Abstract
Background
The technique and safety of transcatheter patent ductus arteriosus (PDA) closure have evolved during the past 20 years. We sought to report a multicenter experience of PDA closure with a focus on the rate of adverse events (AE) and a review of institutional practice differences.
Methods and Results
Outcome data on transcatheter PDA closure were collected at 8 centers prospectively using a multicenter registry (Congenital Cardiac Catheterization Project on Outcome Registry). Between February 2007 and June 2010, 496 PDA closures were recorded using a device in 338 (68%) or coils in 158 (32%). Most patients had an isolated PDA (90%). Fifty percent of patients were between 6 months and 3 years old, with only 40 patients (8%) <6 months old. Median minimum PDA diameter was 2.5 mm (range 1 to 12 mm; IQR 2 to 3 mm) for device closure and 1 mm (range 0.5 to 6 mm; IQR 1 to 2 mm) for coil closure (P<0.001). A device rather than coil was used in patients <3 years, weight <11 kg, and with a PDA minimum diameter >2 mm (all P<0.001). Three of 8 centers exclusively used a device for PDAs with a diameter >1.5 mm. In 9% of cases (n=46), an AE occurred; however, only 11 (2%) were classified as high severity. Younger age was associated with a higher AE rate. Coil‐related AEs were more common than device‐related AEs (10% versus 2%, P<0.001).
Conclusions
PDA closure in the present era has a very low rate of complications, although these are higher in younger children. Technical intervention‐related events were more common in coil procedures compared with device procedures. For PDAs ≤2.5 mm in diameter, institutional differences in preference for device versus coil exist.
Keywords: adverse events, complications, interventional catheterization, PDA, safety
Introduction
In the current era, transcatheter occlusion of the patent ductus arteriosus (PDA) using either coils or device transcatheter therapy is considered to be a well‐established procedure. Single‐center series and multicenter trials, as well as registries, have reported on selection criteria and complication rates.1–4 In practice, operators treat PDAs of a wide range of sizes and in patients at different ages and have access to a variety of devices, coils, and implantation techniques. Using the experience reported in the Congenital Cardiac Catheterization Project on Outcome Registry (C3PO), we sought to determine the current technical success, as well as the incidence and nature of adverse events (AEs) and the relationship to patient or procedural factors. Furthermore, we sought to determine institutional variation in device versus coil treatment for PDA therapy in different sized PDAs.
Methods
Data Collection
Data were collected as part of the Congenital Cardiac Catheterization Outcomes Project (C3PO), a multi‐institutional collaborative registry in which participating centers recorded patient and procedural characteristics and the occurrence of AEs with a web‐based data entry tool. Additional data were entered on specific procedure types (such as PDA closure) to better understand components of efficacy and safety particular to the intervention. Boston Children's Hospital was the sponsor and data‐coordinating center for the project. Data collection started on February 1, 2007, at 6 centers; 2 additional centers joined in May 2008 and July 2009. Data collection, validation, and auditing methods have previously been reported.5 The project had institutional review board approval at all institutions. In accordance with the investigator agreement, all interventional cardiologists who contributed to the data set in this report reviewed and approved the document before peer review submission.
Population
The study population included all patients who underwent transcatheter PDA closure with a device or coil. The cohort was stratified by the first method chosen for therapy, either coil or device. Therefore, if a patient with a PDA underwent attempted coil occlusion complicated by coil embolization and then subsequent device placement, the case was categorized in the coil treatment group. We also report separately on a cohort of patients in whom intervention was considered but abandoned. These patients were not included in the cohort analyzed, as a device or coil was not left in place, and therefore were not considered and counted as an AE. In seeking a population of patients with uncomplicated first cardiovascular intervention for PDA closure, 24 cases with a history of prior catheterization or surgery were excluded. Although either device or coil is reported, the manufacturer was not recorded in the database. During the study period, the only device available at the participating centers was the Amplatzer Device (St Jude Medical Inc). However, in the coil group, numerous types, such as MReye, Gianturco, or Flipper coils (Cook Medical), may have been used but were not specified. In cases in which device or coil embolization occurred, the centers provided additional descriptive data.
