Abstract
Background
Endothelial dysfunction is a key component of vascular vulnerability. Reactive hyperemia index (RHI), as assessed by the peripheral arterial tonometry, can noninvasively evaluate endothelial function. This study was designed to determine the additional prognostic value of endothelial function to the Synergy Between PCI With Taxus and Cardiac Surgery Score (SYNTAXsc) and the Framingham Risk Score (FRS) in predicting cardiovascular events in high‐risk patients.
Methods and Results
We undertook a two‐center prospective study in 528 stable patients at high‐risk for cardiovascular events from the years 2006–2011. The RHI was measured before coronary angiography and coronary complexity was assessed by SYNTAXsc. After optimal therapies including coronary revascularization, there was follow‐up with patients until August 2012. Cardiovascular events consist of cardiovascular death, myocardial infarction, unstable angina, ischemic stroke, coronary revascularization, heart failure‐induced hospitalization, aortic disease, and peripheral arterial disease. During 1468 person‐years of follow‐up, 105 patients developed cardiovascular events. Multivariate Cox proportional hazards analysis identified B‐type natriuretic peptide (BNP), SYNTAXsc, and RHI as independent cardiovascular event predictors (hazard ratio [95% confidence interval]: natural logarithm of BNP per 0.1: 1.019 [1.002 to 1.037]; P=0.023, SYNTAXsc per tertile: 2.426 [1.825 to 3.225]; P<0.0001, RHI per 0.1: 0.761 [0.673 to 0.859]; P<0.0001). When RHI was added to the FRS, BNP, and SYNTAXsc, net reclassification index was significantly improved (27.5%; P<0.0001), with a significant increase in the C‐statistic (from 0.728 [0.679 to 0.778] to 0.766 [0.726 to 0.806]; P=0.031).
Conclusions
Advanced endothelial dysfunction significantly correlated with near future cardiovascular events in high‐risk patients. This physiological vascular measurement improved risk discrimination when added to the FRS, BNP, and SYNTAXsc.
Clinical Trial Registration
URL: clinicaltrials.gov (http://www.clinicaltrials.gov). Unique identifier: NCT00737945.
Keywords: cardiovascular events, endothelial dysfunction, follow‐up study
Introduction
An integrated approach for identifying patients who are at a high risk for near‐future cardiovascular events is desirable for developing proper therapeutic strategies in cardiovascular medicine.1 Cardiovascular risk assessment using established risk factors such as the Framingham Risk Score (FRS) does not exhaustively predict cardiovascular disease development. Endothelial dysfunction is associated with atherosclerotic progression and can often predict future cardiovascular events.2–6
Coronary plaques and morphological findings using coronary angiography (CAG) reportedly improve cardiovascular event prediction. Synergy Between PCI With Taxus and Cardiac Surgery Score (SYNTAXsc) is an angiographic scoring system used to evaluate coronary atherosclerosis and anatomy complexity.7 The SYNTAXsc is reportedly an effective tool for predicting a major cardiovascular event risk in stable patients with multivessel or left main coronary artery disease (CAD) and in patients with acute coronary syndromes,8–9 but not in stable patients with high‐risk condition.
Reactive hyperemia‐peripheral arterial tonometry (RH‐PAT), which is used to measure the digital hyperemic response, is a noninvasive, automatic, and less operator‐dependent test that is clinically used to evaluate endothelial function.10–11 Recently, we reported that the RH‐PAT index (RHI) was useful for identifying female patients who were at high‐risk for ischemic heart disease,12 and Rubinshtein et al reported that the RHI predicted adverse cardiovascular events in patients without known CAD.13
We hypothesized that adding RHI as an assessment of physiological endothelial function to the coronary complexity morphological assessment and classical risk score would provide substantial cardiovascular event prognostic information in high‐risk patients, including stable patients with established CAD.
Methods
Study Design and Population
This is a prospective observational study and we recruited 577 consecutive, stable, high‐risk patients with diabetes mellitus or >2 conventional coronary risk factors without heart failure (left ventricular ejection fraction <50%, cardiomyopathy, and severe valvular heart disease). These patients had been referred to Kumamoto University Hospital and Yokohama City University Medical Center for CAG because of angina‐like chest symptoms and abnormality in electrocardiogram with high‐risk background for CAD between August 2006 and December 2011. RH‐PAT examination and the subsequent CAG were performed. Exclusion criteria were prespecified as a clinical status that could evidently affect their noncardiovascular prognosis and endothelial function, including systemic illness (advanced endocrine disease, hepatic disease, end‐stage renal disease, active inflammatory disease, and cancer), and cerebrovascular disease with residual hemiplegia.
The present study was approved by the Kumamoto University Institutional Review Board and Yokohama City University Institutional Review Board and was conducted in accordance with the guidelines of our institutional ethics committees and the Declaration of Helsinki. Written informed consent was obtained from each patient before participation.
Angiographic Analysis
Based on quantitative CAG analysis, we defined CAD as ≥50% narrowing of coronary artery diameter in at least 1 coronary artery. The SYNTAXsc for each angiogram was independently evaluated by 2 experienced cardiologists who were blinded to the RH‐PAT results. In case of disagreement, consensus was achieved by consulting 2 cardiologists. Briefly, each lesion with ≥50% luminal narrowing in ≥1.5 mm vessels was defined based on the modified American Heart Association coronary tree segment classification and separately scored regarding bifurcations or trifurcations or aortic ostial localization, chronic occlusion, vessel tortuosity, length, calcification, and thrombus formation. Finally, each lesion score was added to obtain the patient's raw SYNTAXsc. Angiographers who calculated SYNTAXsc were blinded to clinical data and RH‐PAT data, and patients with occluded infarct‐related arteries were scored as occlusions of unknown duration. A high SYNTAXsc is indicative of complex coronary disease.7 We assessed reproducibility of the SYNTAXsc in a random sample of 30 patients. The kappa values for SYNTAXsc (tertile partitioning) intra‐ and interobserver agreement were 0.80 and 0.69, respectively.
RH‐PAT Examination
The RH‐PAT studies in all of the patients were uniformly performed early in the morning in the fasted state before medication intake and prior to CAG, but within 7 days of CAG. The PAT method has been previously described.14
RH‐PAT measurements were analyzed with a computerized, automated algorithm to reduce intra‐ and interobserver variability (Endo‐PAT2000 software, version 3.0.4, Itamar Medical Ltd). The RH‐PAT ratio was calculated using the ratio of the average PAT signal amplitude over a 1‐minute time interval, starting 1.5 minutes after cuff deflation (where control arm=A and the occluded arm=C), divided by the average PAT signal amplitude 2.5 minutes before cuff inflation (baseline) (where the control arm=B and the occluded arm=D), and the RH‐PAT ratio=(C/D)/(A/B) (Figure 1A). Because RH‐PAT ratio results have a skewed distribution, we used the Ln_RH‐PAT ratio and the RHI for analyses. The RHI was derived from the following equation: RHI=Ln{[RH‐PAT ratio]×[0.226×Ln (baseline)−0.2]}.13,15 Ln_RH‐PAT ratio and baseline pulse amplitude were retrospectively analyzed using Endo‐PAT2000 software (version 3.4.4); however, reanalysis was impossible in 2 patients for unknown reasons. Peripheral endothelial function as assessed by the RHI was validated by coronary artery response to acetylcholine, which is the gold standard coronary endothelial functional measurement.12,16 Previous studies demonstrated that RH‐PAT technology has excellent reproducibility.14,17–19
Figure 1.
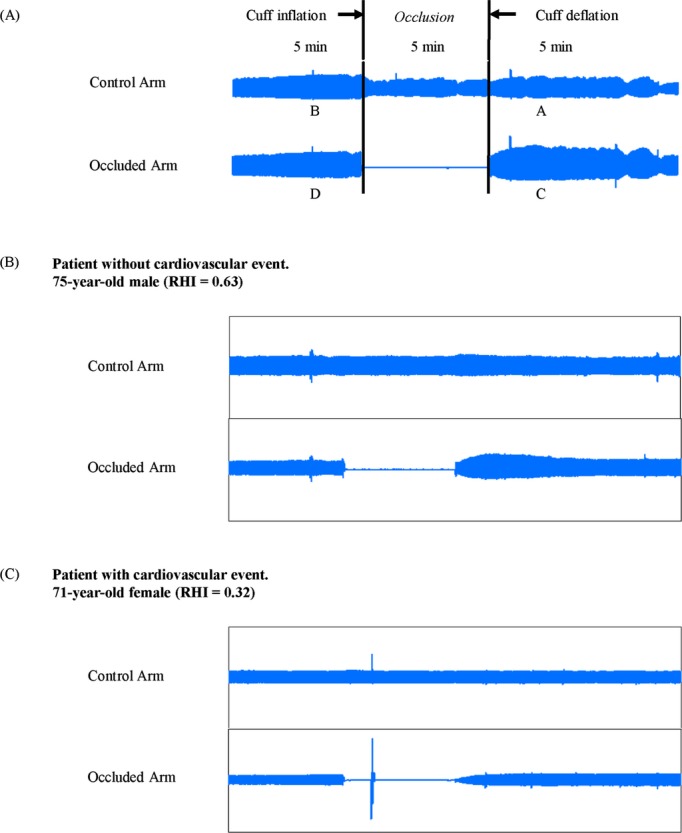
Representative RH‐PAT signals. A, RH‐PAT ratio was calculated with the following equation: RH‐PAT ratio=(C/D)/(A/B). The representative results of RH‐PAT of the patient without cardiovascular event (B) and the patient with cardiovascular event (C). RHI indicates reactive hyperemia‐peripheral arterial tonometry index; RH‐PAT, reactive hyperemia‐peripheral arterial tonometry.
Coronary Risk Factors
Coronary risk factors were defined as current smoking (within 1 year), hypertension (>140/90 mm Hg or antihypertensive medication), dyslipidemia (high‐density lipoprotein [HDL] cholesterol <40 mg/dL, low‐density lipoprotein [LDL] cholesterol ≥140 mg/dL, triglycerides ≥150 mg/dL or medications for dyslipidemia) and, diabetes mellitus (diabetes symptoms and casual plasma glucose concentration ≥200 mg/dL, fasting plasma glucose concentration ≥126 mg/dL, 2‐hour plasma glucose concentration ≥200 mg/dL during 75 g oral glucose tolerance test or hypoglycemic medication), and the presence of a family history of CAD.
Blood Tests and Risk Assessment by Framingham Risk Score (FRS)
Venous blood samples were obtained early in the morning after a 12‐hour fast on the day after admission to measure fasting blood glucose, hemoglobin A1c, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, insulin, creatinine, B‐type natriuretic peptide (BNP), and high‐sensitivity C‐reactive protein (hsCRP) levels. Estimated glomerular filtration rates (eGFRs) were determined using the prediction equation proposed by the Japanese Society of Nephrology and based on the equation described in the Modification of Diet in Renal Disease Study.20 D'Agostino et al reported the FRS for primary and secondary prevention of the composite cardiovascular events.21 In their study, cardiovascular events occurred in 24.7% of patients with coronary heart disease or stroke during 4‐year follow‐up period. We adopted this risk model in our study, and stratified study patients into 1 of 3 risk categories (calculated as a 2‐year score): low‐intermediate risk (<12%), high risk (12% to 25%), or very high risk (>25%).
