Background
Ischemic heart disease (IHD) is the leading cause of death in the United States.1 Cardiac rehabilitation is an evidence‐based, cost‐effective, multidisciplinary program of individual patient risk factor assessment and management, exercise training, and psychosocial support for patients with heart disease that reduces mortality by 12% to 34% (Table 1).2–6 Cardiac rehabilitation is recommended by American Heart Association (AHA) and the American College of Cardiology (ACC) Guidelines for patients after myocardial infarction (MI), percutaneous coronary intervention (PCI), or coronary artery bypass surgery (CABG).7 However, cardiac rehabilitation is dramatically underutilized, with only 14% to 31% of eligible patients participating.8 Barriers to participation include low referral rates, patient difficulty attending center‐based rehabilitation sessions, and cost.9 Recently, an AHA Presidential Advisory called for a reengineering of cardiac rehabilitation to enhance access, adherence, and effectiveness.10 It is clear that new strategies are needed for the delivery of cardiac rehabilitation.
Table 1.
| 1. Patient assessment |
| 2. Nutritional counseling |
| 3. Weight management |
| 4. Blood pressure management |
| 5. Lipid management |
| 6. Diabetes management |
| 7. Tobacco cessation |
| 8. Psychosocial management |
| 9. Physical activity counseling |
| 10. Exercise training |
Mobile technology has the potential to overcome barriers to access to cardiac rehabilitation and may be a useful tool for increasing participation. Mobile health provides the opportunity to improve access to health promotion interventions and has the unique advantage of being able to influence health behaviors in real‐time.11 Of smartphone users, 86% have used their mobile phone to access just‐in‐time information in the past month.12 Through mobile technology, a user can receive and interact with information, record and review data, receive automated feedback, and connect with other users or healthcare providers.
Mobile health interventions also have the potential to reach a wide segment of the population. Among American adults, 91% own a mobile phone and 56% own a smartphone.13 Mobile health applications are increasingly popular, with ≈1 in 5 smartphone users having downloaded a mobile health application.14 Among minorities, a group with traditionally low participation in cardiac rehabilitation, evidence suggests that uptake of smartphones is high, and that minorities are more likely than nonminority populations to use their smartphones to access health information.13–14 In addition, those without home broadband internet access are using their smartphones to access the internet, suggesting that the mobile platform could have even greater penetration than a purely internet‐based platform for reaching disadvantaged populations.15 While older adults are less likely than younger adults to use mobile technology, recent trends have shown significant increases in internet use and mobile phone ownership by older adults.14,16
Use of mobile phone applications can increase motivation and physical activity in generally healthy populations.17–18 Studies of mobile applications have shown a high degree of acceptability and reasonable efficacy for increasing physical activity and weight loss. In patients with diabetes, mobile applications for self‐management have been shown to improve blood glucose control.19 These findings raise the possibility that mobile applications could be used for promoting physical activity and self‐management among patients with IHD who are eligible for cardiac rehabilitation.
However, little is known regarding the use of mobile applications for cardiac rehabilitation. As these mobile applications begin to emerge, it will be important to have a standard framework for their evaluation. In this review, we examine the existing literature on the use of mobile technology for cardiac rehabilitation and propose a framework for developing and evaluating mobile applications for cardiac rehabilitation.
Literature Search
We performed a PubMed search from January 1, 1993 to September 2, 2013 for relevant articles using the following search strategy: (“telemedicine”[Mesh] OR mobile OR internet OR web OR smartphone OR mHealth OR eHealth) AND (“cardiac rehabilitation” OR [{cardiac OR cardiovascular OR heart} AND “secondary prevention”]). The search returned 150 studies. One author (A.B.) reviewed the abstracts of all articles for inclusion and exclusion criteria. Included studies were those that involved mobile phone interventions for cardiac rehabilitation for patients with IHD. Protocols and completed studies were eligible for inclusion. Studies were excluded from this review if they were not available in English, did not include an intervention with evaluation of health outcomes, did not have a mobile phone component, did not enroll adult patients with IHD, or did not have a physical activity component (Figure). Articles reporting content and technical development of included studies were noted. Review articles were excluded from the analysis, but references were examined for other articles meeting inclusion and exclusion criteria. References of included studies were also reviewed to identify other articles meeting inclusion and exclusion criteria.
Figure 1.
