Abstract
Background
Data on cardiovascular diseases (CVD) and cognitive decline are conflicting. Our objective was to investigate if CVD is associated with an increased risk for cognitive decline and to examine whether hypertension, diabetes, or adiposity modify the effect of CVD on cognitive functioning.
Methods and Results
Prospective follow‐up of 6455 cognitively intact, postmenopausal women aged 65 to 79 years old enrolled in the Women's Health Initiative Memory Study (WHIMS). CVD was determined by self‐report. For cognitive decline, we assessed the incidence of mild cognitive impairment (MCI) or probable dementia (PD) via modified mini‐mental state examination (3 MS) score, neurocognitive, and neuropsychiatric examinations. The median follow‐up was 8.4 years. Women with CVD tended to be at increased risk for cognitive decline compared with those free of CVD (hazard ratio [HR], 1.29; 95% CI: 1.00, 1.67). Women with myocardial infarction or other vascular disease were at highest risk (HR, 2.10; 95% CI: 1.40, 3.15 or HR, 1.97; 95% CI: 1.34, 2.87). Angina pectoris was moderately associated with cognitive decline (HR 1.45; 95% CI: 1.05, 2.01) whereas no significant relationships were found for atrial fibrillation or heart failure. Hypertension and diabetes increased the risk for cognitive decline in women without CVD. Diabetes tended to elevate the risk for MCI/PD in women with CVD. No significant trend was seen for adiposity.
Conclusions
CVD is associated with cognitive decline in elderly postmenopausal women. Hypertension and diabetes, but not adiposity, are associated with a higher risk for cognitive decline. More research is warranted on the potential of CVD prevention for preserving cognitive functioning.
Keywords: cardiovascular diseases, cognitive decline, postmenopausal women
Introduction
The number of individuals suffering from cardiovascular diseases has increased over the last few decades making cardiovascular‐related death the leading cause of death in developed countries. Simultaneously, the number of people affected by mild cognitive impairment or dementia has been steadily growing with cognitive decline posing a significant threat and health burden to aging individuals.1
Previous studies have examined the association between atherosclerotic disease and the risk for developing dementia or mild cognitive impairment.2–3 It is now widely accepted that atherosclerosis is an important cause of many vascular diseases that may also alter cognitive functioning by potentially chronically lowering cerebral perfusion and by affecting the neurovascular unit.4 Additionally, several population‐based studies have provided ample evidence that cardiovascular risk factors such as hypertension,5–6 diabetes,7–8 and adiposity9–12 increase the likelihood for dementia. Nevertheless, findings on the relationship between the various sites of atherosclerotic lesions and the risk for developing mild cognitive impairment or dementia among older populations have been controversial.2–3,2–18 Besides, other cardiac conditions such as atrial fibrillation or heart failure are often the consequence of underlying cardiovascular disease. Both result in reduced cardiac output, increase the risk for thromboembolism, and have been associated by some but not all previous studies with cognitive decline.17,19–23
The objective of this study was to assess the association of women with a history of myocardial infarction, angina pectoris, atrial fibrillation, heart failure, other invasive vascular procedures or diseases including coronary bypass surgery, angioplasty, carotid endarterectomy, peripheral vascular disease or with a composite of any cardiovascular disease (CVD) with the incidence of mild cognitive impairment (MCI) or probable dementia (PD) later in life. Second, we examined the effect of hypertension, diabetes, and adiposity on this relationship.
Our study differs from previous reports as CVD was defined broadly to investigate if a more general definition adds more information on MCI/PD risk prediction. We also apply extensive neurocognitive and neuropsychiatric examinations for outcome ascertainment and provide additional data on the effect of hypertension, diabetes, and adiposity on cognitive decline.
Methods
Study Population
The study population consisted of 7479 postmenopausal women enrolled in the Women's Health Initiative Memory Study (WHIMS), an ancillary study of the Women's Health Initiative Hormone Trials (WHI HTs).28–29 The WHIMS was conducted to assess the relative effect of estrogen alone or in combination with progestin on the incidence of probable dementia and mild cognitive impairment.24–26 Recruitment was initiated between May 1996 and December 1999 at 39 US clinical centers from women included in the WHI HTs who were aged ≥65 and were free of dementia at enrollment.24–26 Details of the study population and of the initial screening process have been reported previously.24–29 Participating women were scheduled for in‐clinic visits annually and were sent semiannual questionnaires to ensure the timely update of selected exposures and ascertain medical outcomes.30 Follow‐up of this secondary analysis study was conducted through February 2008 and the median follow‐up was 8.6 years. All protocols were approved by institutional review boards at participating institutions and all participants provided written informed consent.
Exposure Assessment
Exposure status was derived from self‐report questionnaires at baseline.30 The composite variable of any CVD was coded as positive if the participant reported a history of myocardial infarction, angina pectoris, atrial fibrillation, heart failure, peripheral vascular disease, coronary bypass surgery, angioplasty, or carotid endarterectomy at enrollment. Myocardial infarction was defined as women with a reported history of clinical myocardial infarction or evolving Q‐wave myocardial infarction.30 We excluded women with a history of stroke at baseline from our analyses, as stroke is a known independent cause and contributor of cognitive dysfunction.4 Moreover, women with MCI at baseline or missing data were not included in our analysis.
Outcome Assessment
As primary outcome measures for cognitive decline, we assessed the incidence of MCI or PD (MCI/PD). Incident MCI and PD cases were ascertained and centrally adjudicated in all women enrolled in WHIMS.24–26 Specifically, the WHIMS study was designed to detect probable dementia and mild cognitive impairment in 4 phases.
First, the Modified Mini‐Mental State Examination (3MSE) was administered to all study participants as a cognitive screening assessment at baseline and annually thereafter. The 3MSE consisted of 15 items that were summed from 0 to 100, with higher scores reflecting better cognitive functioning.31 Women who scored below an education‐adjusted cut point on the 3MSE were further asked to continue with phase 2 and 3. In phase 2, certified technicians administered a modified Consortium to Establish a Registry for Alzheimer's Disease (CERAD) battery of neuropsychological tests and standardized interviews to assess acquired cognitive and behavioral impairments. Additionally, a designated informant (friend or family member) was interviewed separately regarding acquired cognitive and behavioral impairments in the participant. In phase 3, women were seen by a local physician with expertise in the field of dementia (ie, geriatrician, neurologist, or geriatric psychiatrist). Using a standardized protocol provided by the WHIMS Clinical Coordinating Center (CCC), local physicians reviewed all available data (phases 1 and 2), completed a structured medical history and performed a clinical neuropsychiatric evaluation. The physician then classified the WHIMS participant as having no dementia, MCI, or probable dementia, based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) criteria. MCI was operationally defined as being present if the participant showed poor performance (10th or lower percentile) in at least one area of cognitive function based on the norms established by the Consortium to Establish a Registry for Alzheimer's Disease (CERAD)32 or if a reliable informant reported some functional impairment excluding a basic activity of daily living, and no evidence of medical or psychiatric disorders that could explain the decline in cognitive function was ascertained and the absence of adjudicated dementia was given.24,26 Women suspected of having probable dementia underwent phase 4, including a noncontrast computed tomography brain scan and laboratory blood tests to rule out possible reversible causes of cognitive decline. Finally, a physician was required to provide the most probable etiology of dementia based on the DSM‐IV criteria.
