Abstract
Background
The prevalence and significance of low normal and abnormal ankle brachial index (ABI) values in a community‐dwelling population of sedentary, older individuals is unknown. We describe the prevalence of categories of definite peripheral artery disease (PAD), borderline ABI, low normal ABI, and no PAD and their association with lower‐extremity functional performance in the LIFE Study population.
Methods and Results
Participants age 70 to 89 in the LIFE Study underwent baseline measurement of the ABI, 400‐m walk, and 4‐m walking velocity. Participants were classified as follows: definite PAD (ABI <0.90), borderline PAD (ABI 0.90 to 0.99), low normal ABI (ABI 1.00 to 1.09), and no PAD (ABI 1.10 to 1.40). Of 1566 participants, 220 (14%) had definite PAD, 250 (16%) had borderline PAD, 509 (33%) had low normal ABI, and 587 (37%) had no PAD. Among those with definite PAD, 65% were asymptomatic. Adjusting for age, sex, race, body mass index, smoking, and comorbidities, lower ABI was associated with longer mean 400‐m walk time: (definite PAD=533 seconds; borderline PAD=514 seconds; low normal ABI=503 seconds; and no PAD=498 seconds [P<0.001]). Among asymptomatic participants with and without PAD, lower ABI values were also associated with longer 400‐m walk time (P<0.001) and slower walking velocity (P=0.042).
Conclusion
Among older community‐dwelling men and women, 14% had PAD and 49% had borderline or low normal ABI values. Lower ABI values were associated with greater functional impairment, suggesting that lower extremity atherosclerosis may be a common preventable cause of functional limitations in older people.
Clinical Trial Registration
URL: http://clinicaltrials.gov/ Unique identifier: NCT01072500.
Keywords: aging, exercise, peripheral vascular disease
Introduction
Lower extremity peripheral artery disease (PAD) affects 8 million men and women in the United States (US) and will become more common as the US population survives longer with chronic disease.1 PAD can be detected noninvasively with the ankle brachial index (ABI), a ratio of Doppler‐recorded systolic blood pressures in the lower and upper extremities.2–5 Although intermittent claudication is the classic symptom, PAD is often asymptomatic or can present with atypical leg symptoms.6–7
A normal ABI value is 1.10 to 1.40.2–4,8 This is because among individuals without PAD, lower extremity arterial pressures increase with greater distance from the heart, due to increasing impedance with increasing arterial taper, resulting in higher systolic pressures at the ankle compared with the brachial arteries. Thus, an ABI <0.90 is highly sensitive and specific for PAD, an ABI of 1.10 to 1.40 indicates absence of PAD, and ABI values between 0.90 and 1.09 represent borderline PAD or low normal ABI values.8
It is well established that people with ABIs <0.90 have poorer performance on measures of functional performance. People with ABI <0.90 also have faster functional decline, and increased rates of cardiovascular events, compared with people with an ABI ≥0.90 to 1.40.1,6–7,6–10 However, the prevalence and clinical significance of ABI values between 0.90 and 1.09 are less clear.
We investigated the prevalence of predefined ABI categories in a community‐dwelling cohort of sedentary men and women in the United States participating in the LIFE Study. Based on prior study,11 participants were categorized as follows: Definite PAD (ABI <0.90), borderline PAD (ABI 0.90 to 0.99), low normal ABI (ABI 1.00 to 1.09) and absence of PAD (ABI 1.10 to 1.40). The primary aims of this manuscript were to report the prevalence of each ABI category and associations of each ABI category with measures of functional performance within the entire LIFE cohort and separately among those without exertional leg symptoms (ie, asymptomatic). We hypothesized that borderline and low‐normal ABI values would be common in this population of older sedentary men and women, that borderline and low‐normal ABI values would be associated with poorer performance on measures of lower extremity functioning compared with normal ABI values, and that lower ABI values would be associated with poorer performance on measures of lower extremity functioning even among individuals without exertional leg symptoms (ie, those who are asymptomatic). Prior studies suggest that an ABI >1.40 is associated with lower extremity medial artery calcinosis and inability to accurately determine the presence or severity of PAD.8 However, lower extremity artery stiffness may be associated with impaired functional performance.12–13 Therefore, in exploratory analyses, we evaluated whether an ABI >1.40, which indicates lower extremity arterial stiffness and medial artery calcinosis, is associated with impaired functional performance.
Methods
The LIFE Study, a randomized controlled trial comparing a physical activity and a successful aging intervention, enrolled 1635 community dwelling sedentary men and women age 70 to 89. Methods for the LIFE Study have been described.14 Participants were recruited from 8 field centers: Northwestern University, Chicago, IL; Pennington Biomedical Research Center, Baton Rouge, LA; University of Pittsburgh, Pittsburgh, PA; Stanford University, Palo Alto, CA; Tufts University, Boston, MA; Wake Forest University, Winston‐Salem, NC; Yale University, New Haven, CT; and University of Florida, Gainesville, FL. Institutional Review Board approval was obtained at all sites and all participants gave written informed consent. The LIFE Study will determine whether a supervised moderate‐intensity Physical Activity intervention prevents major mobility disability, as compared with a Successful Aging health education program. The primary outcome is major mobility disability, defined as the inability to walk 400 m during follow‐up. The ankle‐brachial index is a tertiary measure. The current report describes baseline data from the LIFE Study.