Patient and Procedural Characteristics
The following patient characteristics were recorded: age, weight, sex, diagnosis, non‐cardiac problem, genetic syndrome, and hemodynamic data. The admission status (elective or nonelective), airway management method (assisted ventilation or spontaneous respirations), need for inotropic support, case duration, and contrast dose were documented. The PDA minimum lumen diameter was measured. The type of PDA was not reported in the database; however, in cases complicated by embolization or malposition, additional information was reported including the PDA type. If a device was delivered outside a sheath or catheter and then removed from the body, a reason was recorded including embolization, malposition, or another specified reason, in which case additional descriptive data were sought. Embolization was defined as a released coil or device that was no longer located in the PDA. Malposition was defined as a coil or device that was released and then had to be retrieved due to having an abnormal position within the PDA. If a coil or device was placed in the PDA without being released and was removed due to being undersized or oversized or was encroaching on adjacent structures before being released, it was not considered to be in a malposition or to be an AE. All AEs were recorded in the database and classified according to attributability and severity (Table 1) based on previous stratification.6–7 All AEs in the C3PO database have undergone independent review by 2 interventional cardiologists for appropriate categorization of severity level. Technical success was defined as the patient leaving the catheterization laboratory with a coil or device in the PDA with no more than a tiny residual shunt based on angiography. The database did not report whether the PDA was completely closed or there was a tiny residual shunt, and we acknowledge this as one of the limitations of the study. Also, the data were reported as an episode of care, so follow‐up after patient discharge was not available.
Table 1.
Definition of Adverse Events
| Severity Level | Definition | Examples | |
|---|---|---|---|
| Low | 1—None (very mild) | No harm, no change in condition, may have required monitoring to assess for potential change in condition with no intervention indicated. | Imaging equipment problem, medication error, minor bleeding from access site without hematoma resolved with compression, device malfunction removed easily, short self limited arrhythmia from catheter manipulation. |
| 2—Minor | Transient change in condition, not life threatening, condition returns to baseline, required monitoring, required minor intervention such as holding a medication, or obtaining lab test. | Coil malposition or embolization while in the cath lab easily retrieved, device malposition requiring sheath size change or snaring, pulse loss resolved spontaneously, hematoma or large bruising, minor airway problem, transient arrhythmia, allergic reaction | |
| High | 3—Moderate | Transient change in condition may be life threatening if not treated, condition returns to baseline, required monitoring, required intervention such as reversal agent, additional medication, transfer to the intensive care unit for monitoring, or moderate transcatheter intervention to correct condition. | Blood loss, hemothorax, respiratory acidosis, atrial arrhythmia requiring intervention, anesthesia problem requiring reintubation, any device embolization in the catheterization lab or afterward |
| 4—Major | Change in condition, life threatening if not treated, change in condition may be permanent, may have required an intensive care unit admission or emergent readmit to hospital, may have required invasive monitoring, required interventions such as electrical cardioversion or unanticipated intubation or required major invasive procedures or transcatheter interventions to correct condition. | Ventricular arrhythmia requiring medication, vessel dissection, hypotension requiring inotrope support, atrioventricular block, coil or device malposition or embolization requiring surgery | |
| 5—Catastrophic | Any death, and emergent surgery or heart lung bypass support (extracorporeal membrane oxygenation) to prevent death with failure to wean from bypass support. | Death |
AV indicates atrioventricular; ECMO, extra‐corporeal membrane oxygenator.
Statistical Analysis
Median, range, and interquartile range were calculated for all continuous variables and frequency with percentages for categorical variables. A χ2 analysis was used to test for differences in categorical variables, and Wilcoxon rank sum test was used for continuous variables. AEs were tabulated, and frequency and percent were calculated by attributability. Because weight has previously been shown to be associated with worse outcomes,8 the cohort was stratified by weight of <6 kg and >6 kg. In addition, cases were stratified by PDA size <2.5 mm, a threshold previously shown to be associated with effective single coil closure.9 For the incidence of occurrence of an AE, the following patient or procedure characteristics were considered and assessed in a multivariable analysis: age, weight, diagnosis, PDA size ≥2.5 mm, indicators of hemodynamic vulnerability,5–6 and type of device used, either coil or device. Variables significant at P<0.1 in univariate analysis were considered for inclusion and retained if the P value was <0.05 in the model. The single factor that provided the most predictive information about the outcome was included first, and remaining variables were then considered using a forward stepwise method. The size of the PDA and closure method were summarized by institution to explore differences in the size threshold preferred for coil versus device therapy.