Follow‐up and Cardiovascular Events
After optimal therapies including coronary revascularization, patients were followed until August 2012. Cardiovascular events consisted of cardiovascular death, nonfatal myocardial infarction, unstable angina, ischemic stroke, coronary revascularization, heart failure‐induced hospitalization, nonfatal aortic disease, and peripheral arterial disease. Cardiovascular events were documented by phone calls to patients or their families and direct consultation with their physicians. In order to verify diagnosis of the cardiovascular events, 3 independent physicians comprising events committee reviewed all medical records (electrocardiograms, ultrasound echocardiograms, cardiac enzyme data, radiographic images, and death certificates) and validated cardiovascular events. If the reviewing physicians disagreed on the event classification, they adjudicated differences. Cardiovascular death was defined as death because of myocardial infarction (within 28 days), congestive heart failure, or documented sudden death without apparent noncardiovascular causes. Myocardial infarction was diagnosed by a rise or fall in cardiac biomarkers (plasma creatine kinase‐MB or cardiac troponin) above the 99th percentile of the normal range upper limit together with evidence of myocardial ischemia and at least 1 of the following symptoms: electrocardiogram changes (new ST‐T changes, left bundle branch block, or pathological Q wave) or imaging evidence of new viable myocardium loss, or a new regional wall motion abnormality. A diagnosis of unstable angina pectoris was made by new or accelerating myocardial ischemia symptoms accompanied by new ischemic ST‐T‐wave changes. Ischemic stroke diagnosis was based on the documented focal neurologic deficit with radiological evidence of brain infarction excluding intracranial hemorrhage. Hospitalization for heart failure decompensation was made if the patient was admitted with typical heart failure symptoms and had objective signs of worsening heart failure that required intravenous drug administration. A diagnosis of aortic and peripheral arterial disease was based on radiological imaging evidence and surgery requirement or percutaneous intervention.
Statistical Analysis
The continuous variable distributions were tested for normality using the Kolmogorov‐Smirnov test. Continuous variable data with normal distribution were expressed as the mean (standard deviation), and the data with skewed distributions were expressed as medians [interquartile range]. Differences between normally distributed continuous variables were analyzed by an unpaired t test or one‐way analysis of variance (ANOVA) with the post‐hoc Bonferroni test. We used the Mann‐Whitney U test for continuous variables with a skewed distribution. We compared groups using the chi‐squared test. We calculated the cumulative cardiovascular event incidence with the Kaplan‐Meier method and compared cardiovascular event incidence with the log‐rank test. We used the median value RHI of 0.531 to divide patients into 2 groups (high and low RHI). To account for the confounding variables, propensity score was calculated in each patient using a logistic regression model in which the dependent variable was high RHI (>median), high Ln_RH‐PAT ratio (>median), or high baseline pulse amplitude (>median), respectively. Independent variables included in the propensity score model were age, gender, body mass index, hypertension, diabetes, current smoking, family history of CAD, systolic blood pressure, diastolic blood pressure, hemoglobin A1c, total cholesterol, LDL cholesterol, HDL cholesterol, triglyceride, left ventricular ejection fraction, BNP, hsCRP, eGFR, treatment with aspirin, hydroxymethylglutaryl‐CoA reductase inhibitors, calcium channel blockers, angiotensin‐converting enzyme‐inhibitors or angiotensin II receptor blockers, β blocker, and anti‐diabetic drugs, CAD, FRS, and SYNTAXsc. We used Cox proportional hazard models to estimate cardiovascular event hazard ratios (HR) and their 95% confidence intervals (CI) in high‐risk patients by univariate analysis and multivariate analysis with a backward algorithm and forced inclusion models. Multicollinearity between covariates was examined by calculating the mean and individual covariate variance inflation factors. None of the individual covariate variance inflation factors were >2, and the mean variance inflation factor for all covariates included in the Cox hazard model was 1.26.
We confirmed the proportional hazards assumption using Schoenfeld's test. Estimates of C‐statistics for the Cox proportional hazards regression models were calculated.22 The C‐statistics were compared after the addition of BNP levels, the SYNTAXsc, and the RHI to the FRS.21 We also examined whether various combinations of these parameters improved the model's discriminatory power.
We performed likelihood ratio tests to evaluate whether the global model fit improved after RHI addition. We also evaluated whether adding the RHI to the FRS, BNP levels, and SYNTAXscs had an incremental effect in predicting cardiovascular events using the net reclassification index.23 To assess reclassification improvement, we defined 3 risk categories on the basis of the FRS (primary and secondary prevention for 2‐year risk for cardiovascular events)21: low‐intermediate risk; <12%, high risk; 12% to 25%, or very high risk; >25%.
When we performed power analysis, we used the past report from Japan.24 When we fixed parameters as follows, the estimated required patient number was 463: event‐free rate 85%, hazard ratio 2.1, power 0.9, and alpha error 0.05. The number that we enrolled in the present study (n=528) was appropriate when compared with the number that was estimated by the power analysis (n=463). Statistical significance was defined as P<0.05 and all of the tests were 2 tailed. All analyses were performed using PASW 18 for Windows (SPSS Inc), STATA version 11.2 (StataCorp LP), and the SAS 9.2 program for Windows (SAS Institute Inc).
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Patient Enrollment
Figure 2 shows the study flow chart. At baseline, we initially included 577 stable, high‐risk patients with suspected CAD without heart failure. We excluded a total of 49 patients from the analysis on the basis of advanced endocrine disease (n=6), hepatic disease (n=9), renal disease (n=11), active inflammatory disease (n=8), cancer (n=7), and cerebrovascular disease with residual hemiplegia (n=8). The study did not include patients with acute coronary syndromes. After the baseline evaluation that included the RHI, CAG was performed in all of the patients. Four hundred and forty‐two patients had ≥50% coronary artery diameter narrowing and were diagnosed as having stable CAD. Depending on their coronary anatomy, patients with CAD were then treated with percutaneous coronary intervention (PCI) (n=344, 77.8%), coronary artery bypass graft surgery (n=21, 4.8%), or medical therapy alone (n=77, 17.4%). In patients undergoing PCI, a bare metal stent (n=64, 18.6%) or a drug‐eluting stent (n=269, 78.2%) was used per operator discretion. At discharge, 98.4%, 93.4%, 63.6%, and 75.1% of the CAD patients were taking aspirin, hydroxymethylglutaryl‐CoA reductase inhibitors, β‐blockers, and angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers for achieving the optimal medical therapy, respectively.
Figure 2.
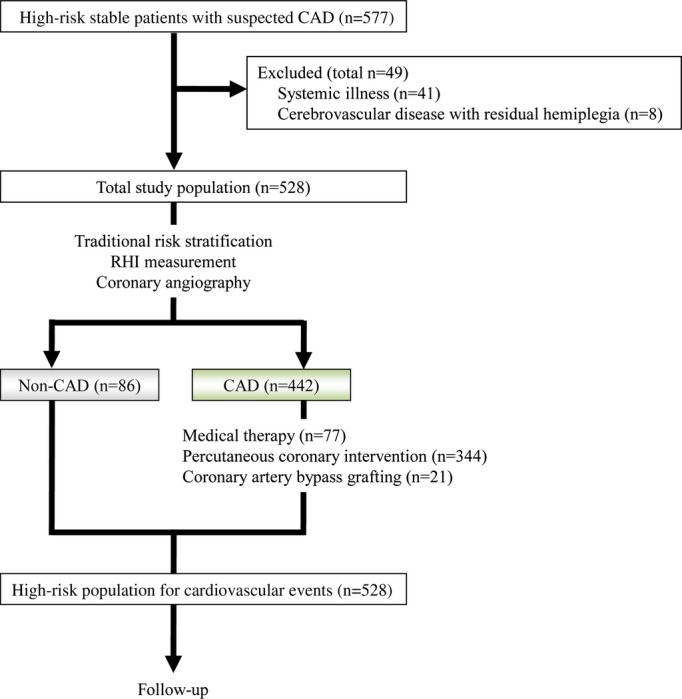
Study protocol flow chart. CAD indicates coronary artery disease; RHI, reactive hyperemia‐peripheral arterial tonometry index.
Baseline Clinical Characteristics
Study patient baseline characteristics are shown in Tables 1 through 4. The mean patient age was 67.2±10.7 years, and coronary risk factor prevalence for hypertension, diabetes, dyslipidemia, current smoking, and family histories of CAD was 81.3%, 47.0%, 83.0%, 21.2%, and 24.6%, respectively. Representative records of RH‐PAT signals in patients with or without cardiovascular events are shown in Figure 1B and 1C.
Table 4.
Baseline Characteristics of 526 High‐risk Patients According to Baseline Pulse Amplitude
| Mean Baseline | P Value | ||
|---|---|---|---|
| High Mean Baseline 857< (n=263) | Low Mean Baseline ≤857 (n=263) | ||
| Age, mean (SD), y | 67.6 (10.3) | 66.7 (11.0) | 0.324 |
| Male sex, no. (%) | 187 (71.1) | 177 (67.3) | 0.395 |
| Body mass index, mean (SD), kg/m2 | 24.4 (3.2) | 24.0 (3.7) | 0.199 |
| Hypertension, no. (%) | 214 (81.4) | 213 (81.0) | >0.99 |
| Diabetes, no. (%) | 127 (48.3) | 119 (45.2) | 0.541 |
| Dyslipidemia, no. (%) | 218 (82.9) | 219 (83.3) | >0.99 |
| Current smoking, no. (%) | 55 (20.9) | 57 (21.7) | 0.915 |
| Family history of CAD, no. (%) | 69 (26.2) | 61 (23.2) | 0.479 |
| Systolic BP, mean (SD), mm Hg | 130.6 (18.2) | 127.7 (18.4) | 0.076 |
| Diastolic BP, mean (SD), mm Hg | 72.8 (11.5) | 72.5 (12.5) | 0.783 |
| Hemoglobin A1c, mean (SD), % | 6.4 (1.0) | 6.4 (1.0) | 0.788 |
| Total/HDL cholesterol ratio, mean (SD) | 3.7 (1.1) | 3.7 (1.1) | 0.974 |
| LDL cholesterol, mean (SD), mg/dL | 104.4 (30.8) | 106.0 (32.4) | 0.576 |
| HDL cholesterol, mean (SD), mg/dL | 51.7 (14.7) | 52.0 (14.6) | 0.814 |
| Triglycerides, median (IQR), mg/dL | 118 (86 to 154) | 113 (80 to 156) | 0.713 |
| LVEF, mean (SD), % | 63.8 (6.6) | 63.5 (7.2) | 0.646 |
| BNP, median (IQR), pg/mL | 28.3 (14.8 to 57.6) | 27.9 (11.7 to 63.4) | 0.482 |
| hsCRP, median (IQR), mg/L | 0.70 (0.30 to 1.80) | 0.78 (0.33 to 1.76) | 0.711 |
| eGFR, mean (SD), mL/min per 1.73 m2 | 66.8 (17.6) | 67.8 (18.8) | 0.565 |
| Aspirin, no. (%) | 222 (84.4) | 211 (80.2) | 0.253 |
| HMG‐CoA RIs, no. (%) | 189 (71.9) | 183 (69.6) | 0.632 |
| CCB, no. (%) | 145 (55.1) | 160 (60.8) | 0.216 |
| ACE‐I or ARB, no. (%) | 147 (55.9) | 148 (56.3) | >0.99 |
| β‐blockers, no. (%) | 121 (46.0) | 106 (40.3) | 0.218 |
| Anti‐diabetic drugs, no. (%) | 91 (34.6) | 82 (31.2) | 0.458 |
| Coronary artery disease, no. (%) | 227 (86.3) | 213 (81.0) | 0.125 |
| FRS, median (IQR), % | 9.0 (5.0 to 11.0) | 8.0 (3.0 to 11.0) | 0.059 |
| SYNTAXsc, median (IQR) | 14.0 (5.0 to 20.0) | 12.0 (5.0 to 19.0) | 0.156 |
| SYNTAXsc ≥23, no. (%) | 51 (19.4) | 40 (15.2) | 0.249 |
| Baseline Pulse Amplitude, mean (SD) | 1226 (304) | 573 (187) | <0.0001 |
| Cardiovascular events, no. (%) | 50 (19.0) | 54 (20.5) | 0.663 |
Data are the mean (SD), median values (25th to 75th percentile range), or no. (%). Significance was assessed by an unpaired t test, the Mann‐Whitney U test or Fisher's exact test. ACE‐I indicates angiotensin‐converting enzyme‐inhibitors; ARB, angiotensin II receptor blockers; BNP, B‐type natriuretic peptide; BP, blood pressure; CAD, coronary artery disease; CCB, calcium channel blockers; eGFR, estimated glomerular filtration rate; FRS, Framingham Risk Score; HDL, high‐density lipoprotein; HMG‐CoA RIs, hydroxymethylglutaryl‐CoA reductase inhibitors; hsCRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; SD, standard deviation; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery score.