The WHIMS Clinical coordinating center adjudication committee (2 neurologists and 1 geriatric psychiatrist) independently reviewed all probable dementia cases identified by the local clinician, a random sample of 50% of MCI cases, and a random sample of 10% of cases without dementia. Details of this review have been published previously.24,27 Agreement between local clinicians and adjudicators ranged between 75% (κ=0.60; 95% CI, 0.52 to 0.68) in the estrogen‐alone trial and 77% in the estrogen plus progestin trial (κ=0.63; 95% CI, 0.58 to 0.69). Results were not affected by treatment assignment (P=0.49).27,33 In cases of disagreement, the diagnosis was most frequently changed to a less serious classification (ie, from probable dementia to MCI or from MCI to no dementia) by the central adjudicators (64% to 74% of cases).27,33
Covariates
Information on all covariates was assessed via self‐report or by physical measure at baseline.30 Blood pressure was measured by certified staff in the WHI clinic using standardized procedures.30 Hypertension was defined as a self‐report of current drug therapy for hypertension or clinic measurement of SBP ≥140 mm Hg or DBP ≥90 mm Hg at enrollment. Women were classified as having diabetes on the basis of self‐report of diabetes or self‐report of diabetes treatment. Body mass index (BMI) was calculated as weight in kg/height in m2. Adiposity was defined as BMI ≥30. Physical activity was assessed with metabolic equivalent tasks (in hours per week) using a 10‐item medical outcome scale.34 Waist circumference at the natural waist or narrowest torso part and maximal hip circumference were measured to the nearest 0.1 cm and used to calculate the waist:hip ratio.
Statistical Analysis
The characteristics of women were compared according to the presence or absence of any CVD at baseline. Differences between women with and without CVD were compared using χ2 statistics for categorized covariates (Table 1). Incidence rates (IR) of PD, MCI, or a composite per 1000 person‐years were calculated as the number of diagnosed cases of MCI or PD (MCI/PD) occurring during the entire follow‐up period of WHIMS divided by person‐years of follow‐up (Tables 2 and 3). For participants who did not develop MCI/PD, the follow‐up time was calculated from the date of baseline interview (ie, study entry) to the date of the last follow‐up cognition test. For participants who developed MCI/PD, the follow‐up time was estimated as the entire time during which the subjects were free of PD plus half of the follow‐up time during which PD developed.17 Hazard ratios (HRs) for the risk of MCI/PD associated with the composite variable of CVD and its subgroups myocardial infarction, angina, atrial fibrillation, heart failure, coronary bypass surgery, angioplasty or carotid endarterectomy, and peripheral vascular disease at baseline were calculated using adjusted Cox proportional hazards analyses (Tables 2 and 3). Two models were formed for each outcome to examine the effect of potential confounding: Model 1 adjusted for WHI Hormone Trial Randomization assignment (HTR arm), age at screening, race, education level, and baseline 3MSE (minimally adjusted model). Model 2 additionally adjusted for smoking status, alcohol intake, physical activity, sleep hours, diabetes status, hypercholesterolemia, hypertension status, BMI, waist–hip ratio, depression, and aspirin use (fully adjusted model). The proportional hazards assumption was tested for the composite variable of CVD and its subgroups for each outcome (PD, MCI, MCI/PD) (Table 4). Thereafter, we conducted additional sensitivity analyses for the effect of CVD and its subgroups on cognitive decline by excluding incident cases of stroke and TIA after baseline from our analysis (Table 5). We also investigated the effect of myocardial infarction on cognitive decline by excluding incident cases of myocardial infarction after baseline from our analysis (data not shown). Moreover, we conducted sensitivity analyses treating death as a competing event. We defined death as a competing event only if death occurred for a PD/MCI‐free woman within a year of the last cognitive examination, because the examination was performed annually. Finally, Cox proportional hazards regression modeling with corresponding forest plots (Figure 2A and 2B) was used to examine the interaction of selected risk factors (hypertension, diabetes, adiposity) to the incidence of MCI/PD. These analyses were based on the fully adjusted models. All analyses were conducted using SAS statistical software (version 9.3; SAS Institute Inc).
Table 1.
Descriptive Characteristics of Postmenopausal Women Enrolled in the Women's Health Initiative Memory Study by CVD at Baseline
| Characteristic | CVD Absent, n (%) | CVD Present, n (%) | P Value |
|---|---|---|---|
| Total | 5560 (100.0) | 895 (100.0) | |
| Age at screening, y | <0.0001 | ||
| 60 to 64 | 131 (2.4) | 12 (1.3) | |
| 65 to 69 | 2739 (49.3) | 348 (38.9) | |
| 70 to 74 | 1903 (34.2) | 338 (37.8) | |
| 75 to 84 | 787 (14.2) | 197 (22.0) | |
| Ethnicity | 0.0021 | ||
| White (not of Hispanic origin) | 4919 (88.5) | 772 (86.3) | |
| Black or African American | 335 (6.0) | 81 (9.1) | |
| Other | 306 (5.5) | 42 (4.7) | |
| Education | <0.0001 | ||
| Less than high school | 355 (6.4) | 92 (10.3) | |
| High school/Vocational or training school | 1934 (34.8) | 315 (35.2) | |
| Some college or associate degree | 1538 (27.7) | 260 (29.1) | |
| College and above | 1733 (31.2) | 228 (25.5) | |
| Smoking | 0.0455 | ||
| Never smoked | 3020 (54.3) | 453 (50.6) | |
| Past smoker | 2174 (39.1) | 389 (43.5) | |
| Current smoker | 366 (6.6) | 53 (5.9) | |
| Servings of alcohol per day | <0.0001 | ||
| None | 2465 (44.3) | 479 (53.5) | |
| <1/day | 2375 (42.7) | 349 (39.0) | |
| 1 to 2/day | 630 (11.3) | 55 (6.2) | |
| ≥3/day | 90 (1.6) | 12 (1.3) | |
| Physical activity (METs), h/week | 0.0002 | ||
| 0 to 1.5 | 1334 (24.0) | 243 (27.2) | |
| >1.5 to 8 | 1579 (28.4) | 296 (33.1) | |
| >8 to 19 | 1516 (27.3) | 211 (23.6) | |
| >19 | 1131 (20.3) | 145 (16.2) | |
| Hours of sleep | 0.0052 | ||
| <6 | 2034 (36.6) | 378 (42.2) | |
| 7 | 2053 (36.9) | 316 (35.3) | |
| 8 | 1261 (22.7) | 167 (18.7) | |
| 9 and above | 212 (3.8) | 34 (3.8) | |
| Depression | <0.0001 | ||
| No | 5171 (93.0) | 785 (87.7) | |
| Yes | 389 (7.0) | 110 (12.3) | |
| BMI, kg/m2 | <0.0001 | ||
| <25 | 1665 (30.0) | 225 (25.1) | |
| 25 to 29 | 2032 (36.6) | 291 (32.5) | |
| >30 | 1863 (33.5) | 379 (42.4) | |
| Hypertension | <0.0001 | ||
| No | 2954 (53.1) | 319 (35.6) | |
| Yes | 2606 (46.9) | 576 (64.4) | |
| Use of aspirin | <0.0001 | ||
| No | 4193 (75.4) | 472 (52.7) | |
| Yes | 1367 (24.6) | 423 (47.3) | |
| Treatment for diabetes | <0.0001 | ||
| No | 5282 (95.0) | 779 (87.0) | |
| Yes | 278 (5.0) | 116 (13.0) | |
| Treatment for hypercholesterolemia | <0.0001 | ||
| No | 4723 (85.0) | 591 (66.0) | |
| Yes | 837 (15.0) | 304 (34.0) | |
| Waist–Hip ratio | <0.0001 | ||
| ≤0.85 | 3615 (65.0) | 515 (57.5) | |
| >0.85 | 1945 (35.0) | 380 (42.5) | |
| WHI Clinical Trial Randomization Assignment | 0.5479 | ||
| Control arm | 2806 (50.5) | 442 (49.4) | |
| Hormone trial arm | 2754 (49.5) | 453 (50.6) | |
| Systolic BP, mm Hg | <0.0001 | ||
| <140 | 3986 (71.7) | 580 (64.8) | |
| ≥140 | 1574 (28.3) | 315 (35.2) | |
| Diastolic BP, mm Hg | 0.0303 | ||
| <70 | 1845 (33.2) | 330 (36.9) | |
| ≥70 | 3715 (66.8) | 565 (63.1) | |
| Diastolic BP, mm Hg | 0.2456 | ||
| <90 | 5314 (95.6) | 863 (96.4) | |
| ≥90 | 246 (4.4) | 32 (3.6) | |
| BP control | <0.0001 | ||
| No treatment and BP <140/90 | 2954 (53.1) | 319 (35.6) | |
| No treatment and BP ≥140/90 | 1064 (19.1) | 121 (13.5) | |
| Treatment and BP <140/90 | 812 (14.6) | 229 (25.6) | |
| Treatment and BP ≥140/90 | 730 (13.1) | 226 (25.3) |
BMI indicates body mass index; BP, blood pressure; CVD, cardiovascular index; MET, metabolic equivalent task; WHI, Women's Health Initiative.
