Introduction to HIF Pathway
The evolutionarily conserved hypoxia‐inducible factor (HIF) pathway is present ubiquitously in mammalian cells and plays a critical role in the regulation of energy metabolism, especially glucose utilization.1–2 HIF is a transcription factor consisting of an O2‐sensitive HIF‐α (HIF‐1α or HIF‐2α) and the O2‐insensitive HIF‐1β subunit.2 Under most physiologically normoxic conditions (>3% O2), HIF‐α is hydroxylated by prolyl hydroxylases (PHD) at 2 proline residues located in the oxygen‐dependent degradation domain (ODD), which leads to interaction with the von Hippel Lindau protein pVHL and subsequent degradation by proteasomes.3–4 When tissue oxygenation decreases to, for example, <2% O2, HIF‐α hydroxylation diminishes and becomes stabilized. While the PHDs themselves can act as oxygen sensors, the mitochondria has also been proposed as a critical organelle responsible for cellular oxygen sensing, presumably through complex III.5 Mitochondrial oxygen sensing may be more critical for acute, rapid physiological changes that are needed to respond to hypoxic conditions. In contrast to hypoxia, under aerobic conditions additional mechanisms have been proposed for increased HIF‐α. In particular, growth factor receptor tyrosine kinase and protooncogene‐mediated pathways can also augment HIF‐α expression through translational regulation.6 Thus, depending on the tissue microenvironment, different mechanisms can be used to increase HIF‐α levels and transcriptional activity.
Obesity and the Tissue Microenvironment
Obese individuals often suffer a myriad of health issues, including type 2 diabetes, cardiomyopathy, and nephropathy. However, the molecular mechanisms linking obesity with cardiomyopathy are probably multifactorial and depend as much on the genetics of the individual as on environmental factors such as circulating cytokines and chemokines. Cardiomyopathy, in particular, has been proposed to result from faulty endothelial function, reactive oxygen species, and inflammation7–11 However, what the causative stimulus is for inflammation and oxidative stress in obese individuals has yet to be clearly determined. In fact, one new hypothesis that explains this link is that microenvironmental changes in the adipose tissue of obese individuals could lead to an inflammatory response, an increase in circulating cytokines, and ultimately to cardiomyopathy and heart failure (Figure). The critical point of this hypothesis is that changes in the adipose tissue lead to cardiomyopathy through secreted factors. Previous studies12–14 have demonstrated that the adipose tissue in both obese humans and mice can become hypoxic. This is a rather surprising finding that has been largely ignored as adipose tissue is often thought to be well vascularized. Could changes in tissue oxygenation result in increased levels of the HIF‐α family in adipose tissue and lead to inflammation and cardiomyopathy?
Figure 1.
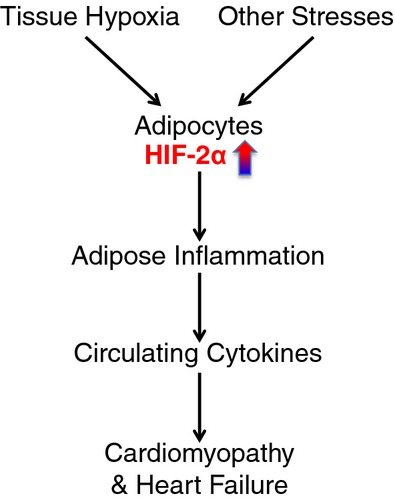
The HIF‐2 pathway in cardiomyopathy.
In this issue of JAHA, Lin et al report a highly novel and important observation regarding HIF function in adipocytes.15 They generated a series of transgenic mouse models through the conditional deletion of Vhl alone or in combination with Hif1a or Hif2a using the adipocyte‐specific aP2 promoter‐driven cre. Mice with adipocyte‐specific Vhl deletion exhibited cardiomegaly with marked ventricular hypertrophy and cardiac dysfunction within a week after birth, suggesting chronic activation of the HIF pathway in adipocytes has deleterious effects remotely in the neonatal heart. Interestingly, the phenotypes of Vhl deletion could be fully rescued by conditionally deleting both Vhl and Hif2a in adipocytes. In contrast, conditional deletion of Hif1a in adipocytes did not only fail to rescue the Vhl‐null phenotypes, and if anything exacerbated the cardiac hypertrophy and dysfunction, which resulted in a shorter lifespan of these mice. The genetic evidence presented by Lin et al clearly demonstrate a critical difference in the abilities of HIF‐1α and HIF‐2α to mediate cardiomyopathy induced by adipocytes, with HIF‐2α being the critical effector of this phenotype. These findings have significant implications in understanding the different roles of HIF in adipocyte function. Previous studies by Lin et al have also demonstrated that HIF‐2α is constitutively expressed in mature adipocytes but not in preadipocytes,16 suggesting that it is the inappropriate expression of HIF‐2α in preadipocytes that is responsible for this phenotype. It is equally possible that loss of VHL in mature adipocytes results in elevated levels of HIF‐2α beyond what is physiologically found, leading to cardiomyopathy. Both of these possibilities should be examined in future studies.