Table 1.
Baseline Characteristics of 528 High‐Risk Patients
| All High‐risk Patients (n=528) | RHI | P Value | ||
|---|---|---|---|---|
| High RHI 0.531< (n=267) | Low RHI ≤0.531 (n=261) | |||
| Age, mean (SD), y | 67.2 (10.7) | 66.2 (11.3) | 68.2 (9.9) | 0.027 |
| Male sex, no. (%) | 365 (69.1) | 168 (62.9) | 197 (75.5) | 0.0019 |
| Body mass index, mean (SD), kg/m2 | 24.2 (3.5) | 24.0 (3.5) | 24.3 (3.4) | 0.297 |
| Hypertension, no. (%) | 429 (81.3) | 210 (78.7) | 219 (83.9) | 0.147 |
| Diabetes, no. (%) | 248 (47.0) | 128 (47.9) | 120 (46.0) | 0.664 |
| Dyslipidemia, no. (%) | 438 (83.0) | 222 (83.1) | 216 (82.8) | 0.908 |
| Current smoking, no. (%) | 112 (21.2) | 47 (17.6) | 65 (24.9) | 0.043 |
| Family history of CAD, no. (%) | 130 (24.6) | 75 (28.1) | 55 (21.1) | 0.069 |
| Systolic BP, mean (SD), mm Hg | 129.3 (18.4) | 129.1 (18.6) | 129.4 (18.2) | 0.890 |
| Diastolic BP, mean (SD), mm Hg | 72.7 (12.1) | 71.9 (12.3) | 73.5 (11.8) | 0.149 |
| Hemoglobin A1c, mean (SD), % | 6.4 (1.0) | 6.4 (1.0) | 6.4 (1.1) | 0.912 |
| Total/HDL cholesterol ratio, mean (SD) | 3.7 (1.1) | 3.6 (1.1) | 3.7 (1.1) | 0.049 |
| LDL cholesterol, mean (SD), mg/dL | 105.2 (31.6) | 106.1 (32.2) | 104.3 (30.9) | 0.525 |
| HDL cholesterol, mean (SD), mg/dL | 51.9 (14.5) | 53.7 (14.6) | 50.1 (14.1) | 0.0042 |
| Triglycerides, median (IQR), mg/dL | 115 (83 to 154) | 115 (80 to 151) | 116 (85 to 160) | 0.561 |
| LVEF, mean (SD), % | 63.6 (6.9) | 64.0 (7.2) | 63.2 (6.8) | 0.220 |
| BNP, median (IQR), pg/mL | 28.6 (13.3 to 62.3) | 28.0 (11.5 to 54.4) | 29.4 (15.0 to 67.9) | 0.019 |
| hsCRP, median (IQR), mg/L | 0.76 (0.30 to 1.80) | 0.70 (0.30 to 1.76) | 0.90 (0.37 to 1.90) | 0.192 |
| eGFR, mean (SD), mL/min per 1.73 m2 | 67.3 (18.1) | 68.8 (17.1) | 65.8 (19.1) | 0.057 |
| Aspirin, no. (%) | 434 (82.2) | 202 (75.7) | 232 (88.9) | 0.0001 |
| HMG‐CoA RIs, no. (%) | 373 (70.6) | 178 (66.7) | 195 (74.7) | 0.045 |
| CCB, no. (%) | 305 (57.8) | 142 (53.2) | 163 (62.5) | 0.035 |
| ACE‐I or ARB, no. (%) | 296 (56.1) | 142 (53.2) | 154 (59.0) | 0.189 |
| β‐blockers, no. (%) | 228 (43.2) | 105 (39.3) | 123 (47.1) | 0.079 |
| Anti‐diabetic drugs, no. (%) | 175 (33.1) | 90 (33.7) | 85 (32.6) | 0.782 |
| Coronary artery disease, no. (%) | 442 (83.7) | 190 (71.2) | 252 (96.6) | <0.0001 |
| FRS, median (IQR), % | 8.0 (4.0 to 11.0) | 7.0 (2.0 to 11.0) | 9.0 (7.0 to 13.0) | <0.0001 |
| SYNTAXsc, median (IQR) | 13.0 (5.0 to 20.0) | 9.0 (0.0 to 18.0) | 16.0 (7.8 to 22.5) | <0.0001 |
| SYNTAXsc ≥23, no. (%) | 91 (17.2) | 34 (12.7) | 57 (21.8) | 0.0058 |
| RHI, mean (SD) | 0.566 (0.210) | 0.725 (0.168) | 0.402 (0.088) | <0.0001 |
| Cardiovascular events, no. (%) | 105 (19.9) | 22 (8.2) | 83 (31.8) | <0.0001 |
Data are the mean (SD), median values (25th to 75th percentile range), or no. (%). Significance was assessed by an unpaired t test, the Mann‐Whitney U test or Fisher's exact test. ACE‐I indicates angiotensin‐converting enzyme‐inhibitors; ARB, angiotensin II receptor blockers; BNP, B‐type natriuretic peptide; BP, blood pressure; CAD, coronary artery disease; CCB, calcium channel blockers; eGFR, estimated glomerular filtration rate; FRS, Framingham Risk Score; HDL, high‐density lipoprotein; HMG‐CoA RIs, hydroxymethylglutaryl‐CoA reductase inhibitors; hsCRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; RHI, reactive hyperemia‐peripheral arterial tonometry index; SD, standard deviation; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery score.
Table 3.
Baseline Characteristics of 526 High‐risk Patients According to Ln_RH‐PAT Ratio
| Ln_RH‐PAT Ratio | P Value | ||
|---|---|---|---|
| High Ln_RH‐PAT Ratio 0.246< (n=263) | Low Ln_RH‐PAT Ratio ≤0.246 (n=263) | ||
| Age, mean (SD), y | 66.3 (11.3) | 68.0 (10.0) | 0.068 |
| Male sex, no. (%) | 174 (66.2) | 190 (72.2) | 0.156 |
| Body mass index, mean (SD), kg/m2 | 23.8 (3.5) | 24.5 (3.4) | 0.024 |
| Hypertension, no. (%) | 204 (77.6) | 223 (84.8) | 0.044 |
| Diabetes, no. (%) | 125 (47.5) | 121 (46.0) | 0.793 |
| Dyslipidemia, no. (%) | 214 (81.4) | 223 (84.8) | 0.352 |
| Current smoking, no. (%) | 48 (18.3) | 64 (24.3) | 0.110 |
| Family history of CAD, no. (%) | 75 (28.5) | 55 (20.9) | 0.055 |
| Systolic BP, mean (SD), mm Hg | 128.5 (18.6) | 129.9 (18.0) | 0.377 |
| Diastolic BP, mean (SD), mm Hg | 72.3 (12.4) | 72.9 (11.6) | 0.547 |
| Hemoglobin A1c, mean (SD), % | 6.4 (1.0) | 6.4 (1.1) | 0.794 |
| Total/HDL cholesterol ratio, mean (SD) | 3.5 (1.0) | 3.8 (1.1) | 0.008 |
| LDL cholesterol, mean (SD), mg/dL | 104.5 (31.5) | 106.0 (31.7) | 0.595 |
| HDL cholesterol, mean (SD), mg/dL | 53.4 (14.2) | 50.4 (14.6) | 0.016 |
| Triglycerides, median (IQR), mg/dL | 114 (78 to 150) | 119 (87 to 161) | 0.100 |
| LVEF, mean (SD), % | 63.8 (7.3) | 63.5 (6.5) | 0.647 |
| BNP, median (IQR), pg/mL | 28.0 (11.2 to 56.8) | 28.0 (14.7 to 63.2) | 0.183 |
| hsCRP, median (IQR), mg/L | 0.70 (0.30 to 1.90) | 0.80 (0.38 to 1.70) | 0.479 |
| eGFR, mean (SD), mL/min per 1.73 m2 | 68.7 (17.6) | 65.9 (18.6) | 0.076 |
| Aspirin, no. (%) | 202 (76.8) | 231 (87.8) | 0.001 |
| HMG‐CoA RIs, no. (%) | 176 (66.9) | 196 (74.5) | 0.068 |
| CCB, no. (%) | 151 (57.4) | 154 (58.6) | 0.792 |
| ACE‐I or ARB, no. (%) | 140 (53.2) | 155 (58.9) | 0.219 |
| β‐blockers, no. (%) | 103 (39.2) | 124 (47.1) | 0.078 |
| Anti‐diabetic drugs, no. (%) | 86 (32.7) | 87 (33.1) | >0.99 |
| Coronary artery disease, no. (%) | 191 (72.6) | 249 (94.7) | <0.0001 |
| FRS, median (IQR), % | 7.0 (2.0 to 11.0) | 9.0 (6.0 to 11.0) | <0.0001 |
| SYNTAXsc, median (IQR) | 11.0 (0.0 to 19.0) | 15.0 (7.0 to 22.0) | <0.0001 |
| SYNTAXsc ≥23, no. (%) | 39 (14.8) | 52 (19.8) | 0.166 |
| Ln_RH‐PAT ratio, mean (SD) | 0.490 (0.213) | 0.094 (0.105) | <0.0001 |
| Cardiovascular events, no. (%) | 29 (11.0) | 75 (28.5) | <0.0001 |
Data are the mean (SD), median values (25th to 75th percentile range), or no. (%). Significance was assessed by an unpaired t test, the Mann‐Whitney U test or Fisher's exact test. ACE‐I indicates angiotensin‐converting enzyme‐inhibitors; ARB, angiotensin II receptor blockers; BNP, B‐type natriuretic peptide; BP, blood pressure; CAD, coronary artery disease; CCB, calcium channel blockers; eGFR, estimated glomerular filtration rate; FRS, Framingham Risk Score; HDL, high‐density lipoprotein; HMG‐CoA RIs, hydroxymethylglutaryl‐CoA reductase inhibitors; hsCRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; RH‐PAT, reactive hyperemia‐peripheral arterial tonometry; SD, standard deviation; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery score.