Table 2.
IR (Per 1000 Person Years) and HR of PD or MCI Related to Cardiovascular Disease at Baseline
| Number of Subjects | PD | MCI | PD or MCI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number Events | Follow‐up Years, Mean (SD) | IR | Min. Adjusted*, HR (95% CI) | Fully Adjusted*, HR (95% CI) | Number of Events | Follow‐up Years, Mean (SD) | IR | Min. Adjusted*, HR (95% CI) | Fully Adjusted*, HR (95% CI) | Number of Events | Follow‐up Years, Mean (SD) | IR | Min. Adjusted*, HR (95% CI) | Fully Adjusted*, HR (95% CI) | ||
| CVD* | ||||||||||||||||
| Absent | 5560 | 153 | 7.8 (2.5) | 3.6 | 1 | 1 | 225 | 7.7 (2.5) | 5.3 | 1 | 1 | 332 | 7.6 (2.6) | 7.8 | 1 | 1 |
| Present | 895 | 35 | 7.2 (2.6) | 5.5 | 1.34 (0.93, 1.95) | 1.25 (0.85, 1.83) | 69 | 7.0 (2.8) | 11.1 | 1.88 (1.43, 2.46) | 1.74 (1.31, 2.32) | 79 | 7.0 (2.8) | 12.7 | 1.43 (1.11, 1.83) | 1.29 (1.00, 1.67) |
| Myocardial infarction | ||||||||||||||||
| Absent | 6246 | 176 | 7.7 (2.5) | 3.7 | 1 | 1 | 271 | 7.6 (2.6) | 5.7 | 1 | 1 | 384 | 7.6 (2.6) | 8.1 | 1 | 1 |
| Present | 209 | 12 | 6.7 (2.8) | 8.6 | 2.19 (1.22, 3.95) | 2.18 (1.19, 3.99) | 23 | 6.5 (2.9) | 17.0 | 2.70 (1.76, 4.14) | 2.56 (1.64, 4.01) | 27 | 6.5 (2.9) | 20.0 | 2.23 (1.51, 3.30) | 2.10 (1.40, 3.15) |
| Angina pectoris | ||||||||||||||||
| Absent | 5978 | 166 | 7.7 (2.5) | 3.6 | 1 | 1 | 248 | 7.6 (2.6) | 5.4 | 1 | 1 | 359 | 7.6 (2.6) | 7.9 | 1 | 1 |
| Present | 465 | 19 | 7.1 (2.7) | 5.7 | 1.39 (0.86, 2.24) | 1.27 (0.77, 2.09) | 43 | 6.9 (2.8) | 13.3 | 2.24 (1.62, 3.10) | 2.03 (1.43, 2.87) | 47 | 6.9 (2.8) | 14.6 | 1.66 (1.22, 2.25) | 1.45 (1.05, 2.01) |
| Atrial fibrillation | ||||||||||||||||
| Absent | 6178 | 176 | 7.7 (2.5) | 3.7 | 1 | 1 | 273 | 7.6 (2.6) | 5.8 | 1 | 1 | 386 | 7.6 (2.6) | 8.2 | 1 | 1 |
| Present | 255 | 10 | 7.1 (2.6) | 5.5 | 1.23 (0.65, 2.33) | 1.12 (0.59, 2.14) | 18 | 6.9 (2.8) | 10.2 | 1.57 (0.97, 2.53) | 1.46 (0.90, 2.37) | 21 | 6.9 (2.8) | 11.9 | 1.29 (0.83, 2.00) | 1.19 (0.76, 1.86) |
| Congestive heart failure | ||||||||||||||||
| Absent | 6384 | 185 | 7.7 (2.5) | 3.8 | 1 | 1 | 287 | 7.6 (2.6) | 5.9 | 1 | 1 | 403 | 7.6 (2.6) | 8.3 | 1 | 1 |
| Present | 71 | 3 | 6.4 (2.8) | 6.6 | 1.45 (0.46, 4.60) | 1.34 (0.42, 4.27) | 7 | 6.1 (2.9) | 16.2 | 2.20 (1.03, 4.72) | 1.89 (0.87, 4.08) | 8 | 6.0 (2.9) | 18.7 | 1.75 (0.86, 3.55) | 1.49 (0.73, 3.04) |
| Other* | ||||||||||||||||
| Absent | 6148 | 173 | 7.7 (2.5) | 3.7 | 1 | 1 | 264 | 7.6 (2.6) | 5.6 | 1 | 1 | 374 | 7.6 (2.6) | 8.0 | 1 | 1 |
| Present | 282 | 13 | 7.1 (2.7) | 6.5 | 1.62 (0.92, 2.85) | 1.60 (0.88, 2.90) | 27 | 6.8 (2.8) | 14.1 | 2.41 (1.62, 3.60) | 2.35 (1.53, 3.61) | 33 | 6.8 (2.8) | 17.3 | 2.02 (1.41, 2.89) | 1.97 (1.34, 2.87) |
BMI indicates body mass index; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; HTR, Hormone Trial Randomization; IR, incidence rate; MCI, mild cognitive impairment; 3MSE, Modified Mini‐Mental State Examination; PD, probable dementia; SD, standard deviation.
Adjusted for age, education, race, HTR arm, and baseline 3MSE.