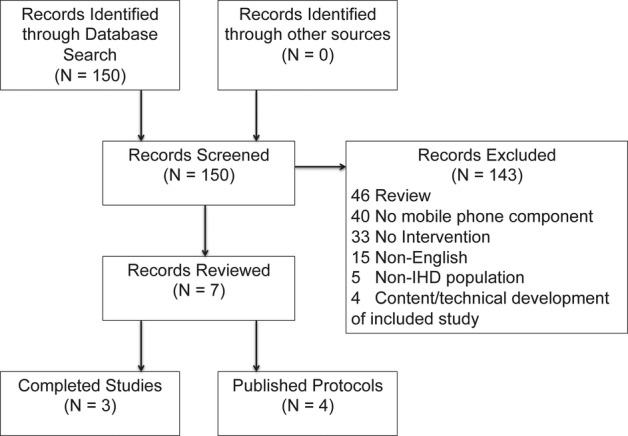
Flow diagram of literature search and selection of studies for review. IHD indicates ischemic heart disease.
Existing Studies
We identified 3 completed, published studies involving mobile phone technology for the delivery of cardiac rehabilitation that evaluated health outcomes in patients with IHD (Table 2).20–22 Though relatively small and not explicitly based on behavior change theory, these studies supported the feasibility and acceptability of the use of mobile technology for cardiac rehabilitation. No studies have evaluated efficacy with regard to cardiovascular events. However, several groups of investigators have published promising study designs for evaluating the use of mobile technology for delivery of cardiac rehabilitation (Table 3).23–26 These studies expand on the existing literature by including the core components of cardiac rehabilitation, basing their interventions on behavior change theory, evaluating a wide array of patient‐centered health outcomes, and employing randomized clinical trial designs (to reduce bias due to confounding from baseline differences in mobile versus traditional groups).
Table 2.
Completed Studies of Mobile Technology for Cardiac Rehabilitation for Ischemic Heart Disease
| Author/Year/Country | Design/Duration | Theoretical Foundation | Non‐mHealth Components | mHealth Components | Intervention | Control | Outcomes |
|---|---|---|---|---|---|---|---|
| Worringham20 2011 Australia |
Observational 6 weeks |
None | Telephone contact pre‐ and postexercise session with provider. | Smartphone, smartphone application, single‐lead ECG, GPS with real‐time transmission to providers. | Monitored exercise training (walking) 3 times weekly assisted by smartphone application. (N=6) | None |
Usability: 80% of sessions no technical problems. Ease of use rated 4.8/5 (95% CI 4.6 to 5.0). Participation: Completed 80% of scheduled exercise sessions. Exercise Capacity: 6MWT improved from 524 to 637 m (P=0.009). Health Status: SF36 Physical Health increased from 50.0 to 78.4 (P=0.03), Mental Health unchanged. Events: None |
| Korzeniowska‐Kubacka21 2011 Poland |
Nonrandomized clinical trial 8 weeks |
None | Supervised exercise sessions at outpatient clinic. No additional intervention specified as adjunct to home sessions. |
Mobile device with preprogrammed exercise training sessions with audio and visual cues for training intensity and 3‐lead ECG monitor. Data transmitted via mobile phone. | 10 clinic supervised exercise sessions followed by 14 home exercise sessions with mobile application (3 sessions per week). (N=30) | 24 clinic supervised exercise sessions (3 sessions per week). (N=32) |
Exercise Capacity: 17.6±16.1% improvement mobile vs 11.5±35.9% control (P>0.05). Risk Factors: BP not significantly changed in either group. Events: not reported |
| Blasco22 2012 Spain |
RCT 12 months |
None | In person assessment. Lifestyle counseling. Intervention participants also supplied with blood pressure cuff, glucose and lipid meter as well as education on use. |
Mobile phone with structured questionnaires for entry and transmission of blood pressure, heart rate, weight, glucose, and lipids. SMS messaging of recommendations. | Lifestyle counseling, mobile intervention, devices for home monitoring. (N=102) | Lifestyle counseling (N=101) |
Usability: mHealth group completed 89% of entries. 5/102 dropped out due to difficulty with mHealth intervention. Physical Activity: 75% met goals in mHealth group vs 73% control. Risk Factors: mHealth group more likely to improve at least 1 risk factor kor (RR 1.4, 95% CI 1.1 to 1.7) (primary outcome). mHealth group more likely to achieve goals for BP (62.1% vs 42.9%), hemoglobin A1c (86.4% vs 54.2%), and BMI (0.37 kg/m2 decrease vs 0.38 increase). No significant differences in smoking cessation, cholesterol, medication adherence. Events: 5 deaths in control group, 0 in mHealth group |
6MWT indicates 6‐minute walk test; CI, confidence interval; BMI, body mass index; BP, blood pressure; ECG, electrocardiogram; GPS, global positioning system; RCT, randomized clinical trial; RR, relative risk; SF‐36, short form 36; SMS, short message service.