Recruitment
Approximately 200 participants were recruited at each field center, using newspaper, radio, and television advertisements and direct mailings to age‐eligible residents living in the community. Participants were also recruited from health fairs, senior centers, medical clinics, and churches.
Inclusion Criteria
Community dwelling men and women age 70 to 89 who were sedentary, defined as a CHAMPS‐18 score indicating ≤125 minutes of physical activity per week,15 and had functional limitations, defined as a Short Physical Performance Battery (SPPB) score <10, were eligible. In our primary analyses, individuals with ABI >1.40 were excluded, since an ABI >1.40 indicates medial calcinosis of the lower extremity arteries and inability to accurately determine the degree of lower extremity obstruction. In separate exploratory analyses we included the ABI >1.40 group to determine whether ABI >1.40 was associated with impaired functioning.
Exclusion Criteria
Exclusion criteria included major mobility disability, measured by inability to complete a 400 m walk within 15 minutes at baseline, significant cognitive impairment, severe psychiatric disorder, presence of severe chronic lung disease, treatment for cancer within the past 3 years (other than non‐melanoma skin cancer), NYHA Class III or IV heart failure, history of cardiac arrest, uncontrolled angina, presence of an implantable cardiac defibrillator, Parkinson's disease, severe arthritis that would interfere with full participation in study interventions, renal disease requiring dialysis, and language barriers.14 We did not systematically evaluate potential participants for the presence of critical limb ischemia. However, all participants underwent a physical examination by a study physician and those found to have clinical examination findings or symptoms that might preclude full participation in an exercise program were excluded. In addition, participants without a detectable lower extremity arterial pressure by Doppler were evaluated by a nurse or physician for possible critical limb ischemia.
Ankle Brachial Index (ABI)
The ABI was measured after the participant rested supine for 5 minutes, using a hand‐held Doppler probe to obtain systolic pressures at the right brachial artery, right posterior tibial artery, left posterior tibial artery, and left brachial artery in the order listed.8 Pressures were repeated in reverse order. The ABI was calculated for each leg by averaging the 2 posterior tibial artery pressures and dividing them by the average of the 4 brachial artery pressures. However, when 1 brachial artery pressure was higher than the alternate brachial artery pressure in both measurement sets, and the difference in the right and left brachial artery pressures differed by at least 10 mm Hg in both measurement sets, then subclavian stenosis was possible and the average brachial artery pressures from the arm with highest pressure was included in analyses.16 ABI categories were defined as described above, using the leg with the lowest ABI value.
Measures of Lower Extremity Functioning
Measures of lower extremity functioning consisted of the 400‐m walk, walking velocity over 4 m, and the short physical performance battery (SPPB). We chose to study the 400‐m walk time and 4‐m walking velocity because oxygen supply to lower extremity muscles during walking is impaired by lower extremity atherosclerosis. Thus, lower extremity atherosclerosis is expected to preferentially impair walking velocity and walking endurance. The SPPB was also studied because it is a well validated measure of lower extremity functioning that combines assessments of leg strength, balance, and walking velocity. Together, the 400‐m walk time, 4‐m walking velocity, and the SPPB are validated measures that predict mobility loss, cardiovascular mortality, and all‐cause mortality.17–21
Four Hundred‐Meter Walk
The 400‐m walk was measured using a 20 m walking course.14 Participants were given standardized instructions, using a script, and were asked to walk back and forth 20 times across the 20‐m walking course until 400 m had been completed. They were allowed to rest standing during the walk. The total time required to complete 400 m was recorded.
Short Physical Performance Battery
The SPPB combines data from time to rise from a seated position 5 times, standing balance, and 4‐m walking velocity. Individuals receive a zero score for each task they are unable to complete. Scores of 1 to 4 are assigned for remaining tasks, based upon quartiles of performance for over 6000 participants in the Established Populations for the Epidemiologic Study of the Elderly.17–18 Scores are summed to obtain the SPPB, ranging from 0 to 12. For the time to rise from a seated position 5 times, participants sit in a straight‐backed chair with arms folded across their chest and stand 5 times consecutively as quickly as possible. Time to complete 5 chair rises is measured.17–18 For standing balance, participants are asked to hold 3 increasingly difficult standing positions for 10 seconds each: standing with feet together side‐by‐side and parallel (side‐by‐side stand), standing with feet parallel with the toes of 1 foot adjacent to and touching the heel of the opposite foot (semi‐tandem stand), and standing with 1 foot directly in front of and touching the other (tandem stand).17–18
Four‐Meter Walking Velocity
Walking velocity was measured with a 4‐m walk performed at usual walking speed.6,10,17–18 The walk was performed twice. The faster speed was used in analyses.6,10,17–18
Leg Symptoms
Leg symptoms were measured using the San Diego Claudication Questionnaire.6,10,22 Participants with any exertional leg symptoms were those who responded “yes” to the first question on the San Diego Claudication Questionnaire, “Do you get pain in either leg or in either buttock when walking?” Participants who responded “no” to this question were classified as “asymptomatic.” Among those with PAD and exertional leg symptoms, classical symptoms of intermittent claudication were defined as exertional calf pain that does not begin at rest, does not resolve if the participant continues walking, and resolves within 10 minutes of rest.