Results
The cohort includes 496 PDA closures performed at 8 institutions and recorded in the database between February 1, 2007, and June 30, 2010. A device was initially used in 338 and a coil was used in 158 cases. The majority of patients (90%) had an isolated PDA with no associated congenital heart disease, and cases were predominantly performed electively (98%) (Table 2). Most procedures were performed under general anesthesia with mechanical ventilation (66%), while inotrope support was rarely necessary (1%). Patients <6 months of age were more likely to undergo device closure: 36 (90%) of 40 cases versus 302 (66%) of 456. Case duration was similar in the 2 treatment groups. More contrast was required for device placement cases compared with coil cases: median 3.9 mL/kg versus 2.3 mL/kg (P<0.001). Coils were predominantly reserved for the smallest PDAs (Figure 1).
Table 2.
Patient and Procedural Characteristics
| Patient and Procedural Characteristics | Total (n=496) | Coil (n=158) | Device (n=338) | P Value |
|---|---|---|---|---|
| Age | <0.001 | |||
| <6 mo | 40 (8) | 4 (3) | 36 (11) | |
| 6 mo to 2 y | 250 (50) | 71 (45) | 179 (53) | |
| 3 to 17 y | 185 (37) | 81 (51) | 104 (31) | |
| ≥18 y | 21 (4) | 2 (1) | 19 (6) | |
| Weight, kg | 12.2 [8.9, 19.5] (2.2, 132.0) | 14.8 [10.4, 27.0] (3.0, 132.0) | 11.2 [8.4, 17.7] (2.2, 107.8) | <0.001 |
| Female sex | 319 (64) | 99 (63) | 220 (65) | 0.62 |
| Diagnosis | 0.14 | |||
| Isolated PDA | 445 (90) | 136 (86) | 309 (91) | |
| Pulmonary hypertension | 6 (1) | 2 (1) | 4 (1) | |
| Intracardiac disease and PDA | 45 (9) | 20 (13) | 25 (7) | |
| Genetic syndrome | 65 (13) | 28 (18) | 37 (11) | 0.05 |
| Noncardiac problem | 113 (23) | 33 (21) | 80 (24) | 0.57 |
| Spontaneous respiration | 171 (34) | 65 (41) | 106 (31) | 0.04 |
| Inotrope support during case | 4 (1) | 2 (1) | 2 (1) | 0.60 |
| Case duration, min | 64 [51, 83] (16, 298) | 62 [47, 84] (16, 289) | 65 [52, 83] (30, 298) | 0.24 |
| <1 h | 209 (42) | 74 (47) | 135 (40) | 0.06 |
| ≥1 h, <2 h | 252 (51) | 70 (44) | 182 (54) | |
| ≥2 h, <3 h | 24 (5) | 12 (8) | 12 (4) | |
| ≥3 h | 10 (2) | 2 (1) | 8 (2) | |
| Contrast dose, mL/kg | 3.5 [2.4, 4.7] (0.0, 14.7) | 2.3 [1.7, 3.4] (0.0, 10.0) | 3.9 [3.0, 5.0] (0.5, 14.7) | <0.001 |
| Minimum lumen diameter of PDA (n=132, 330) | 2.0 [1.7, 3.0] (0.5, 12.0) | 1.0 [1.0, 2.0] (0.5, 6.0) | 2.5 [2.0, 3.0] (1.0, 12.0) | <0.001 |
Number (%) or median [IQR] (range). PDA indicates patent ductus arteriosus.
Figure 1.
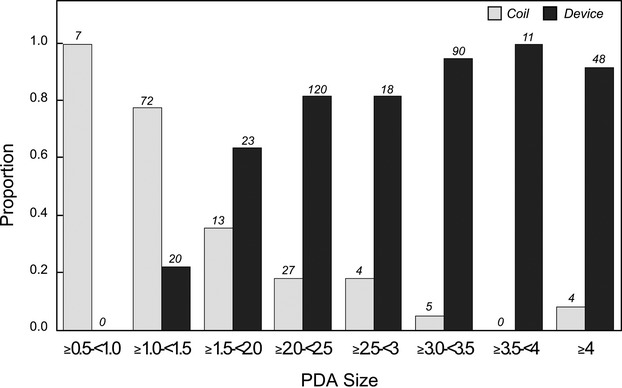
Proportion of 2 PDA closure methods (coils vs devices) based on PDA internal diameter. PDA indicates patent ductus arteriosus.