Table 3.
Ongoing Studies of Mobile Technology for Cardiac Rehabilitation for Ischemic Heart Disease
| Author/Year/Country | Design/Duration | Theoretical Foundation | Non‐mHealth Components | mHealth Components | Intervention | Control | Outcomes |
|---|---|---|---|---|---|---|---|
| Walters23 2010 Australia |
RCT 6 weeks (intensive) 6 months (follow‐up) |
None | In‐person assessment. Individual goal setting with Mentor. Weekly mentoring sessions. Recommendation for walking‐based exercise program. | Smartphone application with step counting, goal setting, diaries (weight, blood pressure, physical activity), visual feedback, text message reminders, educational videos, web portal. Subset will also have ECG and HR monitoring. | Smartphone application plus counseling (N=100). Smartphone application with ECG and HR monitoring plus counseling (N=15) |
Outpatient center‐based CR (N=100) |
Usability: survey Participation: dropout rates Physical Activity: self‐reported and objectively measured (primary outcome). Exercise Capacity: 6MWT Risk Factors: BMI, BP, smoking, alcohol, lipids, HbA1c, med adherence, Diet habits questionnaire Health Status: EQ‐5D, Health Outcome Questionnaire, SAQ, Psychologic functioning Cost: facility, technology, return‐to‐work Events: hospitalizations and death |
| Maddison24 2011 New Zealand |
RCT 24 weeks |
Self‐efficacy Theory | In‐person assessment and exercise prescription. Pedometer provided. Web portal for entry of physical activity, viewing videos, educational material. | SMS messages (personalized) for behavioral support to promote self‐efficacy. | In‐person assessment, personalized SMS messages and web portal. (N=85) | Referral to community‐based CR. (N=85) |
Participation: defined as at least 1 exercise session Physical Activity: IPAQ, Phone diary Exercise Capacity: Treadmill VO2max (primary outcome), 6MWT. Risk Factors: BMI, waist and hip circumference, BP Health Status: self‐efficacy, SF‐36, EQ‐5D Cost: program and medical Events: illness, signs and symptoms |
| Antypas25 2012 Norway |
Cluster RCT 1 year |
Self‐efficacy, Health Action Process Approach, Stages of Change | Completion of 4‐week center‐based CR program. Internet‐based self‐management program. Enhanced version includes tailoring of content and messages. | SMS reminder messages to fill out questionnaires. | Enhanced version of internet‐based self management program. (N=8 clusters of 15 each) | Internet‐based self management program. (N=8 clusters of 15 each) |
Usability: log‐in data, evaluation Physical Activity: IPAQ (primary outcome) Risk Factors: smoking, alcohol use Health Status: self‐efficacy, Hosptial Anxiety and Depression, social support, EQ‐5D Costs: return‐to‐work |
| Alsaleh26 2012 Jordan |
RCT 6 months |
Social Cognitive Theory, Self‐efficacy Theory | In‐person assessment and advice for CR. Physical activity diary. | Personalized SMS motivational messages (1/week×3 months then 1/2 weeks×3 months). | Personalized program and SMS messages. (N=71) | Advice from providers on physical activity. (N=85) |
Usability: evaluation survey Physical Activity: IPAQ (primary outcome) Health Status: self‐efficacy, Mac‐New Heart Disease Questionnaire |
6MWT, 6‐minute walk test; BP, blood pressure; BMI, body mass index; CR, cardiac rehabilitation; ECG, electrocardiogram; EQ‐5D, European quality of life—5 dimensions; HR, heart rate; IPAQ, International Physical Activity Questionnaire; RCT, randomized clinical trial; SAQ, Seattle Angina Questionnaire; SF‐36, short form 36; SMS, short message service.