Comorbidities
Comorbidities were obtained via self‐report using standardized questionnaires. Comorbidities included in the current analyses were diabetes mellitus, heart failure, angina, history of myocardial infarction, stroke, pulmonary disease, cancer, and knee or hip arthritis.
Other Measures
Cigarette smoking was measured using self‐report. Body mass index (BMI) was calculated as kg/m2, based on measures of height and weight obtained at the study visit.
Statistical Analyses
Participant characteristics were compared across ABI categories using chi‐square tests for categorical variables and unadjusted analysis‐of‐variance F‐tests for continuous variables. Associations of ABI categories with 400‐m walk time, 4‐m walking velocity, and the SPPB were assessed using analysis of covariance, adjusting for age (continuous) and sex. Analyses were repeated, adjusting for age, sex, race (African American versus not African American), current smoking status, comorbidities, BMI, and the CHAMPS‐18 measure of physical activity.15 Pairwise comparisons were performed to examine the relationship between each functional measure among participants with ABI 1.10 to 1.40 and each of the remaining ABI categories. All of these analyses were repeated among participants without exertional leg symptoms (ie, among those who were asymptomatic). Associations of absence of PAD, PAD associated with any exertional leg symptoms, and asymptomatic PAD with functional measures were performed using analysis of covariance, adjusting for age, sex, race, smoking, comorbidities, and CHAMPS‐18 measured physical activity, and BMI. Pairwise comparisons were performed to examine relationships between functional performance measures of participants without PAD to participants with PAD who did not have exertional leg symptoms (ie, were asymptomatic) and to participants with PAD who had exertional leg symptoms. In our exploratory analyses, associations of the ABI with functional measures were repeated, including the category of participants with ABI >1.40. Correlation coefficients between the ABI and the 400‐m walk, 4‐m walking velocity, and the SPPB were calculated in the entire cohort and separately among LIFE Study participants with and without exertional leg symptoms.
Results
Of 1635 LIFE participants, 1566 had a valid ABI measurement <1.40 and are included in the primary analyses. An additional 36 LIFE participants had an ABI value >1.40 and were included in separate exploratory analyses. Thirty‐three LIFE participants (2%) had missing ABI data. Fourteen participants had an ABI <0.50 and 5 had an ABI <0.40, consistent with severe PAD.
A total of 587 participants (37%) had a normal ABI value, defined as ABI 1.10 to 1.40. The remainder had ABI values <1.10 or >1.40. Table 1 summarizes the characteristics of participants, according to ABI value. Lower ABI values were associated with older age, lower BMI values, and a higher prevalence of African American race, current smoking, and ever smoking. Participants in the ABI category of 0.90 to 0.99 included the highest proportion of women. Participants with an ABI >1.40 had the highest proportion of men and the lowest proportion of African Americans.
Table 1.
Baseline Characteristics of the Study Population According to Baseline ABI Values
| Definite PAD (ABI <0.90) | Borderline PAD (ABI 0.90 to 0.99) | Low Normal ABI (ABI 1.00 to 1.09) | No PAD (ABI 1.10 to 1.40) | High ABI (ABI >1.40) | |
|---|---|---|---|---|---|
| N (%) | 220 (13.7) | 250 (15.6) | 509 (31.8) | 587 (36.6) | 36 (2.3) |
| Age, y | 80.2±5.5 | 79.2±5.1 | 78.6±5.1 | 78.4±5.2 | 78.7±5.1 |
| Male sex, % | 31.8 | 19.2 | 26.3 | 43.1 | 61.1 |
| African American, % | 24.1 | 23.2 | 19.3 | 14.0 | 8.3 |
| Mean ABI values | 0.75±0.15 | 0.95±0.03 | 1.05±0.03 | 1.18±0.07 | 1.75±0.41 |
| Mean BMI, kg/m2 | 28.7±6.3 | 29.9±6.0 | 30.6±6.1 | 30.7±5.9 | 30.2±6.2 |
| Current smoking, % | 10.2 | 3.3 | 1.8 | 1.6 | 0.0 |
| Ever smoking, % | 57.2 | 44.7 | 49.7 | 45.2 | 44.4 |
| Diabetes mellitus, % | 31.1 | 27.3 | 24.8 | 22.8 | 38.9 |
| Myocardial infarction, % | 11.9 | 9.3 | 6.5 | 6.7 | 11.8 |
| Heart failure, % | 6.4 | 3.6 | 4.1 | 3.6 | 8.6 |
| 20 min/week exercise, % | 22.7 | 15.9 | 17.8 | 19.4 | 25.0 |
| Lung disease, % | 16.9 | 15.3 | 15.8 | 15.5 | 5.6 |
| Mean CHAMPS‐18 Score | 20.0±34.0 | 14.6±30.7 | 15.4±32.1 | 18.1±34.1 | 17.1±32.5 |
ABI indicates ankle brachial index; BMI, body mass index; PAD, peripheral artery disease.