Adjusted for age, education, race, HTR arm, baseline 3MSE, alcohol intake, smoking status, physical activity, diabetes status, sleep hours, hypertension status, BMI, depression, waist‐hip ratio, hypercholesterolemia, and aspirin use.
Composite of myocardial infarction, angina pectoris, atrial fibrillation, congestive heart failure, coronary bypass surgery, angioplasty, carotid endarterectomy, and peripheral vascular disease.
“Other” includes coronary bypass surgery, angioplasty, carotid endarterectomy, and peripheral vascular disease.
Table 3.
IR (Per 1000 Person Years) and HR of PD or MCI Related to Other Invasive Vascular Procedures or Diseases at Baseline
| Number subjects | PD | MCI | PD or MCI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number Events | Follow‐up Years, Mean (SD) | IR | Min. Adjusted*, HR (95% CI) | Fully Adjusted*, HR (95% CI) | Number of Events | Follow‐up Years, Mean (SD) | IR | Min. Adjusted*, HR (95% CI) | Fully Adjusted*, HR (95% CI) | Number of Events | Follow‐up Years, Mean (SD) | IR | Min. Adjusted*, HR (95% CI) | Fully Adjusted*, HR (95% CI) | ||
| Other* | ||||||||||||||||
| Absent | 6148 | 173 | 7.7 (2.5) | 3.7 | 1 | 1 | 264 | 7.6 (2.6) | 5.6 | 1 | 1 | 374 | 7.6 (2.6) | 8.0 | 1 | 1 |
| Present | 282 | 13 | 7.1 (2.7) | 6.5 | 1.62 (0.92, 2.85) | 1.60 (0.88, 2.90) | 27 | 6.8 (2.8) | 14.1 | 2.41 (1.62, 3.60) | 2.35 (1.53, 3.61) | 33 | 6.8 (2.8) | 17.3 | 2.02 (1.41, 2.89) | 1.97 (1.34, 2.87) |
| Coronary bypass surgery | ||||||||||||||||
| Absent | 6356 | 182 | 7.7 (2.5) | 3.7 | 1 | 1 | 280 | 7.6 (2.6) | 5.8 | 1 | 1 | 394 | 7.6 (2.6) | 8.2 | 1 | 1 |
| Present | 77 | 4 | 6.8 (2.9) | 7.7 | 1.81 (0.67, 4.90) | 1.49 (0.53, 4.19) | 11 | 6.4 (3.0) | 22.3 | 3.81 (2.08, 6.99) | 3.20 (1.66, 6.15) | 13 | 6.3 (3.0) | 26.6 | 3.22 (1.85, 5.61) | 2.63 (1.46, 4.75) |
| Angioplasty | ||||||||||||||||
| Absent | 6330 | 181 | 7.7 (2.5) | 3.7 | 1 | 1 | 283 | 7.6 (2.6) | 5.9 | 1 | 1 | 396 | 7.6 (2.6) | 8.3 | 1 | 1 |
| Present | 103 | 5 | 7.2 (2.5) | 6.7 | 1.90 (0.78, 4.64) | 1.76 (0.70, 4.42) | 8 | 7.1 (2.6) | 10.9 | 1.97 (0.97, 3.98) | 1.73 (0.83, 3.62) | 11 | 7.1 (2.6) | 15.1 | 1.89 (1.04, 3.45) | 1.67 (0.89, 3.13) |
| Carotid endarteretomy | ||||||||||||||||
| Absent | 6409 | 183 | 7.7 (2.5) | 3.7 | 1 | 1. | 287 | 7.6 (2.6) | 5.9 | 1 | 1 | 402 | 7.6 (2.6) | 8.3 | 1 | 1 |
| Present | 24 | 3 | 6.9 (2.7) | 18.2 | 3.92 (1.24, 12.4) | 4.28 (1.32, 13.9) | 4 | 6.3 (3.0) | 26.6 | 3.30 (1.22, 8.93) | 3.17 (1.15, 8.74) | 5 | 6.2 (3.0) | 33.4 | 3.11 (1.28, 7.58) | 3.03 (1.23, 7.47) |
| Peripheral vascular disease | ||||||||||||||||
| Absent | 6317 | 182 | 7.7 (2.5) | 3.7 | 1 | 1 | 278 | 7.6 (2.6) | 5.8 | 1 | 1 | 393 | 7.6 (2.6) | 8.2 | 1 | 1 |
| Present | 130 | 5 | 7.0 (2.7) | 5.5 | 1.20 (0.49, 2.92) | 1.22 (0.50, 3.02) | 15 | 6.7 (2.8) | 17.2 | 2.59 (1.54, 4. 37) | 2.44 (1.43, 4. 16) | 16 | 6.7 (2.8) | 18.3 | 1.86 (1.12, 3. 07) | 1.82 (1.09, 3.02) |
CI indicates confidence interval; HR, hazard ratio; HTR, Hormone Trial Randomization; IR, incidence rate; MCI, mild cognitive impairment; 3MSE, Modified Mini‐Mental State Examination; PD, probable dementia; SD, standard deviation.
Adjusted for age, education, race, HTR arm, and baseline 3MSE.
Adjusted for age, education, race, HTR arm, baseline 3MSE, alcohol intake, smoking status, physical activity, diabetes status, sleep hours, hypertension status, BMI, depression, waist‐hip ratio, hypercholesterolemia, and aspirin use.
“Other” includes coronary bypass surgery, angioplasty, carotid endarterectomy, and peripheral vascular disease.
Table 4.
Test Results for Proportionality Hazards Assumption in Cox Models (P Values Reported)
| PD | MCI | PD or MCI | |
|---|---|---|---|
| CVD* | 0.0481 | 0.2218 | 0.4221 |
| Myocardial infarction | 0.3134 | 0.3249 | 0.2493 |
| Angina pectoris | 0.0835 | 0.4631 | 0.5119 |
| Atrial fibrillation | 0.4849 | 0.1149 | 0.2356 |
| Congestive heart failure | 0.6719 | 0.9808 | 0.8037 |
| Other* | 0.1522 | 0.3106 | 0.8043 |
| Coronary bypass surgery | 0.0878 | 0.9750 | 0.7234 |
| Angioplasty | 0.1989 | 0.6293 | 0.2034 |
| Peripheral vascular disease | 0.7606 | 0.4381 | 0.4586 |
| Carotid endarterectomy | 0.5550 | 0.0749 | 0.1146 |
CVD indicates cardiovascular disease; MCI, mild cognitive impairment; PD, probable dementia.
Composite of myocardial infarction, angina pectoris, atrial fibrillation, congestive heart failure, coronary bypass surgery, angioplasty, carotid endarterectomy, and peripheral vascular disease.
“Other” includes coronary bypass surgery, angioplasty, carotid endarterectomy, and peripheral vascular disease.
Table 5.