Technical Success
Successful closure was achieved with the first device chosen in 476 (96%) of 496 cases. In the remaining 20, 16 were closed with a different size coil or device using the same method. In 3 cases, attempted coil closure was abandoned for a device closure. One case was referred to surgery after device embolization. This occurred in a tubular PDA after an initial attempt at closure with a device, followed by a coil, which embolized to the lung and could not be retrieved. Separately, there were 16 cases where a device or coil was never delivered and the procedure was aborted. Among these procedures, 15 of 16 were not electively scheduled in advance and included mostly infants, with a median weight of 5.8 kg (range 2.2 to 13.2). PDA closure was aborted because of pulmonary hypertension (n=5), isthmus narrowing (n=4), unfavorable anatomy (n=2), surgery for another cause (n=2), spontaneous closure (n=2), and pulmonary artery obstruction (n=1). In 5 (31%) of these 16 cases, device closure was attempted and the device was removed while still attached to the cable.
Thus, a total of 512 cases were taken to the catheterization laboratory with an intention to treat. In 16 of 512 cases, the procedure was abandoned. As a device or coil placement was attempted even though not released in 5 of those 16 cases, those 5 patients were added to the total number of cases attempted, making it 501 when looking at technical success. One of the 496 patients in whom PDA closure was performed was sent to surgery, for a transcatheter success rate of 99% (495/501) in cases considered for closure in the catheterization labs at the participating institutions. One could argue that the remaining 11 of 16 patients who were taken to the catheterization lab and did not have a coil or device placed were considered a technical failure, bringing the success rate down to 97% (495/512).
Adverse Events
The incidence of any AEs among patients undergoing transcatheter PDA closure was 9.3% (95% CI 6.4 to 11.5), with a rate of high‐severity AEs of 2.2% (95% CI 1.0 to 3.7), and no difference in high‐severity event rates between treatment groups (Table 3). Among 46 events, 24 were related to device or coil placement and usually reported as embolization or malposition. Intervention‐related AEs were more common in the coil group (10%), with 8 embolization events and 9 recorded malpositions, compared with a rate of 2% in the device treatment group. Other events were related to access, including temporary loss of pulse requiring heparin infusion (n=6) and hematoma or bleeding (n=6), anesthesia or airway related (n=5), or general procedure–related events such as arrhythmias (n=3), allergic reaction (n=1), and medication error (n=1). Event rates were higher among patients weighing <6 kg, with a rate of any event of 27% versus 8% (P=0.002) and high‐severity events of 10% versus 2% (Table 4). In a univariate analysis, AEs were more likely to occur in young patients <6 months, in patients <6 kg, in coil procedures, and in patients with pulmonary hypertension. However, in an attempt to build a multivariable model, after accounting for age, other factors did not add additional independent explanatory information.
Table 3.
Adverse Events (AEs) in the Coil and Device Closure Groups
| AEs | Total (n=496) | Coil (n=158) | Device (n=338) | P Value |
|---|---|---|---|---|
| Any AE, n (%) | 46 (9) | 21 (13) | 25 (7) | 0.02 |
| Any high‐severity AE (levels 3, 4, 5), n (%) | 11 (2) | 2 (1) | 9 (3) | 1.0 |
| Any coil‐ or device‐related AE, n (%) | 24 (5) | 17 (10) | 7 (2) | <0.001 |
| Embolization* | 11 (2) | 8 (5) | 3 (<1) | 0.003 |
| Malposition* | 13 (3) | 9 (6) | 4 (1) | |
| Highest‐severity AE, n (%) | 0.04 | |||
| 0—no AE | 450 (91) | 137 (87) | 313 (93) | |
| 1—very minor | 5 (<1) | 1 (<1) | 4 (<1) | |
| 2—minor | 30 (6) | 18 (11) | 12 (4) | |
| 3—moderate | 9 (2) | 1 (<1) | 8 (2) | |
| 4—major | 2 (<1) | 1 (1) | 1 (<1) | |
Embolization and malposition are subcategories and included in the total of any coil‐ or device‐related AE.
Table 4.