Only 12 (5.5%) of the 220 participants with PAD (ABI <0.90) had classic symptoms of intermittent claudication, compared with 1.6%, 1.4%, and 0.85% of participants with borderline PAD (ABI 0.90 to 0.99), low normal ABI (ABI 1.00 to 1.09), and normal ABI (1.10 to 1.40), respectively (P<0.001). Sixty‐five percent of participants with PAD were asymptomatic (ie, had no exertional leg symptoms), compared with 76.4%, 76.5%, and 74.6% of participants with borderline PAD, low normal ABI, and normal ABI, respectively (P=0.012).
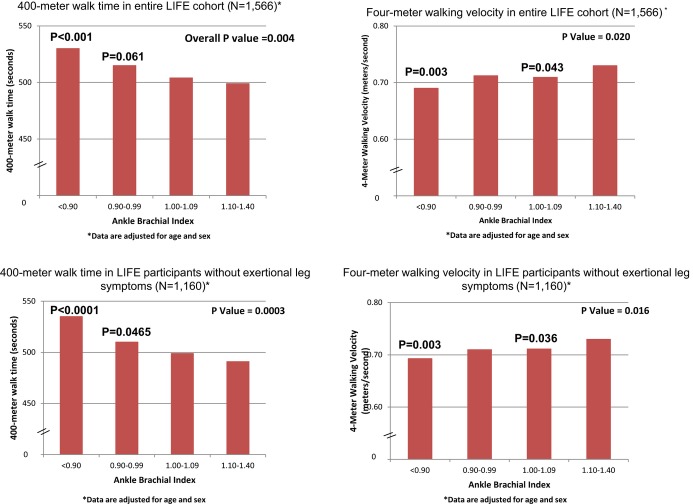
Among all LIFE participants, lower ABI values were associated significantly with longer 400‐m walk time and slower walking velocity, adjusting for age and sex (Figure 1). Those with ABI values <0.90 had slower 400‐m walk time (P<0.001) and slower 4‐m walking velocity (P<0.001) than participants with ABI values of 1.10 to 1.40. Participants with ABI values of 1.00 to 1.09 had slower 4‐m walking velocity than those with ABI of 1.10 to 1.40 (P=0.043). Among participants without exertional leg symptoms, lower ABI values were associated with longer 400‐m walk time and slower walking velocity, adjusting for age and sex (Figure 1). Asymptomatic participants with ABI <0.90 had significantly slower 400‐m walk time (P<0.001) and slower walking velocity (P=0.003), compared to participants with ABI values of 1.10 to 1.40, adjusting for age and sex (Figure 1). Asymptomatic participants with ABI 0.90 to 0.99 had slower 400‐m walking time (P=0.047) and asymptomatic participants with ABI 1.00 to 1.09 had slower 4‐m walking velocity (P=0.036), compared to asymptomatic participants with ABI 1.10 to 1.40 (Figure 1). The ABI was not associated with the SPPB score among all participants or among participants without exertional leg symptoms, adjusting for age and sex (data not shown).
Figure 1.
Age and sex adjusted least square mean values for 400‐meter walk time and 4‐m walking velocity among LIFE Study participants according to their ankle brachial index. *Analyses are adjusted for age and sex. P values shown above individual bars represent statistically significant differences between the ABI group indicated compared with participants with ABI of 1.10 to 1.40. ABI indicates ankle brachial index; LIFE, Lifestyle Interventions and Independence for Elders.
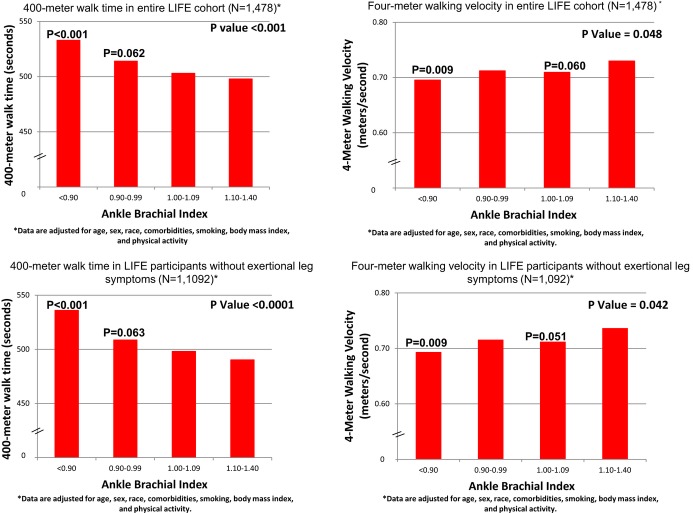
When analyses shown in Figure 1 were repeated with additional adjustment for race, BMI, smoking, physical activity, and comorbidities, findings were not substantially changed within the whole cohort or within the subset of participants without exertional leg symptoms (Figure 2). However, in these fully adjusted analyses, associations of ABI values of 0.90 to 0.99 and ABI‐values of 1.00 to 1.09 with 400‐m walk time and 4‐m walking velocity did not achieve statistical significance. The ABI was not associated with the SPPB score among all participants or among participants without exertional leg symptoms in these fully adjusted analyses (Table 2).
Figure 2.