Sensitivity Analysis: IR (Per 1000 Person Years) and HR of PD or MCI Related to Cardiovascular Disease Excluding Women With Incident Stroke or TIA Events
| Number subjects | PD | MCI | PD or MCI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number Events | Follow‐up Years, Mean (SD) | IR | Min. Adjusted*, HR (95% CI) | Fully Adjusted*, HR (95% CI) | Number of Events | Follow‐up Years, Mean (SD) | IR | Min. Adjusted*, HR (95% CI) | Fully Adjusted*, HR (95% CI) | Number of Events | Follow‐up Years, Mean (SD) | IR | Min. Adjusted*, HR (95% CI) | Fully Adjusted*, HR (95% CI) | ||
| CVD* | ||||||||||||||||
| Absent | 5320 | 142 | 7.8 (2.5) | 3.4 | 1 | 1 | 217 | 7.7 (2.5) | 5.3 | 1 | 1 | 314 | 7.6 (2.6) | 7.7 | 1 | 1 |
| Present | 804 | 29 | 7.1 (2.7) | 5.1 | 1.27 (0.85, 1.91) | 1.14 (0.75, 1.74) | 61 | 6.9 (2.8) | 10.9 | 1.83 (1.37, 2.43) | 1.67 (1.24, 2.26) | 69 | 6.9 (2.8) | 12.4 | 1.41 (1.08, 1.83) | 1.26 (0.96, 1.66) |
| Myocardial infarction | ||||||||||||||||
| Absent | 5933 | 161 | 7.7 (2.5) | 3.5 | 1 | 1 | 258 | 7.6 (2.6) | 5.7 | 1 | 1 | 359 | 7.6 (2.6) | 8.0 | 1 | 1 |
| Present | 191 | 10 | 6.7 (2.9) | 7.9 | 2.00 (1.05, 3.81) | 1.96 (1.01, 3.80) | 20 | 6.5 (2.9) | 16.2 | 2.44 (1.54, 3.86) | 2.30 (1.43, 3.71) | 24 | 6.4 (2.9) | 19.5 | 2.10 (1.38, 3.18) | 1.98 (1.29, 3.04) |
| Angina pectoris | ||||||||||||||||
| Absent | 5699 | 152 | 7.7 (2.5) | 3.5 | 1 | 1 | 236 | 7.6 (2.6) | 5.4 | 1 | 1 | 336 | 7.6 (2.6) | 7.8 | 1 | 1 |
| Present | 413 | 16 | 7.1 (2.7) | 5.4 | 1.33 (0.79, 2.22) | 1.18 (0.69, 2.03) | 39 | 6.9 (2.9) | 13.7 | 2.30 (1.64, 3.23) | 1.50 (1.07, 2.10) | 42 | 6.9 (2.9) | 14.7 | 1.71 (1.24, 2.35) | 1.50 (1.07, 2.10) |
| Atrial fibrillation | ||||||||||||||||
| Absent | 5873 | 161 | 7.7 (2.5) | 3.6 | 1 | 1 | 259 | 7.6 (2.6) | 5.8 | 1 | 1 | 360 | 7.6 (2.6) | 8.1 | 1 | 1 |
| Present | 230 | 8 | 7.0 (2.7) | 4.9 | 1.14 (0.56, 2.33) | 1.01 (0.49, 2.09) | 16 | 6.9 (2.8) | 10.1 | 1.61 (0.97, 2.68) | 1.48 (0.89, 2.48) | 19 | 6.9 (2.8) | 12.0 | 1.37 (0.86, 2.18) | 1.25 (0.78, 2.00) |
| Congestive heart failure | ||||||||||||||||
| Absent | 6057 | 168 | 7.7 (2.5) | 3.6 | 1 | 1 | 271 | 7.6 (2.6) | 5.9 | 1 | 1 | 375 | 7.6 (2.6) | 8.2 | 1 | 1 |
| Present | 67 | 3 | 6.4 (2.9) | 7.0 | 1.56 (0.49, 4.97) | 1.42 (0.44, 4.53) | 7 | 6.1 (3.0) | 17.1 | 2.22 (1.03, 4.77) | 1.94 (0.90, 4.21) | 8 | 6.1 (3.0) | 19.7 | 1.80 (0.88, 3.67) | 1.54 (0.75, 3.16) |
| Other* | ||||||||||||||||
| Absent | 5850 | 158 | 7.7 (2.5) | 3.5 | 1 | 1 | 252 | 7.6 (2.6) | 5.7 | 1 | 1 | 351 | 7.6 (2.6) | 7.9 | 1 | 1 |
| Present | 251 | 11 | 7.0 (2.7) | 6.2 | 1.63 (0.88, 3.01) | 1.51 (0.79, 2.88) | 23 | 6.8 (2.9) | 13.5 | 2.33 (1.52, 3.59) | 2.20 (1.39, 3.49) | 28 | 6.8 2.9) | 16.5 | 1.98 (1.34, 2.91) | 1.88 (1.25, 2.83) |
BMI indicates body mass index; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; HTR, Hormone Trial Randomization; IR, incidence rate; MCI, mild cognitive impairment; 3MSE, Modified Mini‐Mental State Examination; PD, probable dementia; SD, standard deviation; TIA, Transient ischemic attack.
Adjusted for age, education, race, HTR arm, and baseline 3MSE.
Adjusted for age, education, race, HTR arm, baseline 3MSE, alcohol intake, smoking status, physical activity, diabetes status, sleep hours, hypertension status, BMI, depression, waist–hip ratio, hypercholesterolemia, and aspirin use.
Composite of myocardial infarction, angina pectoris, atrial fibrillation, congestive heart failure, coronary bypass surgery, angioplasty, carotid endarterectomy, and peripheral vascular disease.
“Other” includes coronary bypass surgery, angioplasty, carotid endarterectomy, and peripheral vascular disease.
Figure 2.
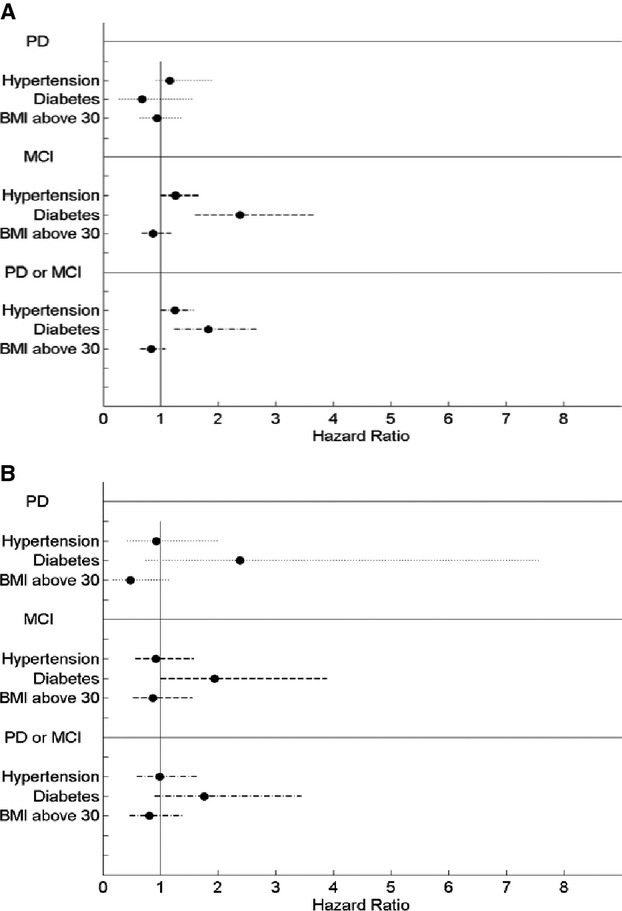
Hazard ratio (95%) of cognition event when CVD (A) absent and (B) present. BMI indicates body mass index; CVD, cardiovascular disease; MCI, mild cognitive impairment; PD, probable dementia.