Cardiovascular Events
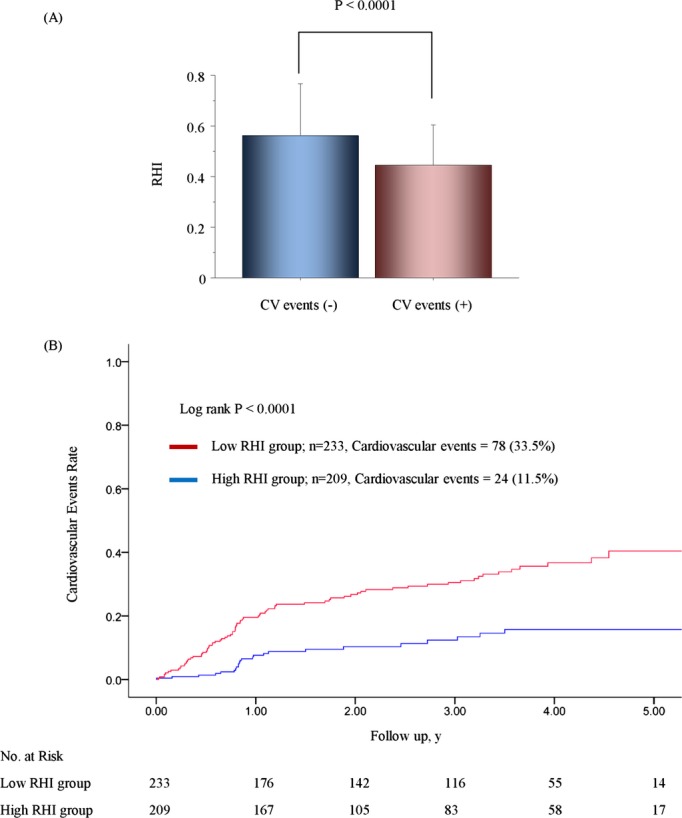
During a mean follow‐up period of 2.8 years with a 1.7‐year SD, which corresponds to 1468 person‐years, 105 patients developed cardiovascular events. Those patients who developed cardiovascular events during the follow‐up period were significantly older and had a lower body mass index, higher BNP levels, higher hsCRP levels, reduced eGFR, increased CAD prevalence, higher FRS, higher SYNTAXsc, and a lower RHI than patients without cardiovascular events (Table 2 and Figure 3A). Patients in the low‐RHI group developed significantly more cardiovascular events (n=83; 31.8% [95% CI, 26.2 to 37.5]) than patients in the high‐RHI group (n=22; 8.2% [95% CI, 4.9 to 11.5]) during the follow‐up period, (P<0.0001) (Table 5). Specifically, acute coronary syndrome, ischemic stroke, coronary revascularization, and heart failure were significantly higher in the low‐RHI group than in the high‐RHI group (Table 5).
Table 2.
Baseline Characteristics of 528 High‐risk Patients Divided by Cardiovascular Events
| All High‐risk Patients (n=528) | Cardiovascular Events During Follow‐up | P Value | ||
|---|---|---|---|---|
| No (n=423) | Yes (n=105) | |||
| Age, mean (SD), y | 67.2 (10.7) | 66.3 (10.8) | 70.7 (9.7) | 0.0002 |
| Male sex, no. (%) | 365 (69.1) | 289 (68.3) | 76 (72.4) | 0.479 |
| Body mass index, mean (SD), kg/m2 | 24.2 (3.5) | 24.4 (3.6) | 23.4 (2.9) | 0.012 |
| Hypertension, no. (%) | 429 (81.3) | 339 (80.1) | 90 (85.7) | 0.211 |
| Diabetes, no. (%) | 248 (47.0) | 197 (46.6) | 51 (48.6) | 0.744 |
| Dyslipidemia, no. (%) | 438 (83.0) | 353 (83.5) | 85 (81.0) | 0.563 |
| Current smoking, no. (%) | 112 (21.2) | 88 (20.8) | 24 (22.9) | 0.689 |
| Family history of CAD, no. (%) | 130 (24.6) | 106 (25.1) | 24 (22.9) | 0.705 |
| Systolic BP, mean (SD), mm Hg | 129.3 (18.4) | 129.4 (18.3) | 128.9 (18.7) | 0.804 |
| Diastolic BP, mean (SD), mm Hg | 72.7 (12.1) | 73.0 (12.3) | 71.4 (10.9) | 0.235 |
| Hemoglobin A1c, mean (SD), % | 6.4 (1.0) | 6.4 (1.0) | 6.5 (1.3) | 0.108 |
| Total/HDL cholesterol ratio, mean (SD) | 3.7 (1.1) | 3.7 (1.1) | 3.6 (1.1) | 0.713 |
| LDL cholesterol, mean (SD), mg/dL | 105.2 (31.6) | 106.2 (32.0) | 101.5 (29.7) | 0.180 |
| HDL cholesterol, mean (SD), mg/dL | 51.9 (14.5) | 52.1 (14.1) | 51.1 (15.8) | 0.556 |
| Triglycerides, median (IQR), mg/dL | 115 (83 to 154) | 117 (85 to 153) | 107 (75 to 157) | 0.234 |
| LVEF, mean (SD), % | 63.6 (6.9) | 63.8 (6.8) | 62.7 (7.4) | 0.150 |
| BNP, median (IQR), pg/mL | 28.6 (13.3 to 62.3) | 26.1 (12.4 to 54.4) | 45.3 (20.8 to 100.2) | <0.0001 |
| hsCRP, median (IQR), mg/L | 0.76 (0.30 to 1.80) | 0.70 (0.30 to 1.70) | 0.90 (0.50 to 2.40) | 0.033 |
| eGFR, mean (SD), mL/min per 1.73 m2 | 67.3 (18.1) | 68.4 (17.8) | 62.8 (18.8) | 0.0048 |
| Aspirin, no. (%) | 434 (82.2) | 342 (80.9) | 92 (87.6) | 0.118 |
| HMG‐CoA RIs, no. (%) | 373 (70.6) | 297 (70.2) | 76 (72.4) | 0.720 |
| CCB, no. (%) | 305 (57.8) | 242 (57.2) | 63 (60.0) | 0.659 |
| ACE‐I or ARB, no. (%) | 296 (56.1) | 233 (55.1) | 63 (60.0) | 0.381 |
| β‐blockers, no. (%) | 228 (43.2) | 174 (41.1) | 54 (51.4) | 0.062 |
| Anti‐diabetic drugs, no. (%) | 175 (33.1) | 132 (31.2) | 43 (41.0) | 0.064 |
| Coronary artery disease, no. (%) | 442 (83.7) | 340 (80.4) | 102 (97.1) | <0.0001 |
| FRS, median (IQR), % | 8.0 (4.0 to 11.0) | 8.0 (3.0 to 11.0) | 9.0 (7.0 to 13.0) | 0.0022 |
| SYNTAXsc, median (IQR) | 13.0 (5.0 to 20.0) | 10.0 (3.0 to 18.0) | 19.0 (15.0 to 25.8) | <0.0001 |
| SYNTAXsc ≥23, no. (%) | 91 (17.2) | 57 (13.5) | 34 (32.4) | <0.0001 |
| Baseline pulse amplitude, mean (SD) | 899 (413) | 910 (418) | 856 (388) | 0.235 |
| Ln_RH‐PAT ratio, mean (SD) | 0.292 (0.260) | 0.322 (0.267) | 0.171 (0.185) | <0.0001 |
| RHI, mean (SD) | 0.566 (0.210) | 0.595 (0.211) | 0.449 (0.163) | <0.0001 |
Data are the mean (SD), median values (25th to 75th percentile range), or no. (%). Significance was assessed by an unpaired t test, the Mann‐Whitney U test or Fisher's exact test. ACE‐I indicates angiotensin‐converting enzyme‐inhibitors; ARB, angiotensin II receptor blockers; BNP, B‐type natriuretic peptide; BP, blood pressure; CAD, coronary artery disease; CCB, calcium channel blockers; eGFR, estimated glomerular filtration rate; FRS, Framingham Risk Score; HDL, high‐density lipoprotein; HMG‐CoA RIs, hydroxymethylglutaryl‐CoA reductase inhibitors; hsCRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; RHI, reactive hyperemia‐peripheral arterial tonometry index; RH‐PAT, reactive hyperemia‐peripheral arterial tonometry; SD, standard deviation; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery score.
Figure 3.
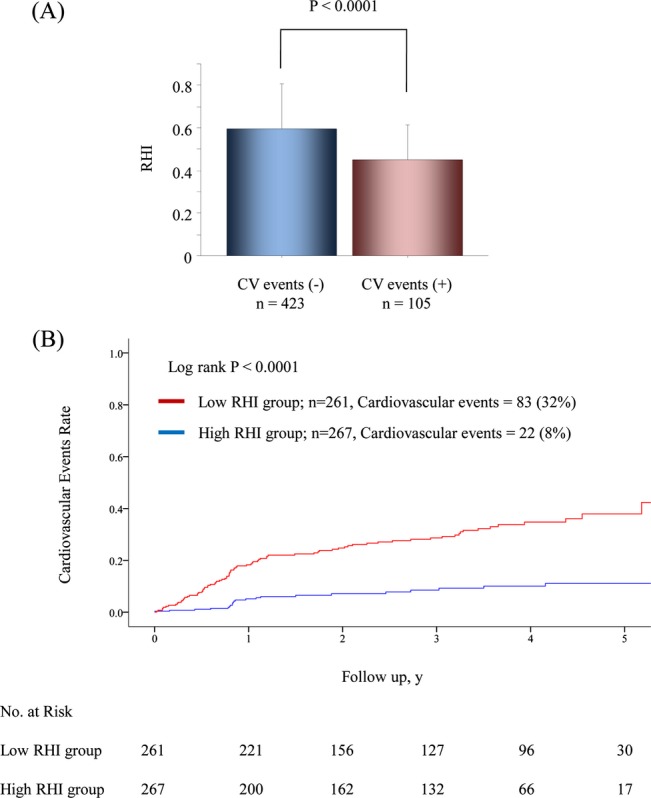
RHI and cardiovascular events. A, These bars represent RHI averages for each group. T‐bars indicate standard deviation. B, Kaplan‐Meier analysis for cardiovascular event probability in high‐risk patients based on a median RHI value of 0.531. CV indicates cardiovascular; RHI, reactive hyperemia‐peripheral arterial tonometry index.
Table 5.
Cardiovascular Events in Patients With Low or High RHI
| All High‐Risk Patients | High RHI Group (n=267) | Low RHI Group (n=261) | P Value |
|---|---|---|---|
| No. (% [95% CI]) | No. (% [95% CI]) | ||
| Total cardiovascular events | 22 (8.2 [4.9 to 11.5]) | 83 (31.8 [26.2 to 37.5]) | <0.0001 |
| Cardiovascular death | 0 (0 [0 to 0]) | 1 (0.4 [−0.4 to 1.1]) | 0.296 |
| AMI or unstable angina | 1 (0.4 [−0.4 to 1.1]) | 28 (10.7 [7.0 to 14.5]) | <0.0001 |
| Heart failure | 2 (0.7 [−0.3 to 1.8]) | 13 (5.0 [2.3 to 7.6]) | 0.0036 |
| Coronary revascularization | 14 (5.2 [2.6 to 7.9]) | 29 (11.1 [7.3 to 14.9]) | 0.0089 |
| Aortic disease or PAD | 4 (1.5 [0.0 to 3.0]) | 6 (2.3 [0.5 to 4.1]) | 0.384 |
| Stroke | 1 (0.4 [−0.4 to 1.1]) | 6 (2.3 [0.5 to 4.1]) | 0.048 |
Significance was assessed by the log rank test. AMI indicates acute myocardial infarction; CI, confidence interval; PAD, peripheral arterial disease; RHI, reactive hyperemia‐peripheral arterial tonometry index.
