventricular end‐systolic volume; RVEF, right ventricular ejection fraction; SA, sleep apnea.
Recurrence of AF
Complete clinical follow‐up was available for 715 patients (99%). There were 245 episodes of late recurrent AF during a median follow‐up time of 42 months (IQR 23 to 50 months). The time to recurrence of late AF ranged from 5 to 57 months. Early recurrence of AF (<3 months) was found in 256 patients (36%). The cumulative incidence of late AF recurrence (>3 month) was higher among patients with an early recurrence of AF (104/256 patients, 41%) than among patients without an early recurrence of AF (141/464 patients, 30%, P=0.006). In a clinical multivariable model, where SA was divided into treated (>4 hours) versus untreated (<4 hours),26 the median duration of CPAP therapy in this study, untreated SA provided the strongest association with late AF recurrence (Table 5). Among all patients with SA, the cumulative incidence of late AF recurrence was 51% compared with an incidence of 30% in patients without SA. Patients below the median CPAP therapy duration of 4 hours had an increased rate of recurrence of late AF compared with patients above the median CPAP therapy duration (68% versus 35%). Kaplan–Meier curves showing differences in late AF recurrence according to the presence or absence of SA and according the median CPAP treatment are presented (Figure1). There was a significant difference in incidence of late AF between patients without SA and patients with SA who were not treated. There was no difference in the cumulative incidence of AF recurrence between patients with treated SA and those without SA.
Table 5.
Multivariable Cox Regression Model 2 for Association With Recurrence of Atrial Fibrillation
| Variable | HR | 95% CI | LRχ2 | P Value |
|---|---|---|---|---|
| Age | 0.99 | 0.98 to 1.01 | 1.09 | 0.30 |
| Male | 0.99 | 0.73 to 1.33 | 0.05 | 0.94 |
| History of hypertension | 1.10 | 0.82 to 1.47 | 0.41 | 0.52 |
| History of diabetes mellitus | 1.13 | 0.80 to 1.59 | 0.45 | 0.50 |
| SA treated* | 1.14 | 0.74 to 1.76 | 0.34 | 0.56 |
| SA untreated* | 2.79 | 1.97 to 3.94 | 33.79 | <0.0001 |
| History of heart failure | 0.82 | 0.59 to 1.13 | 1.48 | 0.22 |
| ACE/ARB Inhibitor | 1.30 | 0.98 to 1.73 | 3.26 | 0.07 |
| Statin use | 0.91 | 0.69 to 1.22 | 0.38 | 0.53 |
| Body mass index | 0.99 | 0.96 to 1.02 | 0.84 | 0.36 |
| Cardiac magnetic resonance parameters | ||||
| LVEF | 1.00 | 0.98 to 1.01 | 0.46 | 0.49 |
| Left atrial dimension | 1.03 | 1.01 to 1.05 | 8.38 | 0.004 |
ACE/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker; HR, hazard ratio; LR, log‐rank; LVEF, left ventricular ejection fraction; SA, sleep apnea.
Reference group=no SA.
Figure 1.
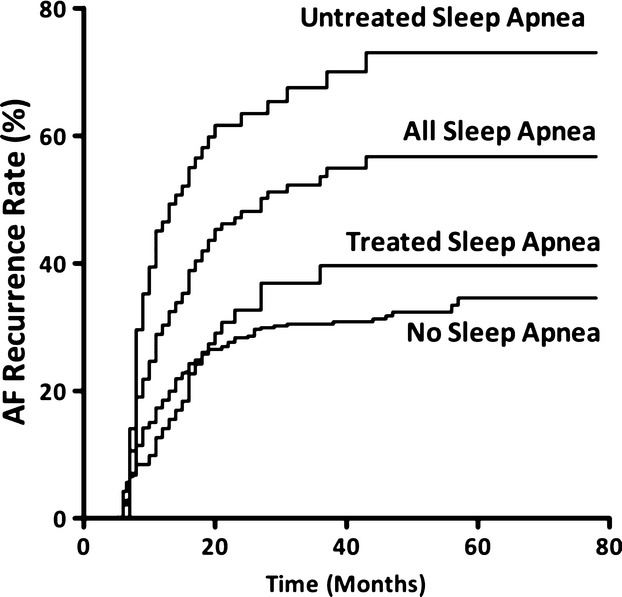
Kaplan–Meier curves displaying probability of AF recurrence according to the both the presence of absence of SA, and according to the SA treatment group. Results were compared using a log‐rank test; P value of <0.0001 for comparison of SA vs no SA and for comparison of SA and treatment vs untreated SA group. AF indicates atrial fibrillation; SA, sleep apnea.