Proposed Framework
Although mobile health applications are increasingly prevalent, they are often not based on evidence‐based practices or rigorously studied with regard to their impact on health outcomes.11,27–30 Based on data from the completed and ongoing studies of the use of mobile technology for cardiac rehabilitation, as well as the principles for establishing evidence for mobile health applications,27,30 we propose a framework for the development and evaluation of mobile applications for cardiac rehabilitation for patients with IHD (Table 4). The design of the mobile application should address the core components of cardiac rehabilitation, be based on behavior change theory, provide tailoring of the mobile application to the individual, and be highly usable. The evaluation of the mobile application should include rigorous study with a randomized clinical trial design comparing the mobile application to usual care and assessment of important patient‐centered outcomes. In addition, the design and reporting of clinical studies of mobile applications for cardiac rehabilitation should adhere to the CONSORT (Consolidated Standards Of Reporting Trials) guidelines for mobile health interventions.31
Table 4.
Framework for Evaluating Mobile Applications for Cardiac Rehabilitation
| 1. Address core components of cardiac rehabilitation: |
| ● Patient assessment |
| ● Exercise training |
| ● Self management, may include: |
| ○ Physical activity |
| ○ Diet |
| ○ Medication adherence |
| ○ Smoking |
| ● Psychosocial Support |
| 2. Apply behavior change theory |
| 3. Enable individual tailoring of features |
| 4. Demonstrate high usability |
| 5. Improve patient‐centered outcomes: |
| ● Participation in cardiac rehabilitation |
| ● Physical activity (energy expenditure) |
| ● Exercise capacity |
| ● Cardiovascular risk factors (nutrition, weight, blood pressure, cholesterol, diabetes, tobacco use) |
| ● Patient‐reported health status (symptoms, functional status, quality of life) |
| ● Cost |
| ● Cardiovascular events |
| 6. Establish efficacy in a randomized clinical trial |
Core Components of Cardiac Rehabilitation
The American Association of Cardiovascular and Pulmonary Rehabilitation specifies several key components that should be included in a cardiac rehabilitation program (Table 1).2–3 However, the optimal components necessary to maximize the effectiveness of cardiac rehabilitation and simplicity of delivery are not entirely clear. Similar mortality benefits have been observed with education plus counseling, exercise training alone, and exercise training combined with additional interventions.4,32 A recent systematic review of alternative approaches to the delivery of cardiac rehabilitation concluded that (1) the most effective interventions combined individual patient risk factor management with psychosocial support, and (2) there was insufficient evidence to support interventions based solely on exercise training.33 Naturally, healthcare providers expect that technology‐based cardiac rehabilitation will include similar components to traditional cardiac rehabilitation and occur in the context of supervision by a healthcare provider.34 However, only one published study of mobile technology for cardiac rehabilitation has included components other than exercise training. Ongoing studies plan to evaluate a more comprehensive program of cardiac rehabilitation.
Based on these findings, we suggest that mobile technology‐based interventions for cardiac rehabilitation should include individual patient risk factor assessment and management, exercise training, self‐management of modifiable risk factors, and psychosocial support. Since the optimal combination of core components for mobile‐delivered cardiac rehabilitation is unknown, this represents an important area for future research.
Theoretical Foundation for Behavior Change
Cardiac rehabilitation can be considered a behavior change intervention to promote healthy behaviors in patients with IHD. Interventions that are based on behavior change theory are more effective than those lacking a theoretical basis.35–36 To date, published studies of mobile cardiac rehabilitation have not specifically addressed behavior change strategies in their design. However, several of the ongoing studies specifically incorporate behavior change strategies, including short‐ and long‐term goal setting,23–24,26 motivational messages and reminders,23,25–26 application of behavior change theories,24–26 and attention to promoting self‐efficacy.24–26 Attention to principles from behavior change theories in the design of mobile interventions for cardiac rehabilitation may significantly increase the likelihood of success. In addition, mobile technology may provide an opportunity for delivering real‐time cues to promote behavior change.11
Individual Tailoring
Content development studies of mobile‐ and web‐based cardiac rehabilitation support designing the intervention to be tailored to the individual.34,37 Both web‐ and mobile‐based systems offer the opportunity to remotely provide programmed feedback based on individually set preferences, short‐ and long‐term goals, and personally tailored feedback from a cardiac rehabilitation provider. However, it appears that access and participation may be superior via a mobile platform.38 All published and planned studies of the use of mobile technology for cardiac rehabilitation include some degree of tailoring the intervention to the individual, further highlighting the importance of tailoring in the design of mobile interventions for cardiac rehabilitation.