Cox Proportional Hazard Analysis for Cardiovascular Events
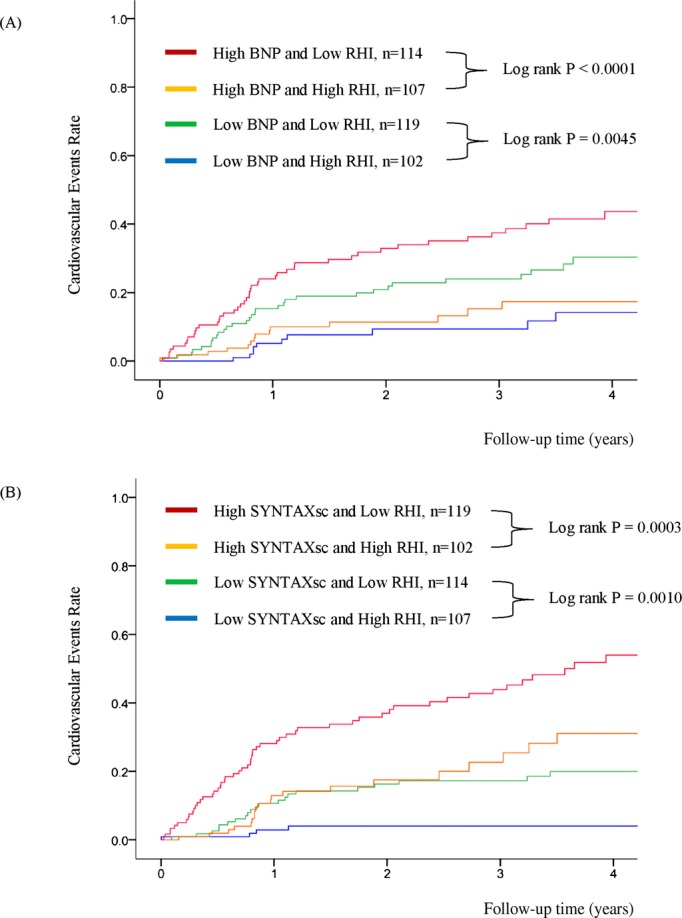
The multivariate analysis with backward algorithms revealed that age, BNP levels, SYNTAXsc, and RHI were independent predictors of cardiovascular events (age HR per 1 year was 1.024 with a 95% CI of 1.000 to 1.048; P=0.046, natural logarithm of BNP level HR per 0.1 was 1.019 with a 95% CI of 1.002 to 1.037; P=0.023, the SYNTAXsc HR per tertile increase was 2.426 with a 95% CI of 1.825 to 3.225; P<0.0001, the RHI HR per 0.1 was 0.761 with a 95% CI of 0.673 to 0.859; P<0.0001) (Table 6, Model 1). RHI was also significantly associated with cardiovascular events in the model with propensity score adjustment (the RHI HR per 0.1 was 0.739 with a 95% CI of 0.649 to 0.842; P<0.0001), and in the forced inclusion model with traditional risk factors, BNP, hsCRP, left ventricular ejection fraction, eGFR, CAD, SYNTAXsc, and RHI (Table 6, Model 2). Furthermore, the FRS was a significant predictor of cardiovascular events (HR for FRS per tertile increase was 1.445, 95% CI: 1.126 to 1.854; P=0.0038), and the forced inclusion model with FRS, BNP, hsCRP, left ventricular ejection fraction, eGFR, CAD, SYNTAXsc, and RHI demonstrated that RHI was independently associated with future cardiovascular events (the RHI HR per 0.1 was 0.771 with a 95% CI of 0.681 to 0.873; P<0.0001) (Table 6, Model 3). Limiting to the coronary heart disease events (n=73), RHI was independently associated with the occurrence of future coronary events by forced‐entry multivariate Cox analysis with FRS, BNP, eGFR, and SYNYAXsc (RHI; per 0.1, HR 0.742, 95% CI; 0.643 to 0.856, P<0.0001). Kaplan‐Meier analysis based on high and low RHI values demonstrated that there was a significantly higher cardiovascular event probability in the low RHI group during the follow‐up period (log‐rank test P<0.0001) (Figure 3B). Kaplan‐Meier estimates for patients in each category by BNP levels and SYNTAXsc confirmed that low RHI values significantly improved cardiovascular events prediction (Figure 4A and 4B).
Table 6.
Cox Proportional Hazards Analysis for Cardiovascular Events
| Variable | Univariate | Multivariate Model 1 | Multivariate Model 2 | Multivariate Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Male sex | 1.211 | 0.789 to 1.859 | 0.381 | Not selected | 1.178 | 0.730 to 1.901 | 0.502 | — | — | — | ||
| Age, per year | 1.043 | 1.022 to 1.065 | <0.0001 | 1.024 | 1.000 to 1.048 | 0.046 | 1.029 | 1.002 to 1.056 | 0.038 | — | — | — |
| Body mass index, per kg/m2 | 0.925 | 0.872 to 0.982 | 0.011 | 0.950 | 0.894 to 1.010 | 0.103 | 0.947 | 0.885 to 1.013 | 0.113 | — | — | — |
| Current smoking | 1.162 | 0.737 to 1.833 | 0.519 | Not selected | 1.284 | 0.780 to 2.113 | 0.325 | — | — | — | ||
| Hypertension | 1.426 | 0.826 to 2.465 | 0.203 | Not selected | 1.306 | 0.731 to 2.335 | 0.367 | — | — | — | ||
| Diabetes mellitus | 1.135 | 0.774 to 1.665 | 0.517 | Not selected | 1.214 | 0.811 to 1.819 | 0.346 | — | — | — | ||
| HDL cholesterol, per mg/dL | 0.994 | 0.980 to 1.007 | 0.376 | Not selected | 1.009 | 0.994 to 1.024 | 0.233 | — | — | — | ||
| LDL cholesterol, per mg/dL | 0.994 | 0.988 to 1.001 | 0.081 | Not selected | 0.999 | 0.992 to 1.006 | 0.775 | — | — | — | ||
| Ln BNP, per 0.1 | 1.036 | 1.019 to 1.052 | <0.0001 | 1.019 | 1.002 to 1.037 | 0.023 | 1.018 | 1.000 to 1.037 | 0.050 | 1.026 | 1.009 to 1.044 | 0.003 |
| Ln hsCRP, per 0.1 | 1.008 | 0.998 to 1.019 | 0.125 | Not selected | 1.004 | 0.993 to 1.015 | 0.514 | 1.001 | 0.991 to 1.012 | 0.813 | ||
| LVEF, per % | 0.977 | 0.952 to 1.003 | 0.081 | Not selected | 0.997 | 0.970 to 1.026 | 0.859 | 1.004 | 0.977 to 1.032 | 0.778 | ||
| eGFR, per mL/min per 1.73 m2 | 0.985 | 0.975 to 0.996 | 0.0067 | Not selected | 1.000 | 0.989 to 1.012 | 0.951 | 0.997 | 0.986 to 1.008 | 0.550 | ||
| Coronary artery disease | 8.587 | 2.720 to 27.11 | 0.0003 | Not selected | 1.110 | 0.310 to 3.980 | 0.873 | 1.577 | 0.433 to 5.747 | 0.490 | ||
| SYNTAXsc, per tertile increment | 2.854 | 2.167 to 3.759 | <0.0001 | 2.426 | 1.825 to 3.225 | <0.0001 | 2.447 | 1.781 to 3.361 | <0.0001 | 2.360 | 1.736 to 3.208 | <0.0001 |
| FRS, per tertile increment | 1.445 | 1.126 to 1.854 | 0.0038 | — | — | — | — | — | — | 0.976 | 0.740 to 1.288 | 0.863 |
| RHI, per 0.1 | 0.693 | 0.614 to 0.781 | <0.0001 | 0.761 | 0.673 to 0.859 | <0.0001 | 0.757 | 0.666 to 0.860 | <0.0001 | 0.771 | 0.681 to 0.873 | <0.0001 |
Multivariate analysis Model 1 was performed using a backward algorithm with a 0.20 significance level. Model 2 and 3 was performed using a forced entry algorithm. Model 2; gender, age, body mass index, smoking, hypertension, diabetes mellitus, HDL cholesterol, LDL cholesterol, BNP, hsCRP, LVEF, eGFR, coronary artery disease, SYNTAXsc, and RHI. Model 3; FRS, BNP, hsCRP, LVEF, eGFR, coronary artery disease, SYNTAXsc, and RHI. BNP indicates B‐type natriuretic peptide; CI, confidence interval; eGFR, estimated glomerular filtration rate; FRS, Framingham Risk Score; HDL, high‐density lipoprotein; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; RHI, reactive hyperemia‐peripheral arterial tonometry index; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery score.
Figure 4.
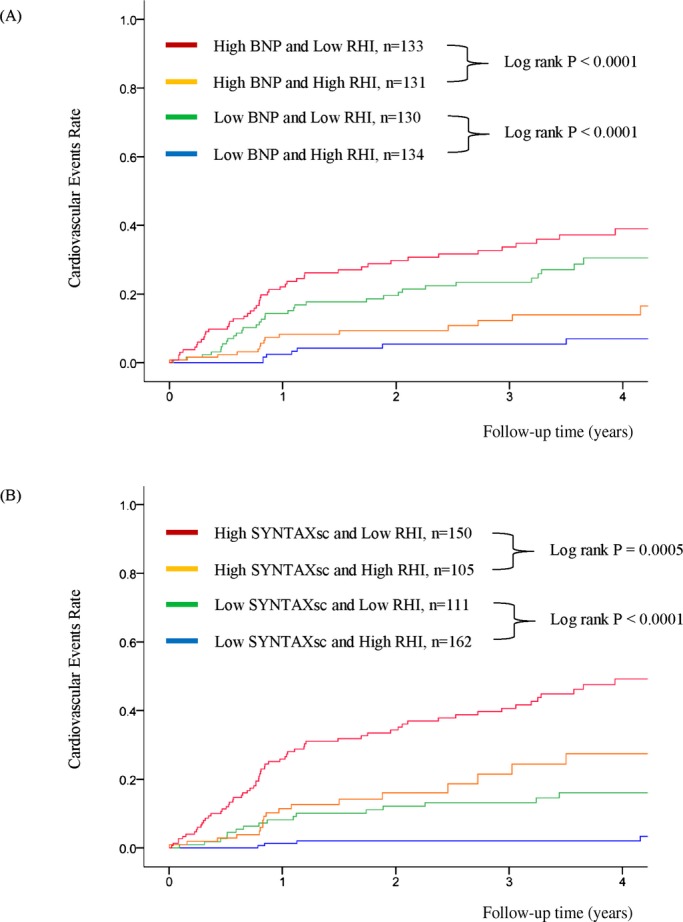
Kaplan‐Meier analysis for cardiovascular event probability in high‐risk patients based on BNP, SYNTAXsc, and RHI. (n=528). A, Analysis in subgroups stratified by RHI and BNP. B, Analysis in subgroups stratified by RHI and SYNTAXsc. Based on each cut‐off point (median value) of RHI, BNP and SYNTAX Score. RHI 0.531, BNP 28.6 pg/mL, and SYNTAX Score 13.0. BNP indicates B‐type natriuretic peptide; RHI, reactive hyperemia‐peripheral arterial tonometry index; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery Score.
Net Reclassification Index and C‐statistics for Cox Proportional Hazard Models to Predict Cardiovascular Events
We treated BNP levels, SYNTAXsc, and RHI values as continuous and reclassified risk scores for the study patients. Significance was achieved in the resultant net reclassification index by adding RHI to the FRS alone (net reclassification index 48.9%; P<0.0001) or to FRS+BNP+SYNTAXsc (net reclassification index 27.5%; P<0.0001) (Table 7).