While the elegant mouse genetics of adipocyte‐induced cardiomyopathy are rigorously demonstrated in this article, the mechanistic basis of this observation is more complex as HIF‐2α is a transcription factor and possesses many target genes that it transcriptionally regulates in a sequence specific dependent manner. Mice with Vhl deletion in adipocytes showed generally lower blood glucose levels and normal responses to either glucose or insulin challenge, suggesting that glucose or insulin homeostasis themselves are not the underlying mechanisms of the cardiomyopathy. If inflammation is the causative factor of the cardiomyopathy, then loss of Vhl should result in increased levels of inflammatory cytokines. In fact, that is what Lin et al found.15 Deletion of Vhl in adipocytes led to highly significant increases in the expression of inflammatory cytokines and chemokines, such as Il1b, Il6, Tnf, Ccl2, Ccl3, Ccl7, and Ccl8, indicating that sustained HIF activation causes strong adipose inflammation. This group further found that serum concentrations of IL‐1 β, CCL2, and IL‐12p70 are also elevated and significantly correlated with cardiomegaly. Consistent with the proposed link between HIF‐2α activation in adipocytes, inflammation, and cardiomyopathy, Lin et al found that deleting Hif2a in VHL‐deficient adipocytes was sufficient to completely normalize both inflammation locally in adipose tissue as well as reducing circulating levels of inflammatory cytokines and chemokines.15 These results are strong evidence that leads us to conclude that adipose inflammation is a major cause of pathological heart hypertrophy and heart failure with HIF‐2 activation in adipocytes as a critical underlying mechanism.
HIF‐2, Obesity and Cardiomyopathy
This work by Lin et al has a potentially significant impact on understanding the etiology of obesity‐associated cardiomyopathy. For decades, it has been known that obesity is closely associated with cardiomyopathy and hypertrophy.17–19 In recent years, chronic inflammation emerged as a potential leading cause of obesity‐associated heart diseases,7–11 but the mechanisms remain to be elucidated.
While the studies performed here have used mouse genetics to demonstrate the importance of HIF‐2α in adipocytes leading to cardiomyopathy, both human and mouse adipose tissue develops hypoxia, which could be the pathophysiological signal for HIF‐2α induction.12–14,20 Furthermore, hypoxia has also been implicated as a regulator of adipokines and inflammatory cytokines in adipose tissue, supporting the link between it, inflammation, and cardiomyopathy.12–13,12–21 The genetic evidence presented by Lin et al has clearly demonstrated that sustained activation of HIF‐2 in adipocytes is both necessary and sufficient to induce chronic inflammation in adipose tissues, and elevated levels of secreted inflammatory cytokines. Vhl deletion in adipocytes led to significantly increased expression of Hmox1, Lep, Vegfd/Figf, and Serpine1/Pai1 in adipose tissue. Interestingly, elevated expression of these genes is often found in adipose tissue of obese subjects.13,22 Among secreted cytokines associated with adipocyte HIF activation, MCP‐1 (CCL2) and IL‐12 levels were elevated in young and/or adult obese patients.23–25 It is also well documented that IL‐1β can induce hypertrophic response in cardiomyocytes.26 It is highly likely that obesity‐associated cardiomyopathy results from concerted actions of multiple secreted cytokines and chemokines either directly by adipose tissue or indirectly by other tissues due to secondary effects of HIF‐induced adipose inflammation. Nonetheless, the work by Lin et al proposes a new paradigm that establishes HIF‐2α as a major driver of the obesity‐induced adipose inflammation and the eventual development of obesity‐associated cardiomyopathy (Figure). Most importantly, since genetic deletion rescued this phenotype, pharmacological inhibitors of HIF‐2α, which are starting to be developed, could have therapeutic benefit to obese patients, if they could be targeted to adipose tissue specifically.