Differences in Patients Weighing <6 kg
| Characteristics | <6 kg (n=37) | ≥6 kg (n=459) | P Value |
|---|---|---|---|
| Any coil intervention | 5 (14) | 153 (33) | 0.01 |
| Any device intervention | 32 (86) | 306 (67) | 0.02 |
| Diagnosis | |||
| Isolated PDA | 22 (59) | 423 (92) | <0.001 |
| Pulmonary hypertension | 4 (11) | 2 (<1) | |
| Intracardiac disease and PDA | 11 (30) | 34 (7) | |
| Genetic syndrome | 14 (38) | 51 (11) | <0.001 |
| Spontaneous respiration | 5 (14) | 166 (36) | 0.004 |
| Case duration, min | 84 [67, 105] (22, 298) | 63 [50, 81] (16, 295) | <0.001 |
| Contrast dose, mL/kg | 6.3 [4.6, 8.2] (0.0, 11.7) | 3.4 [2.3, 4.4] (0.0, 14.7) | <0.001 |
| Minimum lumen diameter of PDA (n=132, 330) | 2.8 [2.0, 3.5] (1.0, 5.0) | 2.0 [1.6, 3.0] (0.5, 12.0) | 0.004 |
| Any adverse event | 9 (27%) | 37 (8%) | 0.002 |
Number (%) or median [IQR] (range). PDA indicates patent ductus arteriosus.
High‐Severity AEs Breakdown
Among patients weighing <6 kg, there were 3 high‐severity AEs: device embolization, difficulty to arouse post anesthesia requiring reintubation, and hypotension requiring inotrope support. In the group weighing >6 kg, 8 high‐severity AEs occurred. Those included 2 device embolization, 1 device malposition requiring additional access and a bail‐out sheath, 1 coil malposition requiring surgery, 1 coil embolization that could not be retrieved and left in a distal segment of the lung, 1 pulse loss requiring heparin, 1 hematoma with retroperitoneal bleed, and 1 anesthesia‐related AE requiring reintubation.
AEs for the <6‐kg Group
AE rates were higher among patients weighing <6 kg, with a rate of any event occurring at 27% versus 8% (P=0.002), and high‐severity events of 10% versus 2% (Table 4). The AEs in the <6 kg group were 3 that were access related, 2 anesthesia related, 1 arrhythmia, 1 coil embolization, 1 coil malposition, 1 device embolization, and no device malposition. There was a tendency toward a higher incidence of device/coil‐related complications in this group, but it did not meet statistical significance. Of note is that in the <6‐kg group, patients had significant comorbid risk factors such as pulmonary hypertension (n=4), genetic syndromes (n=14), and complex congenital heart disease (n=11) (Table 4). This group also had larger PDAs compared with the rest of the cohort (median minimum lumen diameter 2.8 versus 2.0 mm; P=0.004), and a device was more likely to be used (86%) versus a coil (14%) (P=0.02). Case duration was significantly higher (P<0.001). Even though radiation exposure was not reported in the database, one can assume that it was higher. Also, more contrast was used in this group (P<0.001) (Table 4).
Device Embolization
Device embolization was rare, occurring in 3 of 338 procedures (Table 5). The PDAs were all tubular (type C) and large. In cases 1 and 3, an Amplatzer Duct Occluder (ADO) I device was initially attempted and the PDA was then closed with an Amplatzer Vascular Plug II (AVP II). Case 3 was a combined PDA and coarctation, in which the ADO embolized during partial stent inflation in the coarctation and was replaced with an AVP II before complete expansion of the stent. In case 2, 3 different devices (2 muscular VSD devices and 1 AVP II) were attempted before the PDA was finally closed with an ADO I device.
Table 5.
Cases of Device Embolization
| Case # | Age | PDA Type | PDA Size | Initial Device(s) | Fate | Final Device |
|---|---|---|---|---|---|---|
| 1 | 3 mo | C | 3.5 to 4.3 | ADO 6/4 | Emb to LPA/snared | AVP II 4 mm |
| 2 | 6 y | C | 8 | MVSD 10 then MVSD 12 then AVP II 12 | AVP II 12 Emb to RPA/snared | ADO 12/10 |
| 3* | 20 mo | C | 4 to 5 | ADO 8/6 | Emb to LPA/snared | AVP II 8 mm |
7‐LPA indicates left pulmonary artery; ADO, Amplatzer Ductal Occluder; AVP, Amplatzer Vascular Plug; Emb, embolization; MVSD, Amplatzer muscular VSD device; PDA, patent ductus arteriosus; RPA, right pulmonary artery; VSD, ventricular septal defect.
In a case of PDA and coarctation, after partial expansion of the stent, the ADO embolized into the LPA and was retrieved. The partially expanded stent was pulled down the aorta to the level of the diaphragm and an AVP II was placed in the PDA, the partially expanded stent was pushed back up to the level of the coarctation and fully expanded and then the PDA device was released from the cable.