Discussion
In patients with recurrent AF requiring PVI, we aimed to test the effect of SA on cardiac remodeling, to test whether therapy for SA was associated with differences in cardiac structure, and to test whether therapy for SA affected the recurrence rate of AF after PVI. We found that patients with SA had increased LV mass, LA size, estimated pulmonary pressures, and RV size, as well as reduced RV function. We found that therapy for SA was associated with paroxysmal rather than persistent AF, a lower blood pressure, a lower LV mass, and a reduced LA size. Finally, we found that untreated SA increased the incidence of AF recurrence post PVI and that treatment of SA with CPAP is associated with a lower incidence of AF after a successful PVI.
There are limited data comparing the CMR findings in patients with and without SA. We found that patients with SA were more likely to have an increased LV mass, a larger LA, and a lower RVEF. These findings are complementary to echocardiographic data. Arias and colleagues compared LV mass in patients with SA with healthy controls.27 They found that patients with SA had thicker LV walls and a higher LV mass than controls. Otto and colleagues tested the effect of SA on LA size in patients with SA and found that LA size was increased in patients with SA.28 Finally, Shivalkar and colleagues found an increased RV size by echocardiography in patients with SA compared with healthy controls.29 However, patients with SA tend to be overweight and have limited echocardiographic windows. Furthermore, even among nonoverweight subjects, CMR is the gold standard measure of cardiac structure and function.30–31 We also tested the effect of treatment of SA on CMR measures of cardiac structure and function. We found that patients receiving therapy for SA had a lower LV mass and smaller LA. These findings are complementary to prior published data.32 Colish and colleagues tested the effect of CPAP on LV mass and LA size on baseline and follow‐up CMR‐based measurement of LV mass and LA size. Similar to the findings of the present study, they found that therapy with CPAP was associated with a reduction in LV mass and LA size. We extend these findings and show that patients with treated SA had a lower LV mass, a reduced LA size, and a reduction in the recurrence of AF.
We also compared the prevalence and pattern of myocardial scar among patients with and without SA. Compared with patients without SA, patients with SA were more likely to have LGE. Also, the location of LGE was different between groups with and without SA. Patients with SA were more likely to have LGE involving the insertion points of the septal attachments of the RV. Insertion point LGE is a nonspecific CMR finding, occurring in dilated cardiomyopathy,33 hypertrophic cardiomyopathy,34 systemic hypertension,35 and pulmonary hypertension.36 In patients with pulmonary hypertension, insertion‐point LGE is a marker of adverse prognosis.36 The finding of a higher percentage of insertion‐point LGE in patients with SA in this study is likely due to higher pulmonary artery pressure. We also separated patients with SA into duration of treatment. Patients with treated SA were less likely to have sub‐endocardial LGE, a distribution typical of that found after myocardial infarction. We hypothesized that the reduction in an infarct pattern of LGE among patients with treated with SA was related to either a reduction in AF and the associated risk of thrombosis or a direct effect of SA therapy on the risk of myocardial infarction.37–38