Usability
An easy‐to‐use interface is a desired feature of mobile applications for promoting physical activity.37,39 Ongoing studies suggest that mobile applications for cardiac rehabilitation can be highly usable, and that use may be promoted by automatic (preferably wireless) entry of data, such as objectively‐measured physical activity.38 Further study is needed on the features of mobile phone applications for cardiac rehabilitation that promote usability, including the need for integration of sensors for ECG monitoring, physical activity monitoring (via accelerometer and global positioning system [GPS]), and measurement of heart rate, blood pressure, and blood glucose. We propose that formal evaluation of the usability of the mobile application be conducted with user‐testing and field studies to evaluate qualitative and quantitative measures of efficiency, effectiveness, and user satisfaction.40–41
Patient‐Centered Outcomes
Historically, the evaluation of cardiovascular disease interventions has focused on hard cardiovascular events such as death, myocardial infarction, heart failure, and stroke. However, it has become increasingly important to evaluate interventions in the context of patient‐centered outcomes.42–43 Patient‐reported health status includes symptoms, functional status, and health‐related quality of life. These outcomes are influenced by physical, mental, and social health.44 In patients with IHD, there are significant variations in health‐related quality of life, even at similar severity of symptoms.45 Thus, the impact of a mobile application on health outcomes must be examined at multiple levels, including participation in cardiac rehabilitation sessions,46–47 physical activity, exercise capacity, cardiovascular risk factors, patient‐reported health status, costs, and clinical events.
Physical activity reduces risk of secondary cardiovascular events in patients with IHD.48–49 Although patient recall is a common method for evaluating physical activity, it is not as accurate as real‐time reporting of physical activity.50–51 The use of mobile technology offers a promising alternative to traditional recall‐based physical activity questionnaires because physical activity can be reported in real‐time through the mobile device. In one study, mobile‐reported physical activity correlated with both objectively‐measured physical activity and self‐reported physical activity, but there was a large degree of variability in mobile‐reported physical activity at similar levels of objectively‐measured activity.52 Furthermore, mobile technology offers the possibility of interfacing with accelerometers, pedometers, and other wireless devices that track physical activity.
Exercise capacity is also protective against cardiovascular events in patients with IHD.53–57 Measurement of exercise capacity can be undertaken through a variety of methods, including cardiopulmonary exercise testing with expired gas measurement and treadmill exercise testing. The 6‐minute walk test, a test of functional exercise capacity, predicts cardiovascular events similarly to treadmill exercise testing, and offers a simple and less resource‐intensive method for measuring exercise capacity.53 Using mobile technology, patients could conduct their own 6‐minute walk test through device‐based sensors (eg, GPS). Moreover, these measurements could be further integrated with other peripheral sensors (eg, measurement of ECG, heart rate, blood pressure, weight, blood glucose, and more), and with ecologic momentary assessment of behavioral and cognitive phenomena. Future research should include evaluation of the reliability and validity of sensors and ecologic momentary assessment for measuring health outcomes associated with mobile technology.
Cardiac rehabilitation is a cost‐effective intervention for patients with IHD.5 It is unclear what the impact of the use of mobile technology will be on overall costs of care. Although mobile devices and wireless services are expensive, potential savings may include lower travel costs, fewer lost wages, and reduced rates of rehospitalization. Insights gained from the impact of mobile technology on health status may help tailor cardiac rehabilitation to the needs of the individual and ultimately decrease risk of secondary events in patients with IHD.
Efficacy in Randomized Clinical Trial
While observational studies and the analysis of observational data provide important insights about treatment effects, the gold standard for establishing efficacy remains the randomized clinical trial. Of the published studies on the use of mobile technology for cardiac rehabilitation, only 1 employed a randomized design, comparing the mobile intervention to standard risk factor counseling alone.22 Ongoing studies are planning randomized or cluster‐randomized designs, which may provide evidence on the efficacy of mobile interventions for cardiac rehabilitation.23–26
An important consideration in randomized study design is the selection of a comparison group. Since cardiac rehabilitation reduces mortality and is a guideline‐recommended therapy, studies comparing the use of a mobile intervention to no intervention would pose ethical questions. However, standard practices and utilization of cardiac rehabilitation vary from country to country and region to region, creating a practical challenge for standardizing a comparison group. Thus, we recommend that studies of mobile interventions for cardiac rehabilitation be compared with best practices in the setting where the study is being conducted, preferably with referral to formal center‐based or home‐based cardiac rehabilitation, since these interventions have established efficacy.4,6
Conclusions
New strategies for promoting participation in cardiac rehabilitation are desperately needed. Initial evidence supports the feasibility and acceptability of using mobile technology for cardiac rehabilitation in patients with IHD. Whether using mobile technology for cardiac rehabilitation can achieve its potential to improve access, increase participation, and ultimately improve outcomes in patients with IHD, remains to be seen.
