Abstract
Background
Limited information is available on the frequency of pulmonary embolism (PE) in patients with an acute ischemic stroke (AIS). We evaluated clinical characteristics, predisposing factors, and outcomes in AIS patients with PE.
Methods and Results
We included all AIS patients admitted to participating institutions in the Registry of the Canadian Stroke Network. Clinically PE was documented by a physician and confirmed by computed tomography pulmonary angiography within 30 days of the stroke case index. The primary outcome was death or disability at discharge. Secondary outcomes included disposition, length of hospital stay, mortality at 3 months and 1 year. Among 11 287 patients with AIS, PE was found in 89 (0.78%) patients. History of cancer, deep vein thrombosis (DVT)/PE, and DVT during the hospitalization were associated with PE. PE was associated with higher risk of death at 30 days (25.8% versus 13.6%; P<0.001), at 1 year (47.2% versus 24.6%; P<0.001), and disability at discharge (85.4% versus 63.6%; P<0.001). Mean length of stay was longer in stroke patients with PE (36 versus 16 days; P=0.001). After adjusting for age, sex, and stroke severity, PE remained associated with lower survival at 30 days and 1 year, and death or disability at discharge (OR 3.02; 95% CI 1.56 to 5.83).
Conclusions
In this large cohort study, PE occurred in nearly 1% of AIS patients. PE was more common in patients with severe stroke, history of cancer, previous DVT/PE or acute DVT and associated with lower short‐ and long‐term survival, greater disability, and longer length of stay.
Keywords: cerebral infarction, pulmonary embolism, stroke, venous thromboembolism
Introduction
Stroke is a leading cause of death and disability. Medical complications after ischemic stroke contribute substantially to poor stroke outcomes.1–6 Pulmonary embolism (PE) is a serious medical condition with an annual incidence rate of 0.50 to 0.69 per 1000 persons in the general population.7–8 PE carries a high mortality with case fatality at 3 months ranging between 8.6% and 17%.9–10 Longer‐term mortality can be as high as 24%.11 The risk of PE, including fatal cases, in patients with acute ischemic stroke (AIS) is well known but insufficiently examined.3,12 Studies of in‐hospital complications after stroke have often used the combined category of venous thromboembolism (VTE), thus grouping PE with deep venous thrombosis (DVT).13 In the general medical population, immobility, older age, smoking, hypertension, thrombophilia, and cancer are commonly reported risk factors of PE.14 All these factors are frequent in AIS patients; yet, no studies have analyzed how they influence the risk of PE specifically in the AIS population. Limited information is available on the impact of PE on stroke outcomes.
The aim of our study was to assess clinical characteristics, risk factors, and relevant clinical outcomes in patients who developed a PE within 30 days after an AIS.
Methods
We collected data from the Registry of the Canadian Stroke Network (RCSN). The RCSN is a clinical database of consecutive acute stroke patients admitted to 12 stroke centers in Ontario, Canada from July 1, 2003 to June 30, 2008 that has been collected for the purposes of monitoring quality of stroke care.
Study Design and Data Collection
Patients aged 18 years and older with an AIS were included in this analysis. For the purpose of this study, we excluded patients missing a unique health identifier (n=1518) and those with a transient ischemic attack (TIA) (n=268) or hemorrhagic stroke (n=290) as these represent distinct populations. Details of the RCSN can be obtained from http://www.rcsn.org. The cause of poststroke mortality was obtained through linkages of the Ontario Registered Persons Database (RPDB) at the Institute for Clinical Evaluative Sciences. The RPDB is a population‐based administrative database that includes basic demographic data and date of death, and provides complete follow‐up for all residents in the province. Stroke was diagnosed by a physician, and each patient had CT or MRI to confirm diagnosis and rule out other causes of stroke. We recorded demographic data as well as clinical variables including vascular risk factors, past medical history of chronic obstructive pulmonary disease (COPD), cancer, pulmonary embolism, DVT, prothrombotic state, stroke types, Canadian neurological scale (CNS), iScore,15–16 and laboratory on arrival. Prothrombotic states, including antiphopholipid antibody syndrome (APA)/lupus anticoagulant, hyperhomocysteinemia, Protein C or S deficiency, Factor V Leiden, and prothrombin gene mutation were identified from laboratory reports or hematology consult. Patients with history of VTE, cryptogenic stroke, or stroke at young age received these investigations. Clinically suspected PE was defined by documented physician diagnosis and confirmed by CT pulmonary angiography within 30 days of admission at hospital.