Results
Among the 7479 women in the WHIMS trial, 6455 with no history of stroke, MCI and complete follow‐up were included in this study (Figure 1). The characteristics of women in our sample at baseline examination are shown in Table 1. Among the women included, 895 reported any CVD at baseline. Those with CVD were older, more likely to be African‐American, had a higher BMI, were more likely to be a current or past smoker and suffer from depression, whereas they were less likely to have a higher education or be physically active. Women affected by CVD also tended to be hypertensive, under treatment for hypercholesterolemia or diabetes, and more likely to use aspirin. Subjects who screened positive for MCI or PD at follow‐up were significantly older at baseline in the CVD group compared with the non‐CVD group (73.1 versus 71.8 years, P=0.0127) whereas the mean time until diagnosis of MCI or PD was longer in women without CVD (7.64 versus 6.95 years, P<0.0001).
Figure 1.
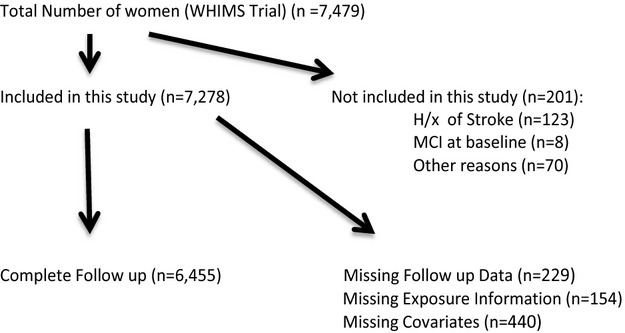
Study inclusion. H/x of stroke indicates history of stroke; MCI, mild cognitive impairment; WHIMS, Women's Health Initiative Memory Study.
The multivariate‐adjusted HRs for incident MCI/PD related to the composite variable of CVD and its various subgroups (MI, angina pectoris, atrial fibrillation, congestive heart failure, and other vascular diseases or procedures) are presented in Tables 2 and 3. Corresponding test results for the proportional hazards assumption for CVD and all subgroups can be found in Table 4. IRs for MCI/PD were significantly higher among women with any CVD (12.7/1000 person years) compared with those without CVD (7.8/1000 person years). After full adjustment the HRs for women with any CVD compared with those free of CVD were 1.29 (95% CI: 1.00, 1.67) for MCI/PD. Among women with myocardial infarction IRs for MCI/PD were higher (20.0/1000 person years) compared with those without myocardial infarction (8.1/1000 person years). This rate corresponds to minimally adjusted HRs of 2.23 (95% CI: 1.51, 3.30). After full adjustment these associations remained statistically significant with little change. Less markedly, rates for angina pectoris were higher (14.6/1000 person years) compared with those without angina pectoris (7.9/1000 person years); the minimally adjusted HR for MCI/PD was moderately elevated with 1.66 (95% CI: 1.22, 2.25) and the fully adjusted model with 1.45 (95% CI: 1.05, 2.01). The IRs of MCI/PD in women with heart failure and atrial fibrillation were 18.7 and 11.9/1000 person years. The corresponding HRs did not show any significant association with MCI/PD. Last, IRs and HRs for MCI/PD were higher for women with any other vascular disease and invasive procedure (17.3/1000 person years and HR, 1.97; 95% CI: 1.34, 2.87) compared with those without (8.0/1000 person years). Among the group of other vascular diseases and invasive procedures coronary bypass surgery (HR, 2.63; 95% CI: 1.46, 4.75), carotid endarterectomy (HR, 3.03; 95% CI: 1.23, 7.47), and peripheral vascular disease (HR, 1.82; 95% CI: 1.09, 3.02) were most strongly associated with cognitive decline whereas no significant relationship was found for women with angioplasty (HR, 1.67; 95% CI: 0.89, 3.13) (Table 3).
When we excluded women with incident stroke or TIA events after baseline (Table 5), we found that a history of myocardial infarction and angina remained strongly associated with cognitive decline (HR, 1.98; 95% CI: 1.29, 3.04 and HR, 1.50; 95% CI: 1.07, 2.10). We also conducted a sensitivity analysis by excluding incident cases of myocardial infarction (n=134) and we found no substantial change of the results (data not shown). Finally, when we conducted sensitivity analyses treating death as a competing event, our results were not materially changed (data not shown).
Interactions on the effect of hypertension, diabetes and adiposity (BMI ≥30) on cognitive decline were examined in women with or without CVD at baseline (Figure 2A and 2B). In women without CVD, prevalent hypertension and diabetes clearly increased the risk for MCI/PD (HR, 1.25; 95% CI: 1.00, 1.57; and HR, 1.83; 95% CI: 1.24, 2.70) (Figure 2A). Adiposity was not related to MCI/PD (HR, 0.84; 95% CI: 0.65, 1.08) in this group. In women with CVD, the presence of diabetes tended to increase women's risk for cognitive decline but this effect was not statistically significant (HR, 1.76; 95% CI: 0.90, 3.44) (Figure 2B). Similarly, no significant effect was found for hypertension and adiposity in these women (HR, 0.99; 95% CI: 0.59, 1.64 and 0.81; 95% CI: 0.47, 1.39).
Discussion
In this large prospective study of 6455 cognitively intact women aged ≥65 years CVD exclusive of stroke was found to be associated with cognitive decline. Among CVD subgroups, a history of myocardial infarction or other invasive vascular procedures or diseases doubled the risk for cognitive decline. These findings support the hypothesis linking clinically manifest CVD to cognitive decline.
The underlying pathophysiological mechanisms by which vascular diseases have been described to alter cognitive functioning are complex: it is well known that cerebral autoregulation keeps cerebral blood flow relatively constant within a range of blood pressures to avoid interruption that may lead to serious consequences, including brain dysfunction and death.35–36 Simultaneously, neurons, glia, perivascular, and vascular cells, systemically often termed as neurovascular unit, work collectively to maintain homeostasis at the micro‐environmental level.4,37–38 Both systems, cerebral blood flow control and neurovascular unit, are essential for protecting the brain from unwanted swings in perfusion pressure and for providing adequate neurovascular functioning.39 Various pathophysiological processes have been shown to target these sensitive regulatory systems separately or in conjunction. Vascular aging as reflected by the degree of atherosclerosis, arteriosclerosis, and inflammation has emerged to provide the underlying complementary mechanistic and pathophysiological pathways between CVD and cognitive decline.35–36,35–41 Several studies with partly controversial findings have been published on the associations between various sites and degrees of atherosclerotic lesions or other surrogate markers and the risk for cognitive decline.2–3,2–17,42,18 In the Rotterdam study as well as the Cardiovascular Health Study Cohort a relationship between preexisting CVD and dementia or Alzheimer disease with highest risk for people suffering from peripheral arterial disease or high common carotid wall thickness has been described.3,15 On the other hand, using autopsy data from the Baltimore Longitudinal Study of Aging, Dolan et al noted that atherosclerosis and dementia were only related to intracranial atherosclerosis, but not to cardiac or aortic atherosclerosis.2 Most recently prospective findings on the effect of coronary artery diseases on cognitive functioning confirmed greater cognitive declines in affected participants.43 However, generalizability of these results may be limited as only 74 patients suffered from vascular disease including 2 with a history of myocardial infarction. Our data on 209 postmenopausal women with a history of myocardial infarction show that these women have double the risk of cognitive decline history compared with their counterparts without myocardial infarction. Additionally, we found high rates of MCI/PD in those with angina pectoris, carotid endarterectomy, peripheral vascular disease, and coronary bypass surgery indicative of a relationship between cardiac and aortic atherosclerosis and cognitive decline.