Fully adjusted least square mean values for 400‐m walk time and 4‐m walking velocity among LIFE Study participants according to their ABI. P values in the figure represent overall statistical significance across all ABI categories. *Analyses are adjusted for age, sex, race, body mass index, smoking, physical activity, and comorbidities. P values shown above individual bars represent statistically significant differences between the ABI group indicated compared with participants with ABI of 1.10 to 1.40. ABI indicates ankle brachial index; LIFE, Lifestyle Interventions and Independence for Elders.
Table 2.
Adjusted Means of the Short Physical Performance Battery Score According to the ABI in the Entire Cohort and in Participants Without Exertional Leg Symptoms*
| Definite PAD (ABI <0.90), Mean (95% Confidence Interval) | Borderline PAD (ABI 0.90 to 0.99), Mean (95% Confidence Interval) | Low Normal ABI (ABI 1.00 to 1.09), Mean (95% Confidence Interval) | No PAD (ABI 1.10 to 1.40), Mean (95% Confidence Interval) | P Value | |
|---|---|---|---|---|---|
| Entire Cohort | N=220 | N=250 | N=509 | N=587 | |
| SPPB score (0 to 12 scale, 12=best) | 7.34 (7.13 to 7.57) | 7.45 (7.25 to 7.66) | 7.36 (7.22 to 7.51) | 7.40 (7.26 to 7.53) | 0.87 |
| Participants Without Exertional Leg Symptoms | N=144 | N=191 | N=388 | N=437 | |
| SPPB score (0 to 12 scale, 12=best) | 7.22 (6.95 to 7.50) | 7.46 (7.22 to 7.69) | 7.38 (7.22 to 7.55) | 7.40 (7.24 to 7.56) | 0.60 |
ABI indicates ankle brachial index; BMI, body mass index; PAD, peripheral artery disease; SPPB, short physical performance battery.
Data are adjusted for adjusted for age, sex, smoking, BMI, race, physical activity, and presence/absence of comorbidities (diabetes, history of heart failure, myocardial infarction, angina, chronic pulmonary disease, knee arthritis, hip arthritis, and cancer).
The ABI was correlated modestly with 400‐m walk time and 4‐m walking velocity in the entire LIFE Study cohort and separately among LIFE Study participants who were asymptomatic (Table 3). There were no significant correlations of the ABI with the SPPB. There were no significant correlations of the ABI with 400‐m walk time or 4‐m walking velocity among LIFE Study participants with exertional leg symptoms (Table 3).
Table 3.
Correlations Between the Ankle Brachial Index and Measures of Functional Performance in LIFE Study participants
| 400‐m Walk Time | 4‐m Walking Velocity | SPPB | |
|---|---|---|---|
| Correlations in the Entire Cohort of LIFE Participants (N=1566) | |||
| Ankle brachial index | −0.115 (P<0.001) | 0.085 (P<0.001) | 0.040 (P=0.113) |
| Correlations in the Subset of LIFE Participants With Exertional Leg Symptoms (N=403) | |||
| Ankle brachial index | −0.0395 (P=0.429) | 0.064 (P=0.202) | 0.014 (P=0.778) |
| Correlations in the Subset of LIFE Participants who are Asymptomatic (No Exertional Leg Symptoms) (N=1160) | |||
| Ankle brachial index | −0.151 (P<0.001) | 0.101 (P<0.001) | 0.053 (P=0.068) |
LIFE indicates Lifestyle Interventions and Independence for Elders; SPPB, short physical performance battery.
Adjusting for age, sex, race, smoking, BMI, physical activity, and comorbidities, 400‐m walk time was significantly different among non‐PAD participants, PAD participants with exertional leg symptoms, and asymptomatic PAD participants (P<0.001). In pairwise comparisons, participants with asymptomatic PAD had slower 400‐m walk time compared with participants without PAD (P<0.001) (Table 4).
Table 4.
Associations of Symptomatic and Asymptomatic Peripheral Artery Disease With 400‐m Walk Time, Walking Velocity, and the Short Physical Performance Battery*
| Participants Without PAD* (N=1346), Mean (95% Confidence Interval) | PAD Participants With Any Exertional Leg Symptoms (N=76), Mean (95% Confidence Interval) | Asymptomatic PAD Participants (N=144), Mean (95% Confidence Interval) | P Value* | |
|---|---|---|---|---|
| 400‐m walk time, second | 503 (497 to 509) | 528 (503 to 552) | 536* (518 to 554) | <0.001 |
| 4‐m walking velocity, m/s | 0.72 (0.70 to 0.73) | 0.70 (0.66 to 0.74) | 0.69 (0.66 to 0.72) | 0.13 |
| SPPB score, 0 to 12 scale | 7.40 (7.31 to 7.48) | 7.55 (7.18 to 7.92) | 7.24 (6.98 to 7.51) | 0.36 |
PAD indicates peripheral artery disease; SPPB, short physical performance battery.
Data are adjusted for adjusted for age, sex, smoking, BMI, race, physical activity, and presence/absence of comorbidities (diabetes, history of heart failure, myocardial infarction, angina, chronic pulmonary disease, knee arthritis, hip arthritis, and cancer).
Participants with any exertional leg symptoms were those who responded “yes” to the question, “Do you get pain in either leg or in either buttock when walking?” Participants who responded “no” to this question were classified as “asymptomatic”.
Overall P value for comparison of 3 groups (non‐PAD participants, PAD participants with exertional leg symptoms, asymptomatic PAD participants).