The data confirming an association between SA and AF complement prior data.2,6,9,11,18 Gami and colleagues tested the association between SA and AF among a cohort of patients referred for cardioversion of AF.2 A diagnosis of SA was suggested using the Berlin questionnaire in almost 50% of patients. In that study, similar to findings noted in the present work, the association between AF and SA was greater than the association with traditional risk factors such as body mass index and hypertension. In an extension of prior work, the same group tested whether there was an association between self‐reported treatment of SA and recurrence of AF after electrical cardioversion.11 In a study of 37 patients with SA, the authors found that the recurrence rate in the 12 patients with treated SA was half that of patients without SA treatment. In patients being referred for PVI, data have shown an association between SA and the presence of AF and an association between SA and recurrence of AF.15–17 There are no prospective randomized data testing whether treatment of SA reduces the risk of AF recurrence post PVI. In the largest observational series by Patel and colleagues,17 50% of patients were not compliant with CPAP therapy. In that group, the recurrence rate was higher than the rate in patients who regularly used CPAP. In a recent study,18 Fein and Colleagues tested the association of SA, treatment of SA, and recurrence of AF post PVI in 62 patients. Similar to this study, they found that CPAP was associated with a 50% reduction in recurrence of AF post PVI. However, important differences exist. We found baseline differences in patients with and without SA for which we able to account. We found that patients with SA were more likely overweight, male, diabetic, and hypertensive and had increased pulmonary artery pressure, RV volume, atrial dimensions, and LV mass. We believe that these baseline differences primarily relate to the larger sample size of this present study. We also found that therapy for SA was associated with differences among patient groups. Based on published data39 and the median duration of CPAP therapy, 4 hours was chosen to separate patients into 2 categories: treated SA and untreated SA. Patients treated with CPAP were more likely to have paroxysmal AF, a lower blood pressure, a lower ventricular mass, and a smaller LA.
This study should be interpreted within the context of the design format. We did not perform serial prolonged monitoring for the recurrence of AF. Confirmation of recurrence was performed based on patient symptoms and underestimated the true incidence of AF recurrence. The study was prospective but observational; therefore, the extent of monitoring was left to the discretion of the primary providers and was not prespecified. Due to the study design, patients were not randomized; therefore, unanticipated variables could have influenced the findings. We did not capture the central apnea indices on all patients and were therefore unable to characterize the patients as having central SA, obstructive SA, or mixed patterns. We did not perform a formal sleep study on all patients referred for PVI. This approach likely yielded a significant underestimation in the prevalence of SA, which is estimated to be close to 50% in this population.2 We also did not randomize patients with SA to duration of CPAP therapy. However, this study may act as a basis for such further work where a formal sleep assessment of all patients being referred to PVI would occur, followed by randomization to CPAP therapy or no therapy, a testing of the effect on cardiac structure, and the inclusion of routine prolonged monitoring for AF.
Among a large cohort of patients with recurrent AF being referred for PVI, we found that 20% had SA, that the presence of SA was associated with adverse cardiac remodeling, that these adverse cardiac structural changes were attenuated among patients treated for SA, and that the presence or absence of therapy for SA provided a very strong association with the incidence of AF recurrence. These data may support further investigation into the role of therapy for SA and the recurrence of AF after PVI.
Sources of Funding
This work was supported by an American Heart Association Fellow to Faculty Grant (12FTF12060588, to Dr Neilan), National Institutes of Health (NIH) T32 Training Grants (T32HL09430101A1 to Dr Neilan and T32 AG000158 to Dr Dodson), and NIH project grants (R01HL090634‐01A1, to Dr Jerosch‐Herold; R01HL091157, to Dr Kwong).
Disclosures
None.