As cognitive decline and atherosclerosis share common cardiovascular risk factors that have been shown to affect cognitive functioning directly, we adjusted our findings accordingly to several important confounding factors resulting in little change in the magnitude of our associations. Nonetheless, besides cerebral malperfusion cerebrovascular events during the study period may provide an alternative mechanism to explain our findings given the absence of regular MRI neuroimaging of our study participants. In fact, multiple studies have reported a gradually progressive course of cognitive decline in association with small, clinically silent infarcts.27,33,44–45 On the other hand, the WHIMS trial was rigorously designed with extensive neurocognitive and neuropsychiatric assessment to potentially identify cognitive decline thereby reducing the possible role of cerebrovascular events. Additionally, our sensitivity analyses confirmed a robust association between myocardial infarction or angina and cognitive decline after excluding women with incident stroke and TIA during the study period. In contrast to previous reports,17,23,46 we did not detect any statistically significant relationship between atrial fibrillation or heart failure and cognitive decline.
Among the many risk factors for cognitive decline, the presence and the time of onset of hypertension, diabetes, or adiposity may be key to the vascular modifications that affect blood flow and cerebral metabolism.5,47 While several longitudinal studies indicate that elevated blood pressure in midlife increases the risk for cognitive decline in later life,48–49 studies on the effect of hypertension on dementia or cognitive impairment occurring in later life are inconsistent.6 We found hypertension to increase the risk for MCI/PD in elderly women without clinical CVD but not in women suffering from CVD most likely due to a limited number of events. Similarly, the presence of diabetes increased the risk for MCI in women without clinical CVD with no statistically significant findings for PD or for women with CVD. These results are consistent with morphological observations of the WHI Memory Study‐Magnetic Resonance Imaging (WHIMS‐MRI) study where participants with poorly controlled BP or diabetes had a higher prevalence of abnormal white matter lesions of the brain or smaller brain volumes with increased ischemic lesions.50–51 Interestingly, adiposity tended to have a protective effect on cognitive decline in our study population. So far, studies examining late‐life BMI and dementia are sparse but current evidence suggests that older persons with a high BMI have less dementia risk than their counterparts with low BMI.52–53 However, a recent meta‐analysis showed that underweight, overweight, or obesity in midlife increase dementia risk.9
The strengths of our analysis include the sample size of our study population, a prospective design with long follow‐up, confirmed and adjudicated assessment of MCI/PD, and the availability of several confounding factors. Nevertheless, our study also exhibits several limitations. Most importantly, the presence of vascular disease, atrial fibrillation, and heart failure was ascertained via self‐report. However, validation studies on the accuracy of self‐reported data in WHI subgroups have already shown good concordance with medication inventories and medical history.54 Furthermore, although a wide range of confounding factors was considered in the analysis, residual confounding cannot be excluded. The relatively low number of incident cases of MCI and PD may have limited the power of our analyses. External validity may be limited as our study population consisted only of relatively healthy postmenopausal women, which may underestimate the true association in the general population. Lastly, women were selected for a more detailed cognitive assessment based on a cut point on the 3MSE score. It may be that individuals with CVD because of greater use of medications as well as the effects of CVD scored below the cut point and became at risk of further testing and to be diagnosed with MCI or PD.
In conclusion, our findings suggest that CVD is associated with cognitive decline in postmenopausal women. A history of myocardial infarction doubled the risk for MCI or PD compared with women without myocardial infarction. Hypertension and diabetes, but not adiposity, are associated with a higher risk for cognitive decline. These results add new impetus to the contribution of vascular diseases and cardiovascular risk factors on cognitive functioning. As dementia is an increasingly significant problem in developed countries, more research is warranted on the potential of CVD prevention for the preservation of cognitive health.
Acknowledgment
The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf.
WHI Short List of Investigators:
Program Office: Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, Maryland).
Clinical Coordinating Center: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, WA).
Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women's Hospital, Harvard Medical School, Boston, MA); Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA); Rebecca Jackson (The Ohio State University, Columbus, OH); Cynthia A. Thomson (University of Arizona, Tucson/Phoenix, AZ); Jean Wactawski‐Wende (University at Buffalo, Buffalo, NY); Marian Limacher (University of Florida, Gainesville/Jacksonville, FL); Robert Wallace (University of Iowa, Iowa City/Davenport, IA); Lewis Kuller (University of Pittsburgh, Pittsburgh, PA); Sally Shumaker (Wake Forest University School of Medicine, Winston‐Salem, NC).
Women's Health Initiative Memory Study: Sally Shumaker (Wake Forest University School of Medicine, Winston‐Salem, NC).
Sources of Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. Open access publishing of this publication was funded by the German Research Foundation (DFG) and the University of Wuerzburg.
Disclosures
Dr Rebecca D. Jackson reports receiving research funding from being a co‐investigator of a Pfizer research grant. She also reports being on a Merck advisory committee on developing education material on fundamentals of clinical research.
References
- 1.WHO. The Top Ten Causes of Death 2008. http://www.who.int/mediacentre/factsheets/fs310/en/index4.html. Accessed October 15, 2012.