Sources of Funding
Dr Beatty is supported by the National Center for Advancing Translational Sciences of the NIH under Award Number KL2TR000143. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. None of these funding sources had any role in the preparation, review, or approval of the manuscript.
Disclosures
Dr Whooley receives research funding from Janssen Research and Development, LLC.
References
- 1.Heron M. Deaths: leading causes for 2009. Natl Vital Stat Rep. 2012; 61:1-96 [PubMed] [Google Scholar]
- 2.Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, Sanderson B, Southard DAmerican Heart Association Exercise, CR, Prevention Committee tCoCC, American Heart Association Council on Cardiovascular N, American Heart Association Council on E, Prevention, American Heart Association Council on Nutrition PA, Metabolism, American Association of C, Pulmonary R Core components of cardiac rehabilitation/secondary prevention programs, 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007; 115:2675-2682 [DOI] [PubMed] [Google Scholar]
- 3.Piepoli MF, Corra U, Benzer W, Bjarnason‐Wehrens B, Dendale P, Gaita D, McGee H, Mendes M, Niebauer J, Zwisler AD, Schmid JPCardiac Rehabilitation Section of the European Association of Cardiovascular P, Rehabilitation Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2010; 17:1-17 [DOI] [PubMed] [Google Scholar]
- 4.Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, Thompson DR, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011. 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong WP, Feng J, Pwee KH, Lim J. A systematic review of economic evaluations of cardiac rehabilitation. BMC Health Serv Res. 2012; 12:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor RS, Dalal H, Jolly K, Moxham T, Zawada A. Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database Syst Rev. 2010. 10.1002/14651858.CD007130.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd‐Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KAWorld Heart F, the Preventive Cardiovascular Nurses A AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011; 124:2458-2473 [DOI] [PubMed] [Google Scholar]
- 8.Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007; 116:1653-1662 [DOI] [PubMed] [Google Scholar]
- 9.Jackson L, Leclerc J, Erskine Y, Linden W. Getting the most out of cardiac rehabilitation: a review of referral and adherence predictors. Heart. 2005; 91:10-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, Tomaselli GF, Yancy CWAmerican Heart Association Science A, Coordinating C Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011; 124:2951-2960 [DOI] [PubMed] [Google Scholar]
- 11.Riley WT, Rivera DE, Atienza AA, Nilsen W, Allison SM, Mermelstein R. Health behavior models in the age of mobile interventions: are our theories up to the task? Transl Behav Med. 2011; 1:53-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rainie L, Fox S. Just in time information through mobile connections. Pew Research Center's Internet x0026 American Life Project [Internet]. 2012. May 11, 2013. Available from: http://pewinternet.org/∼/media//Files/Reports/2012/PIP_Just_In_Time_Info.pdf
- 13.Smith A. Smartphone ownership 2013. 2013. September 24, 2013. Available from: http://pewinternet.org/Reports/2013/Smartphone-Ownership-2013.aspx
- 14.Fox S, Duggan M. Mobile health 2012. Pew Research Center's Internet x0026 American Life Project [Internet]. 2012. December 7, 2012. Available from: http://pewinternet.org/Reports/2012/Mobile-Health.aspx
- 15.Smith A. 35% of American adults own a smartphone. Pew Internet and American Life Project [Internet]. 2011. March 9, 2012. Available from: http://pewinternet.org/Reports/2011/Smartphones.aspx
- 16.Zickuhr K, Madden M. Older adults and internet use. Pew Internet x0026 American Life Project [Internet]. 2012. August 30, 2013. Available from: http://www.pewinternet.org/∼/media//Files/Reports/2012/PIP_Older_adults_and_internet_use.pdf