Table 7.
Reclassification by RHI Addition to FRS Alone and FRS, BNP and SYNTAXsc (n=528)
| Low‐Intermediate Risk | High Risk | Very High Risk | |
|---|---|---|---|
| Risk Category Using FRS Alone | New Risk Category Using FRS+RHI | ||
| Patients Without Cardiovascular Events | |||
| Low‐intermediate risk | 2 | 0 | 0 |
| High risk | 135 | 159 | 93 |
| Very high risk | 6 | 14 | 14 |
| Patients With Cardiovascular Events | |||
| Low‐intermediate risk | 0 | 0 | 0 |
| High risk | 9 | 34 | 48 |
| Very high risk | 0 | 3 | 11 |
| Risk Category Using FRS+BNP+SYNTAXsc | New Risk Category by FRS+BNP+SYNTAXsc+RHI | ||
|---|---|---|---|
| Patients Without Cardiovascular Events | |||
| Low‐intermediate risk | 136 | 27 | 1 |
| High risk | 63 | 78 | 22 |
| Very high risk | 15 | 16 | 65 |
| Patients With Cardiovascular Events | |||
| Low‐intermediate risk | 4 | 6 | 0 |
| High risk | 4 | 15 | 20 |
| Very high risk | 1 | 3 | 52 |
According to the Framingham Risk Score that was calculated for a 2‐year cardiovascular event risk, low‐intermediate risk was <12%, high risk was 12% to 25%, and very high risk was >25%. The overall net reclassification index was 48.9%, P<0.0001 when RHI was used in conjunction with FRS alone, and 27.5%, P<0.0001 when used in conjunction with FRS+BNP+SYNTAXsc. BNP indicates B‐type natriuretic peptide; FRS, Framingham Risk Score; RHI, reactive hyperemia‐peripheral arterial tonometry index; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery score.
We estimated the C‐statistic of the FRS alone. Individual BNP level, SYNTAXsc, and RHI value incorporation into the FRS increased the C‐statistic for cardiovascular event prediction. Adding RHI values to the FRS, BNP level, and SYNTAXsc produced a significant increase in the C‐statistic, from 0.728 to 0.766 (P=0.031, Table 8). We confirmed appropriate proportional hazards assumptions using Schoenfeld's test (P=0.476). RHI addition to the model with FRS, BNP levels, and SYNTAXsc demonstrated a better global fit compared with the model without RHI, as evaluated by the likelihood ratio test (P=0.017). We examined the interaction among all of the variables for effect modification and found that only SYNTAXsc had an interaction with RHI (P=0.005).
Table 8.
C‐Statistics for Cox Proportional Hazards Models to Predict Cardiovascular Events
| C‐Statistics (95% CI) | Increment in C‐Statistics (95% CI) | P Value | |
|---|---|---|---|
| All High‐Risk Patients (n=528) | |||
| FRS | 0.596 (0.540 to 0.653) | 0.0002 | |
| FRS+RHI | 0.699 (0.655 to 0.743) | 0.103 (0.048 to 0.158) | |
| FRS+BNP | 0.640 (0.588 to 0.692) | 0.0055 | |
| FRS+BNP+RHI | 0.706 (0.664 to 0.747) | 0.066 (0.019 to 0.112) | |
| FRS+SYNTAXsc | 0.733 (0.683 to 0.782) | 0.017 | |
| FRS+SYNTAXsc+RHI | 0.774 (0.736 to 0.812) | 0.041 (0.008 to 0.075) | |
| FRS+BNP+SYNTAXsc | 0.728 (0.679 to 0.778) | 0.031 | |
| FRS+BNP+SYNTAXsc+RHI | 0.766 (0.726 to 0.806) | 0.038 (0.004 to 0.072) | |
| CAD Patients (n=442) | |||
| FRS | 0.510 (0.448 to 0.572) | <0.0001 | |
| FRS+RHI | 0.654 (0.603 to 0.704) | 0.144 (0.078 to 0.209) | |
| FRS+BNP | 0.610 (0.553 to 0.667) | 0.0094 | |
| FRS+BNP+RHI | 0.676 (0.631 to 0.720) | 0.066 (0.016 to 0.115) | |
| FRS+SYNTAXsc | 0.682 (0.624 to 0.740) | 0.0070 | |
| FRS+SYNTAXsc+RHI | 0.733 (0.689 to 0.776) | 0.051 (0.014 to 0.088) | |
| FRS+BNP+SYNTAXsc | 0.694 (0.638 to 0.751) | 0.046 | |
| FRS+BNP+SYNTAXsc+RHI | 0.735 (0.692 to 0.779) | 0.042 (0.001 to 0.083) | |
BNP, SYNTAXsc, and RHI were incorporated as continuous variables. We used natural logarithmic transformations of BNP and SYNTAXsc because of skewed distributions. BNP indicates B‐type natriuretic peptide; CAD, coronary artery disease; CI, confidence interval; FRS, Framingham Risk Score; RHI, reactive hyperemia‐peripheral arterial tonometry index; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery score.
We conducted an analysis of a prespecified subgroup of established CAD patients (n=442). CAD patients with cardiovascular events had significantly lower RHI values than those without cardiovascular events (Figure 5A). Kaplan‐Meier estimates for CAD patients in each category by RHI values, BNP levels, and SYNTAXsc revealed that the low RHI values significantly improved cardiovascular event prediction (Figures 5B, 6A, and 6B). Among CAD patients, the net reclassification index and C‐statistics were also significantly improved by adding RHI to the FRS, BNP levels, and SYNTAXsc (net reclassification index was 29.2%; P<0.0001; C‐statistic changed from 0.694 to 0.735; P=0.046) (Tables 8 and 9).
Figure 5.
RHI and cardiovascular events in 442 CAD patients. A, These bars represent averages of the RHI in each group (CV events [−] [n=340], CV events [+] [n=102]). T‐bars indicate standard deviation. B, Kaplan‐Meier analysis for the probability of cardiovascular events in CAD patients based on median value of RHI (0.501). CAD indicates coronary artery disease; CV, cardiovascular; RHI, reactive hyperemia‐peripheral arterial tonometry index.
Figure 6.
Kaplan‐Meier analysis for cardiovascular event probability in CAD patients based on BNP, SYNTAXsc, and RHI. (n=442). A, Analysis in subgroups stratified by RHI and BNP. B, Analysis in subgroups stratified by RHI and SYNTAX score. Based on each cut‐off point (median value) of RHI, BNP, and SYNTAX Score. RHI 0.501, BNP 31.7 pg/mL, and SYNTAX Score 15.8. BNP indicates B‐type natriuretic peptide; CAD, coronary artery disease; RHI, reactive hyperemia‐peripheral arterial tonometry index; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery Score.
Table 9.
Reclassification by RHI Addition to the FRS Alone and the FRS, BNP and SYNTAXsc in CAD Patients (n=442)
| Low‐Intermediate Risk | High Risk | Very High Risk | |
|---|---|---|---|
| Risk Category by FRS Alone | New Risk Category Using FRS+RHI | ||
| Patients Without Cardiovascular Events | |||
| Low‐intermediate risk | 0 | 0 | 0 |
| High risk | 65 | 117 | 88 |
| Very high risk | 4 | 33 | 33 |
| Patients With Cardiovascular Events | |||
| Low‐intermediate risk | 0 | 0 | 0 |
| High risk | 3 | 28 | 41 |
| Very high risk | 2 | 5 | 23 |
| Risk Category Using FRS+BNP+SYNTAXsc | New Risk Category Using FRS+BNP+SYNTAXsc+RHI | ||
|---|---|---|---|
| Patients Without Cardiovascular Events | |||
| Low‐intermediate risk | 54 | 19 | 0 |
| High risk | 59 | 80 | 22 |
| Very high risk | 15 | 23 | 68 |
| Patients With Cardiovascular Events | |||
| Low‐intermediate risk | 2 | 4 | 0 |
| High risk | 4 | 16 | 18 |
| Very high risk | 1 | 4 | 53 |
According to the Framingham Risk Score, which was calculated for a 2‐year cardiovascular event risk, low‐intermediate risk was <12%, high risk was 12% to 25%, and very high risk was more than 25%. The overall net reclassification index was 34.5%, P<0.0001 when used in combination with FRS alone, and 29.2%, P<0.0001 when used in combination with FRS+BNP+SYNTAXsc. BNP indicates B‐type natriuretic peptide; CAD, coronary artery disease; FRS, Framingham Risk Score; RHI, reactive hyperemia‐peripheral arterial tonometry index; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery score.
Ln_RH‐PAT Ratio and Baseline Pulse Amplitude
We also evaluated the prognostic value of baseline pulse amplitude and Ln_RH‐PAT ratio, which is uncorrected for baseline pulse amplitude. The relation of baseline pulse amplitude and Ln_RH‐PAT ratio with cardiovascular risk factors are shown in Table 10. Cox proportional hazard analysis revealed that Ln_RH‐PAT ratio was significantly associated with cardiovascular events in the single model and the model with propensity score adjustment (single analysis, the Ln_RH‐PAT ratio HR per 0.1 was 0.796 with a 95% CI of 0.727 to 0.873; P<0.0001) (the model with propensity score adjustment, the Ln_RH‐PAT ratio HR per 0.1 was 0.816 with a 95% CI of 0.740 to 0.899; P<0.0001). Baseline pulse amplitude did not have significant relation to cardiovascular events in the single and the adjustment model (single analysis, the baseline pulse amplitude HR per 1 was 1.000 with a 95% CI of 0.999 to 1.000; P=0.349) (the model with propensity score adjustment, the baseline pulse amplitude HR per 1 was 1.000 with a 95% CI of 0.999 to 1.000; P=0.233). The addition of Ln_RH‐PAT ratio to the FRS, BNP levels, and SYNTAXsc increased the C‐statistics (Table 11), and the net reclassification indices were significant with the inclusion of Ln_RH‐PAT ratio in the whole study population and in CAD patients (Tables 12 and 13).
Table 10.