P<0.001 as compared with participants without PAD.
In separate exploratory analyses, we evaluated associations of the ABI with functional performance, including the 36 LIFE participants with ABI values >1.40. In these analyses, associations of the ABI with 400‐m walk time remained statistically significant, but the association of the ABI with 4‐m walking velocity was no longer statistically significant (data not shown). There were no differences in 400‐m walk time (521 versus 494 seconds, P=0.162) or 4‐m walking velocity (0.75 versus 0.74 m/s, P=0.068) between participants with ABI >1.40 and those with ABI 1.10 to 1.40. However, participants with ABI >1.40 had a lower SPPB compared to those with ABI ≤1.40 (7.2 versus 7.40, P=0.030).
Discussion
Results reported here document that among sedentary, community dwelling men and women age 70 to 89 with SPPB scores <10, most participants had ABI values below a normal ABI range of 1.10 to 1.40. Fourteen percent had definite PAD (ABI <0.90), 16% had a borderline ABI value of 0.90 to 0.99, and 33% had a low normal ABI value of 1.00 to 1.09. Most participants with definite or borderline PAD were asymptomatic. Among all asymptomatic participants, lower ABI values were associated with slower 400‐m walk time and slower 4‐m walking velocity, even after adjusting for age, sex, race, comorbidities, smoking, BMI, and physical activity. Among asymptomatic participants, those with borderline ABI values had slower 400‐m walk time and those with low normal ABI values had slower 4‐m walking velocity, compared with participants without PAD, adjusting for age and sex. Within the entire cohort, participants with low normal ABI values (ABI 1.00 to 1.09) had slower 4‐m walking velocity, compared with participants without PAD, adjusting for age and sex. However, associations of borderline (ABI 0.90 to 0.99) and low normal (ABI 1.00 to 1.09) ABI values with poorer 400‐m walk time and slower 4‐m walking velocity were not statistically significant after additional adjustment for race, comorbidities, smoking, BMI, and physical activity.
The 400‐m walk, 4‐m walking velocity, and the SPPB are well‐validated measures of functional performance that predict important outcomes in older men and women. Poorer performance on each of these measures is associated with higher rates of mobility loss, all‐cause mortality, and cardiovascular disease mortality.17–21 Our finding that lower ABI values were associated with poorer 400‐m walking performance and slower 4‐m walking velocity, but not lower SPPB scores, likely reflects that lower extremity atherosclerosis results in inadequate oxygen supply to lower extremity skeletal muscle during walking. The SPPB includes measures of balance and leg strength and may be a less specific measure of the functional impairment associated with PAD.
It is well established that people with PAD, defined as ABI <0.90, have an increased rate of all‐cause mortality, cardiovascular events, and functional decline, compared with those without PAD.1,9–10 However, even individuals with low‐normal and borderline ABI values have increased rates of all‐cause mortality, cardiovascular mortality, and mobility loss compared with individuals with normal ABI values of 1.10 to 1.40.9,23 A meta‐analysis of community‐dwelling men found that ABI values of 0.90 to 0.99 and 1.00 to 1.09 were each associated with significantly increased all‐cause and cardiovascular mortality, compared with men with ABI values of 1.10 to 1.20.9 A meta‐analysis of community‐dwelling women found that an ABI value of 0.90 to 0.99 was associated with significantly increased all‐cause and cardiovascular disease mortality and that an ABI value of 1.00 to 1.09 was associated with significantly increased all‐cause mortality.9 In the Walking and Leg Circulation Study (WALCS) cohort of men and women age 55 and older, ABI values of 0.90 to 0.99 and 1.00 to 1.09 were associated with an increased incidence of mobility loss (hazard ratios=3.07 and 2.61, respectively), relative to an ABI value of 1.10 to 1.30 at 5‐year follow‐up.23 Our finding that one third of the LIFE cohort had ABI values between 0.90 and 1.09 suggests that a large proportion of older, sedentary community‐dwelling men and women are at increased risk of all‐cause mortality, cardiovascular mortality, and mobility loss.
Nearly two thirds of the cohort with an ABI <0.90 reported no exertional leg symptoms (ie, were asymptomatic) and >75% of those with borderline or low normal ABI values were asymptomatic. These findings underscore that clinicians cannot rely on the presence of exertional leg symptoms to identify older people with PAD or borderline ABI values. Objective assessment is needed when the diagnosis of PAD is suspected. Absence of exertional leg symptoms in individuals with a low ABI value may be observed if these individuals restrict their physical activity or slow their walking speed to avoid these symptoms. Therefore, it is conceivable that some asymptomatic LIFE Study participants may have experienced ischemic leg symptoms at the end of the 400‐m walk that were not detected because the San Diego Claudication Questionnaire was not re‐administered at the end of the 400‐m walk test, but this was not assessed specifically.
The Women's Health and Aging Study (WHAS) cohort also found a high prevalence of asymptomatic PAD among community‐dwelling disabled women age 65 and older.7 In that cohort of 1002 participants, two‐thirds of those with ABI <0.90 were asymptomatic and within the subset without exertional leg symptoms, lower ABI values were associated with poorer performance on lower‐extremity functional measures. However, the WHAS cohort was limited to women with baseline disability, and did not study the prevalence or significance of ABI values of 0.90 to 0.99 or 1.00 to 1.09. To our knowledge, no prior study has described the prevalence of asymptomatic PAD, borderline PAD, or low normal ABI values among community‐dwelling sedentary men and women age 70 to 89. To our knowledge, no prior studies have assessed associations of borderline or low normal ABI values with functional impairment in any community‐dwelling population.