Institutional Differences
Of a total of 12 143 cases reported by the 8 institutions, 496 (4%) were attempted PDA closures. The annual average PDA case volume per institution was a median of 5% with a range of 2.1% to 7.4% (Figure 2). There was institutional bias for use of device versus coil for PDA minimal diameter <2 mm. Three centers exclusively used a device for PDA diameter >1.5 mm (C, D, and F), while the other 4 centers were more likely to use coils for PDA diameter up to 2.5 mm (Figure 3). There was a significant difference in occurrence of any AE by institution (P<0.0001); however, there was no difference in high‐severity AEs (P=0.75) (Figure 4).
Figure 2.

Average annual PDA cases for each institution. Shaded bars represent the average annual PDA cases, and strippled bars represent the PDA percentage of total cases. PDA indicates patent ductus arteriosus.
Figure 3.

Differences in the preferred PDA closure method (coils vs devices) based on PDA internal diameter in 8 institutions. Figure shows median minimum diameter, IQR, and range by device or coil for each institution. IQR indicates interquartile range; PDA, patent ductus arteriosus.
Figure 4.

Adverse event (AE) rates by institution shown as any event and subdivided showing rates of higher severity level 3 or 4 events.
Discussion
In this multicenter study, we evaluated 496 patients who underwent attempted PDA closure during a period of 41 months and found that the high‐severity AE rate (levels 3, 4, and 5) was only 2% without any mortality.
The success rate of PDA closure was 97% to 99% in our experience, which is not surprising, since this technique has been an established procedure for the past 20 years. Historically, the success rate of PDA closure using only coils was somewhat lower, ranging from 90% to 96%.1–2,1–10 Recently published data from the Mid‐Atlantic Group of Interventional Cardiology (MAGIC) Database data registry comparing Gianturco coils and ADO I devices11 reported similar success rates as our experience. In another large retrospective study spanning several decades, the more recently used Amplatzer devices and Nit‐Occlud coils had a higher reported success rate compared with Gianturco and flipper coils.11 Even though our study might be similar to previously reported ones, it is the largest reported series to date that includes both coils and devices.
There was a difference in terms of device or coil selection depending on the patients' size; interestingly, smaller patients (<6 kg) had larger PDAs in our study, which explains the tendency of using devices rather than coils in these smaller patients. There was an institutional bias of using devices versus coils: some centers used coils only if the minimal lumen diameter of the PDA was <2 mm, and others used coils even in PDAs with a minimal lumen diameter that was >4 mm. The study was observational, and the participating interventional cardiologists had the liberty of using the device or coil of their choice. Despite an overall similar average annual case of PDA, the centers that used coils for larger PDAs had a higher tendency of high‐severity AEs.
It is very interesting that the number of AEs and embolization rate were much lower in our experience compared with a large registry collecting information more than a decade ago1,3,10 (Table 6). In the PDA Coil Registry, coil embolization was reported in 18.7% of 535 cases,10 while in our experience only 5% of coils and <1% of devices were embolized. This historic difference in AE rates may be explained by the use of PDA devices for larger PDAs, prompting less embolization of coils through a large ductus, improved coil and catheter technology available for coil implantation, including detachable coils, and the continuous efforts of investigators to improve the efficacy and safety of coil or device placement. The AE rate of our registry (C3PO) is similar to the complication rate reported in more recent studies4,11–13 (Table 6).
Table 6.
Comparison of Adverse Events in Previous Large Series of PDA Closure
| Report | Timeframe | Subjects | Weight | Device Type | Adverse Event Rate | Major Adverse Event Rate |
|---|---|---|---|---|---|---|
| Tometzki et al1 | 1994–1995 | 71 | 16.4 | Cook coil | 4 (5.6%) | 1 (1.4%) |
| PDA coil registry10 | 1993–1995 | 523 | Gianturco coil | 151 (28.9%) | 116 (22.2%) | |
| Faella and Hijazi2 | 1996–1999 | 316 | 10.7 | ADO | 15 (4.7%) | 1 (0.3%) |
| Pass et al3 | 1999–2002 | 439 | 11 | ADO | 41 (7.1%) | 10 (2.3%) |
| Jang et al4 | 1999–2005 | 117 | 30 | ADO, Nit‐Occlud, Cook | 6 (5.1%) | 4 (3.4%) |
| Dimas et al8 | 1995–2005 | 62 | 4.6 | Gianturco coil, Gianturca‐Grifka VOD, ADO | 8 (13%) | 4 (6.5%) |
| Wang et al13 | 2002–2008 | 46 | 6.3 | ADO | 13 (28.3%) | 3 (6.5%) |
| Ghasemi et al12 | 1994–2007 | 546 | 19.9 | Gianturco coil, Flipper coil, ADO, Nit‐Occlud | 47 (8.6%) | 21 (3.8%) |
| Brunetti et al11 | 2005–2008 | 359 | 18.7 | Gianturco coils, Flipper coils, ADO, other devices | 16 (4.5%) | 8 (2.2%) |
ADO indicates Amplatzer Ductal Occluder; PDA, patent ductus arteriosus; VOD, vascular occlusion device.