The Relation of Baseline Pulse Amplitude, Ln_RH‐PAT Ratio, and RHI With Cardiovascular Risk Factors
| Variables | Baseline Pulse Amplitude | Ln_RH‐PAT Ratio | RHI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Β (SE) | Partial R2 | P Value | Β (SE) | Partial R2 | P Value | Β (SE) | Partial R2 | P Value | |
| Age | 0.104 (0.044) | 0.011 | 0.018 | −0.110 (0.044) | 0.012 | 0.012 | −0.125 (0.043) | 0.016 | 0.004 |
| Male | 0.203 (0.095) | 0.009 | 0.033 | −0.280 (0.094) | 0.017 | 0.003 | −0.294 (0.094) | 0.018 | 0.002 |
| Currently smoking | 0.046 (0.113) | <0.001 | 0.68 | −0.121 (0.112) | 0.002 | 0.28 | −0.090 (0.112) | 0.001 | 0.42 |
| Diabetes | 0.131 (0.087) | 0.004 | 0.13 | −0.044 (0.087) | <0.001 | 0.61 | −0.009 (0.086) | <0.001 | 0.92 |
| Hypertension | −0.032 (0.113) | <0.001 | 0.78 | −0.114 (0.112) | 0.002 | 0.31 | −0.133 (0.112) | 0.003 | 0.24 |
| Body mass index | 0.174 (0.044) | 0.017 | 0.003 | −0.113 (0.044) | 0.013 | 0.011 | −0.072 (0.044) | 0.005 | 0.11 |
| Systolic blood pressure | 0.104 (0.043) | 0.011 | 0.017 | −0.004 (0.043) | <0.001 | 0.93 | 0.003 (0.043) | <0.001 | 0.94 |
| Diastolic blood pressure | −0.013 (0.044) | <0.001 | 0.76 | −0.052 (0.044) | 0.003 | 0.24 | −0.102 (0.043) | 0.010 | 0.020 |
| Fasting blood glucose | 0.049 (0.044) | 0.002 | 0.27 | −0.016 (0.044) | <0.001 | 0.72 | −0.007 (0.043) | <0.001 | 0.87 |
| Hemoglobin A1c | 0.001 (0.044) | <0.001 | 0.98 | −0.020 (0.044) | <0.001 | 0.64 | −0.030 (0.043) | <0.001 | 0.49 |
| Total/HDL cholesterol ratio | 0.030 (0.044) | <0.001 | 0.50 | −0.114 (0.044) | 0.013 | 0.009 | −0.112 (0.043) | 0.013 | 0.010 |
| Triglycerides | 0.040 (0.044) | 0.002 | 0.37 | −0.031 (0.044) | <0.001 | 0.48 | −0.028 (0.044) | <0.001 | 0.53 |
| High‐sensitivity CRP | −0.063 (0.043) | 0.004 | 0.15 | 0.036 (0.043) | 0.001 | 0.41 | 0.034 (0.043) | 0.001 | 0.44 |
| Anti‐hypertensive drugs | −0.038 (0.119) | <0.001 | 0.75 | −0.333 (0.118) | 0.016 | 0.005 | −0.380 (0.117) | 0.021 | 0.001 |
| HMG‐CoA RIs | 0.102 (0.096) | 0.002 | 0.29 | −0.158 (0.095) | 0.005 | 0.097 | −0.123 (0.095) | 0.003 | 0.20 |
| Coronary artery disease | 0.132 (0.123) | 0.002 | 0.29 | −0.836 (0.117) | 0.095 | <0.001 | −0.838 (0.117) | 0.096 | <0.001 |
| SYNTAXsc | −0.003 (0.045) | <0.001 | 0.95 | −0.181 (0.044) | 0.033 | <0.001 | −0.186 (0.044) | 0.035 | <0.001 |
The first 2 rows present models for age and sex separately, with no adjustment for the other variable. Age and sex were forced into all other models. Continuous variables were standardized to mean of 0 and SD of 1, and all categorical variables were coded 1=presence and 0=absence of factor. CRP indicates C‐reactive protein; HDL, high‐density lipoprotein; HMG‐CoA RIs, hydroxymethylglutaryl‐CoA reductase inhibitors; RHI, reactive hyperemia‐peripheral arterial tonometry index; RH‐PAT, reactive hyperemia‐peripheral arterial tonometry; SE, standard error; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery score.
Table 11.
C‐Statistics for Cox Proportional Hazards Models to Predict Cardiovascular Events
| C‐Statistics (95% CI) | Increment in C‐Statistics (95% CI) | P Value | |
|---|---|---|---|
| All High‐Risk Patients (n=526) | |||
| FRS | 0.596 (0.539 to 0.659) | 0.022 | |
| FRS+Ln_RH‐PAT ratio | 0.659 (0.608 to 0.709) | 0.062 (0.009 to 0.116) | |
| FRS+BNP | 0.652 (0.570 to 0.733) | 0.080 | |
| FRS+BNP+Ln_RH‐PAT ratio | 0.688 (0.623 to 0.753) | 0.036 (−0.004 to 0.076) | |
| FRS+SYNTAXsc | 0.733 (0.690 to 0.776) | 0.119 | |
| FRS+SYNTAXsc+Ln_RH‐PAT ratio | 0.753 (0.709 to 0.797) | 0.020 (−0.005 to 0.045) | |
| FRS+BNP+SYNTAXsc | 0.740 (0.679 to 0.801) | 0.072 | |
| FRS+BNP+SYNTAXsc+Ln_RH‐PAT ratio | 0.760 (0.704 to 0.816) | 0.020 (−0.002 to 0.041) | |
| CAD Patients (n=440) | |||
| FRS | 0.510 (0.440 to 0.580) | 0.0030 | |
| FRS+Ln_RH‐PAT ratio | 0.603 (0.545 to 0.661) | 0.093 (0.032 to 0.154) | |
| FRS+BNP | 0.610 (0.511 to 0.709) | 0.0998 | |
| FRS+BNP+Ln_RH‐PAT ratio | 0.645 (0.570 to 0.719) | 0.035 (−0.007 to 0.076) | |
| FRS+SYNTAXsc | 0.682 (0.630 to 0.733) | 0.0596 | |
| FRS+SYNTAXsc+Ln_RH‐PAT ratio | 0.709 (0.657 to 0.761) | 0.028 (−0.001 to 0.056) | |
| FRS+BNP+SYNTAXsc | 0.694 (0.622 to 0.766) | 0.0693 | |
| FRS+BNP+SYNTAXsc+Ln_RH‐PAT ratio | 0.717 (0.650 to 0.784) | 0.023 (−0.002 to 0.047) | |
BNP, SYNTAXsc, and RHI were incorporated as continuous variables. We used natural logarithmic transformations of BNP and SYNTAXsc because of skewed distributions. BNP indicates B‐type natriuretic peptide; CAD, coronary artery disease; CI, confidence interval; FRS, Framingham Risk Score; RHI, reactive hyperemia‐peripheral arterial tonometry index; RH‐PAT, reactive hyperemia‐peripheral arterial tonometry; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery score.
Table 12.
Reclassification by Ln_RH‐PAT Ratio Addition to FRS Alone and FRS, BNP, and SYNTAXsc (n=526)
| Low‐Intermediate Risk | High Risk | Very High Risk | |
|---|---|---|---|
| Risk Category Using FRS Alone | New Risk Category Using FRS+Ln_RH‐PAT Ratio | ||
| Patients Without Cardiovascular Events | |||
| Low‐intermediate risk | 2 | 0 | 0 |
| High risk | 128 | 175 | 93 |
| Very high risk | 6 | 13 | 15 |
| Patients With Cardiovascular Events | |||
| Low‐intermediate risk | 0 | 0 | 0 |
| High risk | 8 | 40 | 43 |
| Very high risk | 0 | 5 | 8 |
| Risk Category Using FRS+BNP+SYNTAXsc | New Risk Category by FRS+BNP+SYNTAXsc+Ln_RH‐PAT Ratio | ||
|---|---|---|---|
| Patients Without Cardiovascular Events | |||
| Low‐intermediate risk | 134 | 30 | 0 |
| High risk | 45 | 92 | 25 |
| Very high risk | 7 | 26 | 63 |
| Patients With Cardiovascular Events | |||
| Low‐intermediate risk | 6 | 4 | 0 |
| High risk | 2 | 19 | 18 |
| Very high risk | 1 | 3 | 51 |
According to the Framingham Risk Score that was calculated for a 2‐year cardiovascular event risk, low‐intermediate risk was <12%, high risk was 12% to 25%, and very high risk was >25%. The overall net reclassification index was 41.3%, P<0.0001 when Ln_RH‐PAT ratio was used in conjunction with FRS alone, and 20.8%, P=0.0003 when used in conjunction with FRS+BNP+SYNTAXsc. BNP indicates B‐type natriuretic peptide; FRS, Framingham Risk Score; RH‐PAT, reactive hyperemia‐peripheral arterial tonometry; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery score.
Table 13.
Reclassification by Ln_RH‐PAT Ratio Addition to the FRS Alone and the FRS, BNP, and SYNTAXsc in CAD Patients (n=440)
| Low‐Intermediate Risk | High Risk | Very High Risk | |
|---|---|---|---|
| Risk Category by FRS Alone | New Risk Category Using FRS+Ln_RH‐PAT Ratio | ||
| Patients Without Cardiovascular Events | |||
| Low‐intermediate risk | 0 | 0 | 0 |
| High risk | 46 | 132 | 91 |
| Very high risk | 2 | 28 | 40 |
| Patients With Cardiovascular Events | |||
| Low‐intermediate risk | 0 | 0 | 0 |
| High risk | 2 | 29 | 41 |
| Very high risk | 1 | 10 | 18 |
| Risk Category Using FRS+BNP+SYNTAXsc | New Risk Category Using FRS+BNP+Syntaxsc+Ln_RH‐PAT Ratio | ||
|---|---|---|---|
| Patients Without Cardiovascular Events | |||
| Low‐intermediate risk | 54 | 19 | 0 |
| High risk | 41 | 95 | 24 |
| Very high risk | 5 | 36 | 65 |
| Patients With Cardiovascular Events | |||
| Low‐intermediate risk | 3 | 3 | 0 |
| High risk | 1 | 21 | 16 |
| Very high risk | 1 | 4 | 52 |
According to the Framingham Risk Score, which was calculated for a 2‐year cardiovascular event risk, low‐intermediate risk was <12%, high risk was 12% to 25%, and very high risk was more than 25%. The overall net reclassification index was 23.3%, P=0.0045 when used in combination with FRS alone, and 24.4%, P<0.0001 when used in combination with FRS+BNP+SYNTAXsc. BNP indicates B‐type natriuretic peptide; CAD, coronary artery disease; FRS, Framingham Risk Score; RH‐PAT, reactive hyperemia‐peripheral arterial tonometry; SYNTAXsc, Synergy Between PCI With Taxus and Cardiac Surgery score.
Discussion
Among patients who were high risk for a cardiovascular event, RHI values successfully and incrementally predicted future cardiovascular events. We demonstrated that adding the RHI value to the Framingham risk model, SYNTAXsc anatomical assessment, and BNP levels improved risk classification, as evidenced by a net reclassification index and a significant increase in the C‐statistics. These findings indicated that an additional physiological assessment of endothelial function could be clinically valuable to identify vulnerable patients who may develop near‐future cardiovascular events.
Atherosclerotic lesions with a high probability of acute thrombotic complications because of plaque rupture or superficial endothelial erosion should be considered “vulnerable plaques.” Currently, cardiovascular risk stratification with established coronary risk factors cannot fully predict the development of acute cardiovascular complications, especially the near‐future cardiovascular events.1,25 The cardiovascular events frequently occurred in the highest‐risk population with history of CAD and those with the established evidence of athero‐thrombotic diseases. Thus, these populations should be the most clinically important patients to prevent the occurrence of cardiovascular events, however the practical risk stratification strategy for these patients has not been proposed.1 Most plaque disruption occurs in moderately stenotic plaques that contain a soft, lipid‐rich core covered by a thin, inflamed fibrous cap, which often accompanies endothelial dysfunction.26 Severe endothelial dysfunction predisposes an individual to vulnerable endothelium, which could lead to plaque disruption and thrombosis. In the present study, we showed that using the RHI value in conjunction with the FRS improved future cardiovascular event prediction. Based on these findings, we propose an additional physiological biomarker assessing endothelial dysfunction, which could be an integrated measurement of all atherogenic and atheroprotective factors. A combined approach with an FRS‐based risk classification, noninvasive physiological RHI, anatomical coronary plaque complexity, and BNP levels might be clinically valuable and could be an integrative strategy for cardiovascular risk assessment in high‐risk patients. Complementing unknown risk conditions assessed by RH‐PAT would open a new era of patient risk stratification in cardiovascular medicine.