References
- 1.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005; 353:2034-2041 [DOI] [PubMed] [Google Scholar]
- 2.Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, Malouf JF, Ammash NM, Friedman PA, Somers VK. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004; 110:364-367 [DOI] [PubMed] [Google Scholar]
- 3.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep‐disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep‐disordered breathing. JAMA. 2003; 289:2230-2237 [DOI] [PubMed] [Google Scholar]
- 4.Young T, Peppard PE, Taheri S. Excess weight and sleep‐disordered breathing. J Appl Physiol. 2005; 99:1592-1599 [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000; 342:1378-1384 [DOI] [PubMed] [Google Scholar]
- 6.Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, Roselle GA. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998; 97:2154-2159 [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001; 285:2370-2375 [DOI] [PubMed] [Google Scholar]
- 8.Estes NA, III, Sacco RL, Al‐Khatib SM, Ellinor PT, Bezanson J, Alonso A, Antzelevitch C, Brockman RG, Chen PS, Chugh SS, Curtis AB, DiMarco JP, Ellenbogen KA, Epstein AE, Ezekowitz MD, Fayad P, Gage BF, Go AS, Hlatky MA, Hylek EM, Jerosch‐Herold M, Konstam MA, Lee R, Packer DL, Po SS, Prystowsky EN, Redline S, Rosenberg Y, Van Wagoner DR, Wood KA, Yue L, Benjamin EJ. American Heart Association atrial fibrillation research summit: a conference report from the American Heart Association. Circulation. 2011; 124:363-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008; 118:1080-1111 [DOI] [PubMed] [Google Scholar]
- 10.Monahan K, Brewster J, Wang L, Parvez B, Goyal S, Roden DM, Darbar D. Relation of the severity of obstructive sleep apnea in response to anti‐arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol. 2012; 110:369-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman AS, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003; 107:2589-2594 [DOI] [PubMed] [Google Scholar]
- 12.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998; 339:659-666 [DOI] [PubMed] [Google Scholar]
- 13.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC, Jr, Priori SG, Estes NA, III, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Ohman EM, Stevenson WG, Tarkington LG, Yancy CW. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011; 123:e269-e367 [DOI] [PubMed] [Google Scholar]
- 14.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2011; 3:32-38 [DOI] [PubMed] [Google Scholar]
- 15.Matiello M, Nadal M, Tamborero D, Berruezo A, Montserrat J, Embid C, Rios J, Villacastin J, Brugada J, Mont L. Low efficacy of atrial fibrillation ablation in severe obstructive sleep apnoea patients. Europace. 2010; 12:1084-1089 [DOI] [PubMed] [Google Scholar]
- 16.Chilukuri K, Dalal D, Marine JE, Scherr D, Henrikson CA, Cheng A, Nazarian S, Spragg D, Berger R, Calkins H. Predictive value of obstructive sleep apnoea assessed by the Berlin Questionnaire for outcomes after the catheter ablation of atrial fibrillation. Europace. 2009; 11:896-901 [DOI] [PubMed] [Google Scholar]
- 17.Patel D, Mohanty P, Di Biase L, Shaheen M, Lewis WR, Quan K, Cummings JE, Wang P, Al‐Ahmad A, Venkatraman P, Nashawati E, Lakkireddy D, Schweikert R, Horton R, Sanchez J, Gallinghouse J, Hao S, Beheiry S, Cardinal DS, Zagrodzky J, Canby R, Bailey S, Burkhardt JD, Natale A. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol. 2010; 3:445-451 [DOI] [PubMed] [Google Scholar]
- 18.Fein AS, Shvilkin A, Shah D, Haffajee CI, Das S, Kumar K, Kramer DB, Zimetbaum PJ, Buxton AE, Josephson ME, Anter E. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence following catheter ablation. J Am Coll Cardiol. 2013; 62:300-305 [DOI] [PubMed] [Google Scholar]
- 19.Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, Gerstad NA, Gillam LD, Hodgson JM, Kim RJ, Kramer CM, Lesser JR, Martin ET, Messer JV, Redberg RF, Rubin GD, Rumsfeld JS, Taylor AJ, Weigold WG, Woodard PK, Brindis RG, Hendel RC, Douglas PS, Peterson ED, Wolk MJ, Allen JM, Patel MR. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006; 48:1475-1497 [DOI] [PubMed] [Google Scholar]