In the LIFE Study cohort, we observed correlations between the ABI and functional performance that were stronger among LIFE Study participants who were asymptomatic compared with those who were symptomatic. This phenomenon may have occurred if symptomatic LIFE Study participants had a higher prevalence of comorbid disease affecting the lower extremities, such as spinal stenosis, disk disease, or osteoarthritis. A higher prevalence of these comorbidities may reduce the association of the ABI with functional performance and also contribute to the presence of exertional leg pain. In addition, since individuals unable to walk 400 m were excluded from the LIFE Study, and those unable to walk 400 m may more frequently have symptoms limiting their mobility, the LIFE Study cohort may include a narrower range of 400‐m walk performance among participants with leg symptoms. This phenomenon may have limited our ability to observe an association of the ABI with 400 m walk performance among individuals with symptoms. Overall, however, correlations of the ABI with functional performance were lower in the LIFE Study cohort than previously reported in a population of individuals with PAD.24
In exploratory analyses, LIFE participants with ABI >1.40 had a lower SPPB than those with ABI of 1.10 to 1.40, but there were no differences in 400‐m walk time or 4‐m walking velocity between participants with ABI >1.40 and those with ABI of 1.10 to 1.40. These results are only somewhat consistent with previous studies demonstrating that stiff peripheral arterial vessels are associated with greater impairment in functional performance both in a vascular laboratory setting and in the Health ABC study.12–13 The relatively small sample size of LIFE participants with ABI >1.40 and the exclusion of individuals who are not sedentary and had an SPPB ≥10 may have contributed to the lack of a consistent association of ABI >1.40 with impaired functioning compared with individuals with ABI of 1.10 to 1.40. In addition, ABI >1.40 may not be as sensitive as pulse‐wave velocity measures for identifying stiff lower extremity arteries.
This study has limitations. First, participants were enrolled in the LIFE Study, a randomized trial designed to prevent mobility loss among older, sedentary men and women with an SPPB <10. Findings may not be generalizable to individuals not meeting inclusion criteria for the LIFE Study. Nevertheless, because many older men and women in the US are sedentary and many have some functional limitations, our findings are likely to be representative of a large proportion of community‐dwelling, older men and women. Second, the study design is cross‐sectional. Longitudinal data are needed to establish the prognostic significance of the ABI categories defined here. Third, random blood pressure measurement error leads to minor biases in calculated ABI and may result in misclassification of some individuals within ABI categories. However, duplicate blood pressure measures were used and this method is known to reduce bias by 30% to 40% and achieves sensitivities that exceed 90%.25 Fourth, the LIFE Study exclusion of individuals with SPPB scores ≥10 may have limited our ability to identify a significant association of the ABI with the SPPB, since SPPB scores were truncated at ≤9. Fifth, the smaller sample size of the group with ABI 0.90 to 0.99, compared with those with ABI of 1.00 to 1.09 may have limited the statistical power to detect significant associations of borderline ABI values with poorer functional performance, compared with the normal ABI group. Sixth, the large number of comparisons may result in some of the statistical tests being statistically significant by chance. However, our findings were generally consistent regarding the association of lower ABI values with slower walking velocity and slower 400‐m walk times. Seventh, we did not re‐administer the San Diego claudication questionnaire at the conclusion of the 400‐m walk. Eighth, we did not measure the Gardner treadmill stress test in the LIFE Study, which may have identified more participants with symptomatic PAD. Ninth, small meaningful differences for the 4‐m walking velocity have been identified as ranging from 0.04 to 0.06 m/s.26 Differences in walking velocity between the ABI 1.10 to 1.40 and the normal ABI groups did not achieve this level of clinical significance. However, clinically meaningful differences in the 400‐m walk time have been defined as 20 to 30 seconds,27 and several comparisons across ABI categories met this definition of clinical significance.
In conclusion, approximately only one third of sedentary, community‐dwelling older men and women with some functional limitation have a completely normal ABI value. Many have borderline and low normal ABI values. These findings underscore a significant and growing public health challenge in a large proportion of community‐dwelling older men and women.
Sources of Funding
Funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376‐05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH. Supported in part by the NIH/National Center for Advancing Translational Science Clinical and Translational Science Award to the University of Florida UL1 TR000064. Dr Fielding's contribution is partially supported by the US Department of Agriculture, under agreement No. 58‐1950‐7‐707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Dept of Agriculture. This research is also supported by the Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679). This research is partially supported by the University of Florida Claude D. Pepper Older Americans Independence Center (1 P30 AG028740). The Pittsburgh Field Center is partially supported by the Pittsburgh Claude D. Pepper Older Americans Independence Center (P30 AG024827). The Wake Forest University Field Center is, in part, supported by the Claude D. Pepper Older Americans Independence Center (1 P30 AG21332). Dr Gill is the recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging. The Yale Field Center is partially supported by the Claude D. Pepper Older Americans Independence Center (P30AG021342). Dr Fragoso is the recipient of a Career Development Award from the Department of Veterans Affairs.