- 17.Fukuoka Y, Vittinghoff E, Jong SS, Haskell W. Innovation to motivation—pilot study of a mobile phone intervention to increase physical activity among sedentary women. Prev Med. 2010; 51:287-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephens J, Allen J. Mobile phone interventions to increase physical activity and reduce weight: a systematic review. J Cardiovasc Nurs. 2013; 28:320-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal K, Eastwood SV, Michie S, Farmer AJ, Barnard ML, Peacock R, Wood B, Inniss JD, Murray E. Computer‐based diabetes self‐management interventions for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2013; 3:CD008776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worringham C, Rojek A, Stewart I. Development and feasibility of a smartphone, ECG and GPS based system for remotely monitoring exercise in cardiac rehabilitation. PLoS One. 2011; 6:e14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korzeniowska‐Kubacka I, Dobraszkiewicz‐Wasilewska B, Bilinska M, Rydzewska E, Piotrowicz R. Two models of early cardiac rehabilitation in male patients after myocardial infarction with preserved left ventricular function: comparison of standard out‐patient versus hybrid training programmes. Kardiol Pol. 2011; 69:220-226 [PubMed] [Google Scholar]
- 22.Blasco A, Carmona M, Fernandez‐Lozano I, Salvador CH, Pascual M, Sagredo PG, Somolinos R, Munoz A, Garcia‐Lopez F, Escudier JM, Mingo S, Toquero J, Monivas V, Gonzalez MA, Fragua JA, Lopez‐Rodriguez F, Monteagudo JL, Alonso‐Pulpon L. Evaluation of a telemedicine service for the secondary prevention of coronary artery disease. J Cardiopulm Rehabil Prev. 2012; 32:25-31 [DOI] [PubMed] [Google Scholar]
- 23.Walters DL, Sarela A, Fairfull A, Neighbour K, Cowen C, Stephens B, Sellwood T, Sellwood B, Steer M, Aust M, Francis R, Lee CK, Hoffman S, Brealey G, Karunanithi M. A mobile phone‐based care model for outpatient cardiac rehabilitation: the care assessment platform (CAP). BMC Cardiovas Disord. 2010; 10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maddison R, Whittaker R, Stewart R, Kerr A, Jiang Y, Kira G, Carter KH, Pfaeffli L. HEART: heart exercise and remote technologies: a randomized controlled trial study protocol. BMC Cardiovas Disord. 2011; 11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antypas K, Wangberg SC. E‐rehabilitation—an internet and mobile phone based tailored intervention to enhance self‐management of cardiovascular disease: study protocol for a randomized controlled trial. BMC Cardiovas Disord. 2012; 12:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsaleh E, Blake H, Windle R. Behavioural intervention to increase physical activity among patients with coronary heart disease: protocol for a randomised controlled trial. Int J Nurs Stud. 2012; 49:1489-1493 [DOI] [PubMed] [Google Scholar]
- 27.Nilsen W, Kumar S, Shar A, Varoquiers C, Wiley T, Riley WT, Pavel M, Atienza AA. Advancing the science of mhealth. J Health Commun. 2012; 17suppl 1:5-10 [DOI] [PubMed] [Google Scholar]
- 28.Breton ER, Fuemmeler BF, Abroms LC. Weight loss—there is an app for that! But does it adhere to evidence‐informed practices? Transl Behav Med. 2011; 1:523-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomlinson M, Rotheram‐Borus MJ, Swartz L, Tsai AC. Scaling up mhealth: where is the evidence? PLoS Med. 2013; 10:e1001382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Nilsen WJ, Abernethy A, Atienza A, Patrick K, Pavel M, Riley WT, Shar A, Spring B, Spruijt‐Metz D, Hedeker D, Honavar V, Kravitz R, Craig Lefebvre R, Mohr DC, Murphy SA, Quinn C, Shusterman V, Swendeman D. Mobile health technology evaluation: the mhealth evidence workshop. Am J Prev Med. 2013; 45:228-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eysenbach G, Group C‐E. CONSORT‐EHEALTH: improving and standardizing evaluation reports of Web‐based and mobile health interventions. J Med Internet Res. 2011; 13:e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta‐analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med. 2005; 143:659-672 [DOI] [PubMed] [Google Scholar]
- 33.Clark RA, Conway A, Poulsen V, Keech W, Tirimacco R, Tideman P. Alternative models of cardiac rehabilitation: a systematic review. Eur J Prev Cardiol. 2013 [DOI] [PubMed] [Google Scholar]
- 34.Vandelanotte C, Dwyer T, Van Itallie A, Hanley C, Mummery WK. The development of an internet‐based outpatient cardiac rehabilitation intervention: a Delphi study. BMC Cardiovas Disord. 2010; 10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010; 31:399-418 [DOI] [PubMed] [Google Scholar]