- 20.Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA, III, Page RL, Ezekowitz MD, Slotwiner DJ, Jackman WM, Stevenson WG, Tracy CM, Fuster V, Ryden LE, Cannom DS, Le Heuzey JY, Crijns HJ, Lowe JE, Curtis AB, Olsson SB, Ellenbogen KA, Prystowsky EN, Halperin JL, Tamargo JL, Kay GN, Wann LS, Jacobs AK, Anderson JL, Albert N, Hochman JS, Buller CE, Kushner FG, Creager MA, Ohman EM, Ettinger SM, Stevenson WG, Guyton RA, Tarkington LG, Halperin JL, Yancy CW. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011; 123:104-12321173346 [Google Scholar]
- 21.Blackburn H, Keys A, Simonson E, Rautaharju P, Punsar S. The electrocardiogram in population studies. A classification system. Circulation. 1960; 21:1160-1175 [DOI] [PubMed] [Google Scholar]
- 22.Morgenthaler TI, Kapen S, Lee‐Chiong T, Alessi C, Boehlecke B, Brown T, Coleman J, Friedman L, Kapur V, Owens J, Pancer J, Swick T. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006; 29:1031-1035 [PubMed] [Google Scholar]
- 23.Farzaneh‐Far A, Ariyarajah V, Shenoy C, Dorval JF, Kaminski M, Curillova Z, Wu H, Brown KB, Kwong RY. Left atrial passive emptying function during dobutamine stress MR imaging is a predictor of cardiac events in patients with suspected myocardial ischemia. JACC Cardiovasc Imaging. 2011; 4:378-388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging. 2011; 4:150-156 [DOI] [PubMed] [Google Scholar]
- 25.Michaud GF, John R. Percutaneous pulmonary vein isolation for atrial fibrillation ablation. Circulation. 2011; 123:e596-e601 [DOI] [PubMed] [Google Scholar]
- 26.National government services, Inc LCD for positive airway pressure (PAP) devices for the treatment of obstructive sleep apnea (l27230): American Medical Association; center for medicaid and medicare. 2010. Available at http://www.cms.gov/medicare-coverage-database Accessed March 22, 2011.
- 27.Arias MA, Garcia‐Rio F, Alonso‐Fernandez A, Mediano O, Martinez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005; 112:375-383 [DOI] [PubMed] [Google Scholar]
- 28.Otto ME, Belohlavek M, Romero‐Corral A, Gami AS, Gilman G, Svatikova A, Amin RS, Lopez‐Jimenez F, Khandheria BK, Somers VK. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007; 99:1298-1302 [DOI] [PubMed] [Google Scholar]
- 29.Shivalkar B, Van de Heyning C, Kerremans M, Rinkevich D, Verbraecken J, De Backer W, Vrints C. Obstructive sleep apnea syndrome: more insights on structural and functional cardiac alterations, and the effects of treatment with continuous positive airway pressure. J Am Coll Cardiol. 2006; 47:1433-1439 [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Jerosch‐Herold M, Jacobs DR, Jr, Shahar E, Folsom AR. Coronary risk factors and myocardial perfusion in asymptomatic adults: the multi‐ethnic study of atherosclerosis (MESA). J Am Coll Cardiol. 2006; 47:565-572 [DOI] [PubMed] [Google Scholar]
- 31.Missouris CG, Forbat SM, Singer DR, Markandu ND, Underwood R, MacGregor GA. Echocardiography overestimates left ventricular mass: a comparative study with magnetic resonance imaging in patients with hypertension. J Hypertens. 1996; 14:1005-1010 [PubMed] [Google Scholar]
- 32.Colish J, Walker JR, Elmayergi N, Almutairi S, Alharbi F, Lytwyn M, Francis A, Bohonis S, Zeglinski M, Kirkpatrick ID, Sharma S, Jassal DS. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012; 141:674-681 [DOI] [PubMed] [Google Scholar]
- 33.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marban E, Tomaselli GF, Lima JA. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008; 51:2414-2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli‐Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010; 56:867-874 [DOI] [PubMed] [Google Scholar]
- 35.Rudolph A, Abdel‐Aty H, Bohl S, Boye P, Zagrosek A, Dietz R, Schulz‐Menger J. Noninvasive detection of fibrosis applying contrast‐enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009; 53:284-291 [DOI] [PubMed] [Google Scholar]
- 36.Freed BH, Gomberg‐Maitland M, Chandra S, Mor‐Avi V, Rich S, Archer SL, Jamison EB, Jr, Lang RM, Patel AR. Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J Cardiovasc Magn Reson. 2012; 14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990; 336:261-264 [DOI] [PubMed] [Google Scholar]
- 38.Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener‐West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010; 122:352-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campos‐Rodriguez F, Martinez‐Garcia MA, de la Cruz‐Moron I, Almeida‐Gonzalez C, Catalan‐Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012; 156:115-122 [DOI] [PubMed] [Google Scholar]