The majority of the AEs recorded in this study were related to the device or coil placement itself. Device or coil malposition and embolization are the most common problems encountered by the interventional cardiologists in PDA closure. The incidence of overall AEs was higher among the coil closure group (13%) compared with device closure group (7%); however, the high‐severity AE rates were 1% to 3% in both groups, with no significant difference. Similarly, the difference in embolization rate was higher in patients with coil closure (5%) compared with patients with device closure (<1%). This difference of AEs and embolization stems from the fact that PDA closure with a device on a cable is a more controlled procedure, while the technique of freehand PDA coil delivery is unpredictable in terms of final device positioning.
Overall rates of AEs, including high‐severity AEs (levels 3, 4, and 5), were higher in patients weighing <6 kg. In smaller patients, vascular access is more difficult and the rate of compromised limb circulation or hematoma is higher than in larger patients. Given the higher incidence of AEs when a coil was used in the group <6 kg, it would be preferable to use a device; this is made feasible by the fact that the PDAs tended to be larger in this group. One could argue that surgery might be preferred for this age group, but surgery is not without complications, including bleeding, pneumothorax, vocal cord paresis, diaphragm paralysis, bronchial compression, and ligation of left pulmonary artery and aorta.14–16 The best univariate predictor of AEs was young age (<6 months). Interestingly, there was a trend of increased AEs with low body weight (<6 kg), pulmonary hypertension, and a complex cardiac defect besides a PDA. When considered along with age <6 months in a multivariate model, other patient and procedural characteristics did not prove to be independent predictors of AEs.
Because transcatheter closure of PDA is the treatment of choice outside of the newborn period, a clear understanding of the AEs of this procedure has become even more important. The results of the current study improve our understanding of patient and procedure characteristics associated with the AEs in PDA closure. These data should assist in informing families and preparing physicians for safer procedures.
This report presents the largest cohort of patients with PDA closure recorded in a prospective multicenter study unbiased to patient or device selection. The rate of high‐severity complications is so low in the current era of interventional cardiology that only large cohorts can estimate accurately the nature and frequency of AEs. This study has the advantage to compare several centers and eliminate referral bias; however, the study is limited by a treatment bias posed by the practice preference of certain operators. We acknowledge that this report focuses primarily on AEs and not on efficacy and that the database did not capture whether there were small residual shunts. Our study was also limited by late event reporting. Another potential limitation might be that the sample size was inadequate to assess variables of rare frequency. Also, there may have been institutional bias on reporting some AEs.
In conclusion, PDA closure in our era is among the safest interventional procedures with low frequency of complications but with a higher rate among younger patients (<6 months). Technical intervention–related events were more common in coil procedures compared with device procedures. For PDAs ≤2.5 mm in diameter, institutional practice patterns vary for device versus coil closure.
Sources of Funding
Grant support was provided between 2006 and 2010 by the American Heart Association Pharmaceutical Round Table award (AHA‐PRA) and Children's Heart Foundation.
Disclosures
None.
Acknowledgments
We would like to acknowledge the Congenital Cardiac Catheterization Project on Outcomes (C3PO) participating institutions.