- 36.Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta‐analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010; 12:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaeffli L, Maddison R, Whittaker R, Stewart R, Kerr A, Jiang Y, Kira G, Carter K, Dalleck L. A mhealth cardiac rehabilitation exercise intervention: findings from content development studies. BMC Cardiovas Disord. 2012; 12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varnfield M, Karunanithi MK, Sarela A, Garcia E, Fairfull A, Oldenburg BF, Walters DL. Uptake of a technology‐assisted home‐care cardiac rehabilitation program. Med J Aust. 2011; 194:S15-S19 [DOI] [PubMed] [Google Scholar]
- 39.Rabin C, Bock B. Desired features of smartphone applications promoting physical activity. Telemed J E health. 2011; 17:801-803 [DOI] [PubMed] [Google Scholar]
- 40.Zhang D, Adipat B. Challenges, methodologies, and issues in the usability testing of mobile applications. Int J Hum Comput Interact. 2005; 18:293-308 [Google Scholar]
- 41.Bastien JM. Usability testing: a review of some methodological and technical aspects of the method. Int J Med Informatics. 2010; 79:e18-e23 [DOI] [PubMed] [Google Scholar]
- 42.Institute of Medicine Crossing the Quality Chasm: a New Health System for the 21st Century. 2001Washington, DC.The National Academies Press; [PubMed] [Google Scholar]
- 43.Rumsfeld JS, Alexander KP, Goff DC, Jr, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat‐Jacobson D, Zerwic JJAmerican Heart Association Council on Quality of C, Outcomes Research CoC, Stroke Nursing CoE, Prevention CoPVD, Stroke C Cardiovascular health: the importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation. 2013; 127:2233-2249 [DOI] [PubMed] [Google Scholar]
- 44.Sherbourne CD, Sturm R, Wells KB. What outcomes matter to patients? J Gen Intern Med. 1999; 14:357-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nease RF, Jr, Kneeland T, O'Connor GT, Sumner W, Lumpkins C, Shaw L, Pryor D, Sox HC. Variation in patient utilities for outcomes of the management of chronic stable angina. Implications for clinical practice guidelines. Ischemic Heart Disease Patient Outcomes Research Team. JAMA. 1995; 273:1185-1190 [PubMed] [Google Scholar]
- 46.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long‐term risks of death and myocardial infarction among elderly medicare beneficiaries. Circulation. 2010; 121:63-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009; 54:25-33 [DOI] [PubMed] [Google Scholar]
- 48.Apullan FJ, Bourassa MG, Tardif JC, Fortier A, Gayda M, Nigam A. Usefulness of self‐reported leisure‐time physical activity to predict long‐term survival in patients with coronary heart disease. Am J Cardiol. 2008; 102:375-379 [DOI] [PubMed] [Google Scholar]
- 49.Janssen I, Jolliffe CJ. Influence of physical activity on mortality in elderly with coronary artery disease. Med Sci Sports Exerc. 2006; 38:418-427 [DOI] [PubMed] [Google Scholar]
- 50.Loney T, Standage M, Thompson D, Sebire SJ, Cumming S. Self‐report vs. objectively assessed physical activity: which is right for public health? J Phys Act Health. 2011; 8:62-70 [DOI] [PubMed] [Google Scholar]
- 51.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the united states measured by accelerometer. Med Sci Sports Exerc. 2008; 40:181-188 [DOI] [PubMed] [Google Scholar]
- 52.Pfaeffli L, Maddison R, Jiang Y, Dalleck L, Lof M. Measuring physical activity in a cardiac rehabilitation population using a smartphone‐based questionnaire. J Med Internet Res. 2013; 15:e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beatty AL, Schiller NB, Whooley MA. Six‐minute walk test as a prognostic tool in stable coronary heart disease: data from the heart and soul study. Arch Intern Med. 2012; 172:1096-1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanhees L, Fagard R, Thijs L, Staessen J, Amery A. Prognostic significance of peak exercise capacity in patients with coronary artery disease. J Am Coll Cardiol. 1994; 23:358-363 [DOI] [PubMed] [Google Scholar]
- 55.Ghayoumi A, Raxwal V, Cho S, Myers J, Chun S, Froelicher VF. Prognostic value of exercise tests in male veterans with chronic coronary artery disease. J Cardpulm Rehabil. 2002; 22:399-407 [DOI] [PubMed] [Google Scholar]
- 56.Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, Shephard RJ. Prediction of long‐term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002; 106:666-671 [DOI] [PubMed] [Google Scholar]
- 57.Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, Shephard RJ. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J Am Coll Cardiol. 2003; 42:2139-2143 [DOI] [PubMed] [Google Scholar]