Disclosures
None.
References
- 1.Aragones J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009; 9:11-22 [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia‐inducible factor 1. Annu Rev Cell Dev Biol. 1999; 15:551-578 [DOI] [PubMed] [Google Scholar]
- 3.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia‐inducible factors for oxygen‐dependent proteolysis. Nature. 1999; 399:271-275 [DOI] [PubMed] [Google Scholar]
- 4.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia‐inducible factor requires direct binding to the β‐domain of the von Hippel‐Lindau protein. Nat Cell Biol. 2000; 2:423-427 [DOI] [PubMed] [Google Scholar]
- 5.Chandel NS. Mitochondrial regulation of oxygen sensing. Adv Exp Med Biol. 2010; 661:339-354 [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Defining the role of hypoxia‐inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010; 29:625-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860-867 [DOI] [PubMed] [Google Scholar]
- 8.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009; 53:1925-1932 [DOI] [PubMed] [Google Scholar]
- 9.Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005; 307:373-375 [DOI] [PubMed] [Google Scholar]
- 10.Litwin SE. The growing problem of obesity and the heart: the plot “thickens.”. J Am Coll Cardiol. 2006; 47:617-619 [DOI] [PubMed] [Google Scholar]
- 11.Walsh K. Adipokines, myokines and cardiovascular disease. Circ J. 2009; 73:13-18 [DOI] [PubMed] [Google Scholar]
- 12.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009; 58:718-725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007; 293:E1118-E1128 [DOI] [PubMed] [Google Scholar]
- 14.Halberg N, Khan T, Trujillo ME, Wernstedt‐Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner‐Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia‐inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009; 29:4467-4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Q, Huang Y, Booth C, Haase V, Johbnson R, Simon C, Giordano F, Yun Z. Activation of hypoxia‐inducible factor‐2 in adipocytes results in pathological cardiac hypertrophy. J Am Heart Assoc. 2013; 2:e00054810.1161/JAHA.113.000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006; 281:30678-30683 [DOI] [PubMed] [Google Scholar]
- 17.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008; 88:389-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001; 321:225-236 [DOI] [PubMed] [Google Scholar]
- 19.Amad KH, Brennan JC, Alexander JK. The cardiac pathology of chronic exogenous obesity. Circulation. 1965; 32:740-745 [DOI] [PubMed] [Google Scholar]
- 20.Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr. 2008; 100:227-235 [DOI] [PubMed] [Google Scholar]
- 21.Yin J, Gao Z, He Q, Zhou D, Guo Z, Ye J. Role of hypoxia in obesity‐induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab. 2009; 296:E333-E342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y, Park T. DNA microarrays to define and search for genes associated with obesity. Biotechnol J. 2010; 5:99-112 [DOI] [PubMed] [Google Scholar]
- 23.Breslin WL, Johnston CA, Strohacker K, Carpenter KC, Davidson TR, Moreno JP, Foreyt JP, McFarlin BK. Obese Mexican American children have elevated MCP‐1, TNF‐alpha, monocyte concentration, and dyslipidemia. Pediatrics. 2012; 129:e1180-e1186 [DOI] [PubMed] [Google Scholar]
- 24.Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon BS, Erickson KL, Yu R. Circulating levels of MCP‐1 and IL‐8 are elevated in human obese subjects and associated with obesity‐related parameters. Int J Obes (Lond). 2006; 30:1347-1355 [DOI] [PubMed] [Google Scholar]
- 25.Suarez‐Alvarez K, Solis‐Lozano L, Leon‐Cabrera S, Gonzalez‐Chavez A, Gomez‐Hernandez G, Quinones‐Alvarez MS, Serralde‐Zuniga AE, Hernandez‐Ruiz J, Ramirez‐Velasquez J, Galindo‐Gonzalez FJ, Zavala‐Castillo JC, De Leon‐Nava MA, Robles‐Diaz G, Escobedo G. Serum IL‐12 is increased in Mexican obese subjects and associated with low‐grade inflammation and obesity‐related parameters. Mediators Inflamm. 2013; 2013:967067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Li T, Wang Y, Li J, Guo L, Wu M, Shan X, Que L, Ha T, Chen Q, Kelley J, Li Y. Tollip attenuated the hypertrophic response of cardiomyocytes induced by IL‐1beta. Front Biosci. 2009; 14:2747-2756 [DOI] [PubMed] [Google Scholar]