References
- 1.Tometzki AJ, Arnold R, Peart I, Sreeram N, Abdulhamed JM, Godman MJ, Patel RG, Kitchiner DJ, Bu'Lock FA, Walsh KP. Transcatheter occlusion of the patent ductus arteriosus with Cook detachable coils. Heart. 1996; 76:531-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faella HJ, Hijazi ZM. Closure of the patent ductus arteriosus with the Amplatzer PDA device: immediate results of the international clinical trial. Catheter Cardiovasc Interv. 2000; 51:50-54 [DOI] [PubMed] [Google Scholar]
- 3.Pass RH, Hijazi Z, Hsu DT, Lewis V, Hellenbrand WE. Multicenter USA Amplatzer patent ductus arteriosus occlusion device trail: initial and one‐year results. J Am Coll Cardiol. 2004; 44:513-519 [DOI] [PubMed] [Google Scholar]
- 4.Jang GY, Son CS, Lee JW, Lee JY, Kim SJ. Complications after transcatheter closure of patent ductus arteriosus. J Korean Med Sci. 2007; 12:484-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergersen L, Gauvreau K, Jenkins KJ, Lock JE. Adverse event rates in congenital cardiac catheterization: a new understanding of risks. Congenit Heart Dis. 2008; 3:90-105 [DOI] [PubMed] [Google Scholar]
- 6.Bergersen L, Gauvreau K, Foerster SR, Marshall AC, McElhinney DB, Beekman RH, Hirsch R, Kreutzer J, Balzer D, Vincent J, Hellenbrand WE, Holzer R, Cheatham JP, Moore JW, Burch G, Armsby L, Lock JE, Jenkins KJ. Catheterization for congenital heart disease adjustment for risk method (CHARM). JACC Cardiovasc Interv. 2011; 4:1037-1046 [DOI] [PubMed] [Google Scholar]
- 7.Bergersen L, Giroud JM, Jacobs JP, Franklin RC, Beland MJ, Krogmann ON, Aiello VD, Colan SD, Elliott MJ, Gaynor JW, Kurosawa H, Maruszewski B, Stellin G, Tchervenkov CI, Walters HL, Weinberg P, Everette AD. Report from The International Society for Nomenclature of Paediatric and Congenital Heart Disease: cardiovascular catheterization for congenital and pediatric cardiac disease (Part 2—Nomenclature of complications associated with interventional cardiology). Cardiol Young. 2011; 21:260-265 [DOI] [PubMed] [Google Scholar]
- 8.Dimas VV, Takao C, Ing FF, Mattamal R, Nugent AW, Grifka RG, Mullins CE, Justino H. Outcomes of transcatheter occlusion of patent ductus arteriosus in infants weighing <6 kg. JACC Cardiovasc Interv. 2010; 12:1295-1299 [DOI] [PubMed] [Google Scholar]
- 9.Hijazi ZM, Lloyd TR, Beekman RH, III, Geggel RL. Transcatheter closure with single or multiple Gianturco coils of patent ductus arteriosus in infants weighing < or = 8 kg: retrograde versus antegrade approach. Am Heart J. 1996; 132:827-835 [DOI] [PubMed] [Google Scholar]
- 10.Lloyd TR. The PDA Coil Registry Available at: http://www.med.umich.edu/pdc/pdacoil/pda_main.htm. Accessed November 6, 2013.
- 11.Brunetti M, Ringel R, Owada C, Coulson J, Jennings JM, Hoyer MH, Everett AD. Percutaneous closure of patent ductus arteriosus: a multi‐institutional registry comparing multiple devices. Catheter Cardiovasc Interv. 2010; 76:696-702 [DOI] [PubMed] [Google Scholar]
- 12.Ghasemi A, Pandya S, Reddy SV, Turner DR, Du W, Navabi MY, Mirzaaghayan MR, Kiani A, Sloan K, Forbes TJ. Trans‐catheter closure of patent ductus arteriosus‐What Is the Best Device? Catheter Cardiovasc Interv. 2010; 76:687-695 [DOI] [PubMed] [Google Scholar]
- 13.Wang JK, Wu MH, Lin MT, Chiu SN, Chen CA, Chiu HH. Transcatheter closure of moderate‐to‐large patent ductus arteriosus in infants using Amplatzer duct occluder. Circ J. 2010; 74:361-364 [DOI] [PubMed] [Google Scholar]
- 14.Mavroudis C, Backer CL, Gevitz M. Forty‐six years of patent ductus arteriosus division at Children's Memorial Hospital of Chicago: standards for comparison. Ann Surg. 1994; 220:402-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghani SA, Hashim R. Surgical management of patent ductus arteriosus: a review of 413 cases. J R Coll Surg Edinb. 1989; 34:33-36 [PubMed] [Google Scholar]
- 16.Galal O, Nehgme R, Al‐Fadley A, deMoor M, Abbag FI, Al‐Oufi SH, Williams E, Fawzy ME, al‐Halees Z. The role of surgical ligation of patent ductus arteriosus in the era of the Rashkind device. Ann Thorac Surg. 1997; 63:434-437 [DOI] [PubMed] [Google Scholar]