Disclosures
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Batchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics‐2013 update: a report from the American Heart Association. Circulation. 2013; 127:143-152 [DOI] [PubMed] [Google Scholar]
- 2.Fung YC. In: Fung YC. (ed.). Blood flow in arteries: pressure and velocity waves in large arteries and the effects of geometric nonuniformity. In Biodynamics—Circulation. 1984New York, NY: Springer‐Verlag; 133-136 [Google Scholar]
- 3.Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Br J Surg. 1969; 56:676-679 [DOI] [PubMed] [Google Scholar]
- 4.Ouriel K, Zarins CK. Doppler ankle pressure: an evaluation of three methods of expression. Arch Surg. 1982; 117:1297-1300 [DOI] [PubMed] [Google Scholar]
- 5.Lijmer JG, Hunink MG, van den Dungen JJ, Loonstra J, Smit AJ. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol. 1996; 22:391-398 [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001; 286:1599-1606 [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the Women's Health and Aging Study. Circulation. 2000; 101:1007-1012 [DOI] [PubMed] [Google Scholar]
- 8.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat‐Jacobson DAmerican Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia Measurement and interpretation of the ankle‐brachial index: a scientific statement from the American Heart Association. Circulation. 2012; 126:2890-2909 [DOI] [PubMed] [Google Scholar]
- 9.Ankle Brachial Index Collaboration Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton‐Tyrrell K, Fowkes FG, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodríguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta‐analysis. JAMA. 2008; 300:197-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004; 292:453-461 [DOI] [PubMed] [Google Scholar]
- 11.McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle‐brachial index and subclinical cardiac and carotid disease: the Multi‐Ethnic Study of Atherosclerosis. Am J Epidemiol. 2005; 162:33-41 [DOI] [PubMed] [Google Scholar]
- 12.Amoh‐Tonto CA, Malik AR, Kondragunta V, Ali Z, Kullo IJ. Brachial‐ankle pulse wave velocity is associated with walking distance in patients referred for peripheral arterial disease evaluation. Atherosclerosis. 2009; 206:173-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson NL, Sutton‐Tyrell K, Youk AO, Boudreau RM, Mackey RH, Simonsick EM, Rosano C, Hardy SE, Windham BG, Harris TB, Najjar SS, Lakatta EG, Atkinson HH, Johnson KC, Bauer DC, Newman ABHealth ABC Study Arterial stiffness and gait speed in older adults with and without peripheral arterial disease. Am J Hypertens. 2011; 24:90-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fielding RA, Rejeski WJ, Blair S, Church T, Espeland MA, Gill TM, Guralnik JM, Hsu FC, Katula J, King AC, Kritchevsky SB, McDermott MM, Miller ME, Nayfield S, Newman AB, Williamson JD, Bonds D, Romashkan S, Hadley E, Pahor MLIFE Research Group The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci. 2011; 66:1226-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart AL, Verboncoeur CJ, McLellan BY, Gillis DE, Rush S, Mills KM, King AC, Ritter P, Brown BW, Jr, Bortz WM., II Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001; 56:M65-M70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Garnst AC, McDermott MM. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004; 44:618-623 [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000; 55:M221-M231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guralnik JM, Ferrucci L, Simonsick E, Salive ME, Wallace RB. Lower extremity function in persons over 70 years as a predictor of subsequent disability. N Engl J Med. 1995; 332:556-561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchesvsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long‐distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006; 295:2018-2026 [DOI] [PubMed] [Google Scholar]
- 20.McDermott MM, Guralnik JM, Tian L, Ferrucci L, Liu K, Liao Y, Criqui MH. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007; 50:974-982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermott MM, Tian L, Liu K, Guralnik JM, Ferrucci L, Tan J, Pearce WH, Schneider JR, Criqui MH. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008; 51:1482-1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non‐invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996; 1:65-71 [DOI] [PubMed] [Google Scholar]
- 23.McDermott MM, Guralnik JM, Liu K, Ferrucci L, Liao Y, Sharma L, Criqui MH. Associations of borderline and low normal ankle‐brachial index values with functional decline at 5‐year follow‐up: the WALCS (Walking and Leg Circulation Study). J Am Coll Cardiol. 2009; 53:1056-1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott MM, Ferrucci L, Guralnik JM, Dyer AR, Liu K, Pearce WH, Clark E, Liao Y, Criqui MH. The ankle‐brachial index is associated with the magnitude of impaired walking endurance among men and women with peripheral arterial disease. Vasc Med. 2010; 15:251-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espeland MA, Regensteiner JG, Jaramillo SA, Gregg E, Knowler WC, Wagenknecht LE, Bahnson J, Haffner S, Hill J, Hiatt WRLook AHEAD Study Group Measurement characteristics of the ankle‐brachial index: results from the Action for Health in Diabetes study. Vasc Med. 2008; 13:225-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006; 54:743-749 [DOI] [PubMed] [Google Scholar]
- 27.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, Studenski SA. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE‐P Study). J Nutr Health Aging. 2009; 13:538-544 [DOI] [PMC free article] [PubMed] [Google Scholar]