- 2.Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging Cohort. Ann Neurol. 2010; 68:231-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Atherosclerosis and risk for dementia. Ann Neurol. 2007; 61:403-410 [DOI] [PubMed] [Google Scholar]
- 4.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42:2672-2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Razay G, Williams J, King E, Smith AD, Wilcock G. Blood pressure, dementia and Alzheimer's disease: the optima longitudinal study. Dement Geriatr Cogn Disord. 2009; 28:70-74 [DOI] [PubMed] [Google Scholar]
- 6.McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev. 2009:CD004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia: a population‐based neuropathologic study. Neurology. 2010; 75:1195-1202 [DOI] [PubMed] [Google Scholar]
- 8.Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology. 2010; 75:764-770 [DOI] [PubMed] [Google Scholar]
- 9.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late‐life as a risk factor for dementia: a meta‐analysis of prospective studies. Obes Rev. 2011; 12:e426-e437 [DOI] [PubMed] [Google Scholar]
- 10.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta‐analysis. Obes Rev. 2008; 9:204-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O'Meara ES, Longstreth WT, Jr, Luchsinger JA. Midlife and late‐life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009; 66:336-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007; 4:103-109 [DOI] [PubMed] [Google Scholar]
- 13.Arntzen KA, Schirmer H, Johnsen SH, Wilsgaard T, Mathiesen EB. Carotid atherosclerosis predicts lower cognitive test results: a 7‐year follow‐up study of 4,371 stroke‐free subjects—the Tromso study. Cerebrovasc Dis. 2012; 33:159-165 [DOI] [PubMed] [Google Scholar]
- 14.Rafnsson SB, Deary IJ, Fowkes FG. Peripheral arterial disease and cognitive function. Vasc Med. 2009; 14:51-61 [DOI] [PubMed] [Google Scholar]
- 15.Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, Ives D, Dekosky ST, Kuller LH. Dementia and Alzheimer's disease incidence in relationship to cardiovascular disease in the cardiovascular health study cohort. J Am Geriatr Soc. 2005; 53:1101-1107 [DOI] [PubMed] [Google Scholar]
- 16.Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, van Duijn CN, Van Broeckhoven C, Grobbee DE. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam study. Lancet. 1997; 349:151-154 [DOI] [PubMed] [Google Scholar]
- 17.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population‐based cohort study. Arch Intern Med. 2006; 166:1003-1008 [DOI] [PubMed] [Google Scholar]
- 18.Almeida OP, Garrido GJ, Beer C, Lautenschlager NT, Arnolda L, Flicker L. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur Heart J. 2012; 33:1769-1776 [DOI] [PubMed] [Google Scholar]
- 19.Marzona I, O'Donnell M, Teo K, Gao P, Anderson C, Bosch J, Yusuf S. Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. CMAJ. 2012; 184:E329-E336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyasaka Y, Barnes ME, Petersen RC, Cha SS, Bailey KR, Gersh BJ, Casaclang‐Verzosa G, Abhayaratna WP, Seward JB, Iwasaka T, Tsang TS. Risk of dementia in stroke‐free patients diagnosed with atrial fibrillation: data from a community‐based cohort. Eur Heart J. 2007; 28:1962-1967 [DOI] [PubMed] [Google Scholar]
- 21.Park H, Hildreth A, Thomson R, O'Connell J. Non‐valvular atrial fibrillation and cognitive decline: a longitudinal cohort study. Age Ageing. 2007; 36:157-163 [DOI] [PubMed] [Google Scholar]
- 22.Sabatini T, Frisoni GB, Barbisoni P, Bellelli G, Rozzini R, Trabucchi M. Atrial fibrillation and cognitive disorders in older people. J Am Geriatr Soc. 2000; 48:387-390 [DOI] [PubMed] [Google Scholar]
- 23.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population‐based study. The Rotterdam study. Stroke. 1997; 28:316-321 [DOI] [PubMed] [Google Scholar]
- 24.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003; 289:2663-2672 [DOI] [PubMed] [Google Scholar]
- 25.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD, Kotchen JM, Wassertheil‐Smoller S, Wactawski‐Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003; 289:2651-2662 [DOI] [PubMed] [Google Scholar]
- 26.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004; 291:2959-2968 [DOI] [PubMed] [Google Scholar]
- 27.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007; 6:611-619 [DOI] [PubMed] [Google Scholar]
- 28.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski‐Wende J, Wallace R, Wassertheil‐Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative Randomized Controlled Trial. JAMA. 2004; 291:1701-1712 [DOI] [PubMed] [Google Scholar]
- 29.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative Randomized Controlled Trial. JAMA. 2002; 288:321-333 [DOI] [PubMed] [Google Scholar]
- 30. Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998; 19:61-109 [DOI] [PubMed] [Google Scholar]
- 31.Teng EL, Chui HC. The Modified Mini‐Mental State (3MS) Examination. J Clin Psychiatry. 1987; 48:314-318 [PubMed] [Google Scholar]
- 32.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The consortium to establish a registry for Alzheimer's disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989; 39:1159-1165 [DOI] [PubMed] [Google Scholar]
- 33.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly‐Hayes M, Romero JR, Kase CS, Wolf PA, Seshadri S. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring study. Stroke. 2010; 41:600-606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ware JE, Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992; 30:473-483 [PubMed] [Google Scholar]
- 35.Poels MM, Ikram MA, Vernooij MW, Krestin GP, Hofman A, Niessen WJ, van der Lugt A, Breteler MM. Total cerebral blood flow in relation to cognitive function: the Rotterdam scan study. J Cereb Blood Flow Metab. 2008; 28:1652-1655 [DOI] [PubMed] [Google Scholar]
- 36.van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008; 28:1071-1085 [DOI] [PubMed] [Google Scholar]
- 37.Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf). 2011; 203:47-59 [DOI] [PubMed] [Google Scholar]
- 38.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011; 12:723-738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010; 120:287-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan ZS, Beiser AS, Fox CS, Au R, Himali JJ, Debette S, Decarli C, Vasan RS, Wolf PA, Seshadri S. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle‐aged adults: the Framingham Offspring Study. Diabetes Care. 2011; 34:1766-1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007; 68:1902-1908 [DOI] [PubMed] [Google Scholar]
- 42.Viticchi G, Falsetti L, Vernieri F, Altamura C, Bartolini M, Luzzi S, Provinciali L, Silvestrini M. Vascular predictors of cognitive decline in patients with mild cognitive impairment. Neurobiol Aging. 2012; 33:1127.e1-1127.e9 [DOI] [PubMed] [Google Scholar]
- 43.Zheng L, Mack WJ, Chui HC, Heflin L, Mungas D, Reed B, DeCarli C, Weiner MW, Kramer JH. Coronary artery disease is associated with cognitive decline independent of changes on magnetic resonance imaging in cognitively normal elderly adults. J Am Geriatr Soc. 2012; 60:499-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeCarli C. Clinically asymptomatic vascular brain injury: a potent cause of cognitive impairment among older individuals. J Alzheimers Dis. 2013; 33suppl 1:S417-S426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003; 348:1215-1222 [DOI] [PubMed] [Google Scholar]
- 46.Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, Day JD. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer's dementia. Heart Rhythm. 2010; 7:433-437 [DOI] [PubMed] [Google Scholar]
- 47.Duron E, Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag. 2008; 4:363-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia: a double edged sword. Ageing Res Rev. 2009; 8:61-70 [DOI] [PubMed] [Google Scholar]
- 49.Qiu C, Winblad B, Fratiglioni L. The age‐dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005; 4:487-499 [DOI] [PubMed] [Google Scholar]
- 50.Kuller LH, Margolis KL, Gaussoin SA, Bryan NR, Kerwin D, Limacher M, Wassertheil‐Smoller S, Williamson J, Robinson JG. Relationship of hypertension, blood pressure, and blood pressure control with white matter abnormalities in the Women's Health Initiative Memory Study (WHIMS)‐MRI trial. J Clin Hypertens (Greenwich). 2010; 12:203-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tooze JA, Gaussoin SA, Resnick SM, Fischbein NJ, Robinson JG, Bryan RN, An Y, Espeland MA. A uniform approach to modeling risk factor relationships for ischemic lesion prevalence and extent: the Women's Health Initiative magnetic resonance imaging study. Neuroepidemiology. 2010; 34:55-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB. Association between late‐life body mass index and dementia: the Kame Project. Neurology. 2009; 72:1741-1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahl AK, Lopponen M, Isoaho R, Berg S, Kivela SL. Overweight and obesity in old age are not associated with greater dementia risk. J Am Geriatr Soc. 2008; 56:2261-2266 [DOI] [PubMed] [Google Scholar]
- 54.Margolis KL, Lihong Q, Brzyski R, Bonds DE, Howard BV, Kempainen S, Simin L, Robinson JG, Safford MM, Tinker LT, Phillips LS. Validity of diabetes self‐reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008; 5:240-247 [DOI] [PMC free article] [PubMed] [Google Scholar]