Although endothelial function testing is expectantly desired in clinical practice, endothelial function as determined by brachial artery flow‐dependent vasodilation (FMD) has not been successfully incorporated into the current integrative risk stratification system because of its operator dependency and technical problems.18,27 The additional benefits of FMD to traditional risk factors in the cardiovascular risk reclassification have not been established.4,6 Practically with clinical utility, digital RHI is a reproducible and less operator‐dependent technique for peripheral endothelial function assessment10,12,14–15,18 that noninvasively reflects coronary endothelial function.12,16 FMD attenuates markedly with advancing age. In contrast, digital vascular function (RHI) well reflects metabolic risk factors including obesity, high cholesterol, diabetes, and smoking.28 Rubinshtein et al demonstrated a significant association between RHI and future cardiovascular events during a 7‐year follow‐up period among patients without CAD.13 In the high‐risk population and in CAD patients, accurate classification of the near‐future event risk is clinically imperative.1 We demonstrated that low RHI values were significantly associated with the near‐future cardiovascular events independent from FRS and coronary plaque complexity, as assessed by SYNTAXsc in high‐risk patients, which indicated the usefulness of the RH‐PAT test in the practical medicine. Compared with medical therapy for atherosclerosis risk factors, prompt coronary revascularization did not effectively reduce cardiovascular event risk.29 Although invasive coronary revascularization can anatomically treat local coronary stenotic plaques, it cannot treat physiological vascular disorders, such as endothelial dysfunction in the systemic vasculature. Thus, effective identification of vulnerable patients with severe endothelial dysfunction is important to investigate potential treatments and improve prognosis. Use of the RHI as a noninvasive assessment of endothelial function could represent an important advance in comprehensive clinical cardiovascular risk evaluation, even after invasive coronary revascularization with optimal medical treatments. Recently, Matsubara et al reported that the new treatment strategy for diabetes improved endothelial function in CAD patients with lower RHI and uncontrolled diabetes.30 We can introduce and evaluate the clinical efficacy of new approaches to achieve optimal therapies with improving endothelial function.
RH‐PAT reflects changes in flow and digital microvessel dilation.18 Validation studies have shown that impairment in peripheral finger endothelial function measured with RH‐PAT is correlated with coronary microvascular function.16 In this study, we clarified the new aspect that peripheral microvascular endothelial function as assessed by RHI can predict cardiovascular events in conductance vessels. The fact that endothelial dysfunction is a systemic condition may explain why peripheral microvascular endothelial function correlates with endothelial function in the coronary arteries (conductance vessels).13,31–33 Taken together, we suggest that peripheral microvascular endothelial dysfunction could associate with the burden of cardiovascular risk and could be considered a barometer of the total risk burden (the risk of the risk factors).
Endothelial dysfunction, as assessed by RHI, could be modestly explained by clinically available risk factors,34 which suggests that the majority of contributing pathogenic factors to endothelial dysfunction have not yet been fully elucidated. The medical community really needs to recognize that a clinical assessment of endothelial function could be an integrated parameter reflecting unknown atherogenic factors, including mental stress, environmental, and genetic background. The RH‐PAT technique is less operator‐dependent and has good reproducibility.35 We would potentially introduce the noninvasive endothelial function tests in the future practical medicine.18
Past studies, including the Framingham study, demonstrated that baseline pulse amplitude was positively related to most cardiovascular risk factors and Ln_RH‐PAT ratio was negatively associated with most cardiovascular disease risk factors.15,28 Whereas in this study, significant positive relation to baseline pulse amplitude was observed only in age, male sex, body mass index, and systolic blood pressure. Age, male sex, body mass index, total/HDL cholesterol ratio, use of antihypertensive drugs, CAD, and SYNTAXsc were negatively correlated with Ln_RH‐PAT ratio. There are several plausible explanations for the discrepancies with prior reports. The high‐aged study patients, the small sample size, and the high prevalence of cardiovascular risk factors (hypertension, dyslipidemia, diabetes, and male) and CAD could cause these discrepancies. These high‐risk profiles could also be one reason for the lower Ln_RH‐PAT ratio in this study compared to previous studies.15,28
The present trial was limited because it was a 2‐center design with a small patient population. Further multicenter studies will be required to confirm our results in a larger patient population.
In conclusion, advanced endothelial dysfunction was significantly associated with adverse cardiovascular events in high‐risk patients. Patients with advanced endothelial dysfunction as identified by the lower RHI might have vulnerable vasculature and endothelium. Clinical evaluation of endothelial function with RHI could provide useful and complementary prognostic information to improve risk assessment for the near‐future cardiovascular events in high‐risk patients.
Role of the Sponsors
The Ministry of Education, Science, and Culture in Japan and West Japan Vascular Function Society had no role in the process of designing, implementing, and reporting of the study apart from its financial contribution, nor in drafting of the manuscript.
Sources of Funding
This study was funded in part by a grant‐in‐aid for Scientific Research (No. C22590786 and No. C25461086 for S. Sugiyama) from the Ministry of Education, Science, and Culture in Japan and a grant from the West Japan Vascular Function Society.
Disclosures
None.
References
- 1.Eagle KA, Ginsburg GS, Musunuru K, Aird WC, Balaban RS, Bennett SK, Blumenthal RS, Coughlin SR, Davidson KW, Frohlich ED, Greenland P, Jarvik GP, Libby P, Pepine CJ, Ruskin JN, Stillman AE, Van Eyk JE, Tolunay HE, McDonald CL, Smith SC., Jr Identifying patients at high risk of a cardiovascular event in the near future: current status and future directions: report of a national heart, lung, and blood institute working group. Circulation. 2010; 121:1447-1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long‐term follow‐up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000; 101:948-954 [DOI] [PubMed] [Google Scholar]
- 3.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow‐mediated dilation to ankle‐brachial pressure index. Circulation. 2003; 108:2093-2098 [DOI] [PubMed] [Google Scholar]
- 4.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow‐mediated dilation for incident cardiovascular events in a population‐based study: the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2009; 120:502-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau KK, Wong YK, Chan YH, Yiu KH, Teo KC, Li LS, Ho SL, Chan KH, Siu CW, Tse HF. Prognostic implications of surrogate markers of atherosclerosis in low to intermediate risk patients with type 2 diabetes. Cardiovasc Diabetol. 2012; 11:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ras RT, Streppel MT, Draijer R, Zock PL. Flow‐mediated dilation and cardiovascular risk prediction: a systematic review with meta‐analysis. Int J Cardiol. 2012; 168:344-351 [DOI] [PubMed] [Google Scholar]
- 7.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005; 1:219-227 [PubMed] [Google Scholar]
- 8.Serruys PW, Onuma Y, Garg S, Vranckx P, De Bruyne B, Morice MC, Colombo A, Macaya C, Richardt G, Fajadet J, Hamm C, Schuijer M, Rademaker T, Wittebols K, Stoll HP. 5‐year clinical outcomes of the ARTS II (arterial revascularization therapies study II) of the sirolimus‐eluting stent in the treatment of patients with multivessel de novo coronary artery lesions. J Am Coll Cardiol. 2010; 55:1093-1101 [DOI] [PubMed] [Google Scholar]
- 9.Palmerini T, Genereux P, Caixeta A, Cristea E, Lansky A, Mehran R, Dangas G, Lazar D, Sanchez R, Fahy M, Xu K, Stone GW. Prognostic value of the SYNTAX score in patients with acute coronary syndromes undergoing percutaneous coronary intervention: analysis from the ACUITY (acute catheterization and urgent intervention triage strategy) trial. J Am Coll Cardiol. 2011; 57:2389-2397 [DOI] [PubMed] [Google Scholar]
- 10.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003; 146:168-174 [DOI] [PubMed] [Google Scholar]
- 11.Nohria A, Gerhard‐Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006; 101:545-548 [DOI] [PubMed] [Google Scholar]
- 12.Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, Matsubara J, Sumida H, Kaikita K, Kojima S, Nagayoshi Y, Yamamuro M, Izumiya Y, Iwashita S, Matsui K, Jinnouchi H, Kimura K, Umemura S, Ogawa H. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010; 55:1688-1696 [DOI] [PubMed] [Google Scholar]
- 13.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010; 31:1142-1148 [DOI] [PubMed] [Google Scholar]
- 14.Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, Schnall RP, Holmes DR, Higano ST, Lerman A. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003; 41:1761-1768 [DOI] [PubMed] [Google Scholar]
- 15.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross‐sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008; 117:2467-2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004; 44:2137-2141 [DOI] [PubMed] [Google Scholar]
- 17.Selamet Tierney ES, Newburger JW, Gauvreau K, Geva J, Coogan E, Colan SD, De Ferranti SD. Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. J Pediatr. 2009; 154:901-905 [DOI] [PubMed] [Google Scholar]
- 18.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012; 126:753-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S, Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012; 60:1778-1786 [DOI] [PubMed] [Google Scholar]
- 20.Imai E, Matsuo S, Makino H, Watanabe T, Akizawa T, Nitta K, Iimuro S, Ohashi Y, Hishida A. Chronic kidney disease Japan cohort (CKD‐JAC) study: design and methods. Hypertens Res. 2008; 31:1101-1107 [DOI] [PubMed] [Google Scholar]
- 21.D'Agostino RB, Russell MW, Huse DM, Ellison RC, Silbershatz H, Wilson PW, Hartz SC. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J. 2000; 139:272-281 [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004; 23:2109-2123 [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008; 27:157-172‐ [DOI] [PubMed] [Google Scholar]
- 24.Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata K, Sano K, Kobayashi T, Yano T, Nakamura K, Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009; 53:323-330 [DOI] [PubMed] [Google Scholar]
- 25.Helfand M, Buckley DI, Freeman M, Fu R, Rogers K, Fleming C, Humphrey LL. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009; 151:496-507 [DOI] [PubMed] [Google Scholar]
- 26.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, De Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003; 108:1664-1672 [DOI] [PubMed] [Google Scholar]
- 27.Vlachopoulos C. Progress towards identifying biomarkers of vascular aging for total cardiovascular risk prediction. J Hypertens. 2012; 30suppl:S19-S26 [DOI] [PubMed] [Google Scholar]
- 28.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: the Framingham Heart Study. Hypertension. 2011; 57:390-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009; 360:2503-2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, Maeda H, Fujisue K, Yamamoto E, Kaikita K, Hokimoto S, Jinnouchi H, Ogawa H. Dipeptidyl peptidase‐4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti‐inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J. 2013; 77:1337-1344 [DOI] [PubMed] [Google Scholar]
- 31.Anderson TJ, Gerhard MD, Meredith IT, Charbonneau F, Delagrange D, Creager MA, Selwyn AP, Ganz P. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995; 75:71B-74B [DOI] [PubMed] [Google Scholar]
- 32.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995; 26:1235-1241 [DOI] [PubMed] [Google Scholar]
- 33.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium‐dependent flow‐mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998; 82:1535-1539 [DOI] [PubMed] [Google Scholar]
- 34.Schnabel RB, Schulz A, Wild PS, Sinning CR, Wilde S, Eleftheriadis M, Herkenhoff S, Zeller T, Lubos E, Lackner KJ, Warnholtz A, Gori T, Blankenberg S, Munzel T. Noninvasive vascular function measurement in the community: cross‐sectional relations and comparison of methods. Circ Cardiovasc Imaging. 2011; 4:371-380 [DOI] [PubMed] [Google Scholar]
- 35.Brant LC, Barreto SM, Passos VM, Ribeiro AL. Reproducibility of peripheral arterial tonometry for the assessment of endothelial function in adults. J Hypertens. 2013; 31:1984-1990 [DOI] [PubMed] [Google Scholar]