Outcome Measures
The primary outcome was death or disability at discharge defined as the modified Rankin scale ≥3. Secondary outcomes included disposition status, length of hospital stay (LOS), and death at 3 months and at 1 year.
Statistical Analysis
Chi squared tests were used to compare categorical variables, and analysis of variance (ANOVA) or Kruskal–Wallis tests were used to compare mean and median differences for continuous variables. As differences may be significant but not clinically meaningful, we used standardized differences to compare baseline characteristics between those with and without PE. Standardized differences reflect the mean difference as a percentage of the standard deviation. Effect sizes greater than 0.1 are typically felt to be clinically meaningful.17 Multivariate logistic regression analysis using backward selection was used to identify variables associated with the occurrence of PE and poor outcomes in ischemic stroke patients. Survival function is represented using Kaplan Meyer curves with differences between groups using the log‐rank test. Statistical analysis was performed using SAS statistical software version 9.2.2 (SAS Institute Inc). All tests were 2‐tailed, and P values <0.05 were considered significant. Approvals from the St. Michael's Hospital review board and the RCSN Publications Committee were obtained.
Results
Overall, there were 11 287 patients with AIS included in the study. PE within 30 days after admission at hospital was found in 89 (0.78%) patients. AIS patients with PE were most common (53.9%) in the age group ranging 60 to 79 years. Differences in baseline characteristics between stroke patients with and without PE are represented in Table 1. AIS patients with PE were more likely to have history of cancer (29.2% versus 9.9%, P<0.001), previous DVT/PE (10.1% versus 2.7%, P<0.001), and documented prothrombotic state (4.5% versus 0.6%, P<0.001) as compared to patients without PE. Patients with PE were also likely to have a more severe stroke (CNS≤4.5: 30.3% versus 17.6%, P<0.001) and coma 5.6% versus 3.1%, P<0.001). No difference was found in stroke subtypes, iScore, or rates of intravenous recombinant tissue plasminogen activator (rtPA) administration between PE and non‐PE groups. DVT during hospitalization was diagnosed in 0.9% of AIS patients without PE and in 36% of stroke patients with PE (P<0.001). DVT, cancer, and prothrombotic state were the only 3 variables with P values <0.05 and standardized differences > 0.1, suggestimg that they were clinically meaningful. In the multivariable analysis, age, sex, stroke severity, past history of cancer (OR 3.25, 95% CI 1.94 to 5.45, P<0.0001), past history of DVT/PE (OR 2.41, 95% CI 1.07 to 5.42, P=0.03), and the development of in‐hospital DVT (OR 71.3, 95% CI 42.5 to 119.5, P<0.0001) were significant factors associated with PE.
Table 1.
Clinical Characteristics of Patients With and Without PE
| Total (n=11 287), % | Stroke With PE (n=89), % | Stroke Without PE (n=11 198), % | SD | P Value | |
|---|---|---|---|---|---|
| Age, y | |||||
| <60 | 2063 (18.3) | 17 (19.1) | 2046 (18.3) | 0.02 | 0.28 |
| 60 to 79 | 5297 (46.9) | 48 (53.9) | 5249 (46.9) | 0.14 | |
| ≥80 | 3927 (34.8) | 24 (27.0) | 3903 (34.9) | 0.17 | |
| Male | 5888 (52.2) | 46 (51.7) | 5842 (52.2) | 0.01 | 0.99 |
| Risk factors | |||||
| Diabetes mellitus | 2904 (25.7) | 19 (21.3) | 2885 (25.8) | 0.1 | 0.34 |
| Hypertension | 7663 (67.9) | 61 (68.5) | 7602 (67.9) | 0.01 | 0.9 |
| Hyperlipidemia | 3910 (34.6) | 23 (25.8) | 3887 (34.7) | 0.19 | 0.08 |
| Coronary artery disease | 2737 (24.2) | 21 (23.6) | 2716 (24.3) | 0.02 | 0.89 |
| Atrial fibrillation or atrial flutter | 2043 (18.1) | 11 (12.4) | 2032 (18.1) | 0.15 | 0.16 |
| Congestive heart failure | 1062 (9.4) | 5 (5.6) | 1057 (9.4) | 0.13 | 0.22 |
| Peripheral vascular disease | 759 (6.7) | <5 | 756 (6.8) | 0.13 | 0.21 |
| Asthma or COPD | 1426 (12.6) | 13 (14.6) | 1413 (12.6) | 0.06 | 0.57 |
| Cancer | 1138 (10.1) | 26 (29.2) | 1112 (9.9) | 0.64 | <0.001 |
| Previous DVT/PE | 312 (2.8) | 9 (10.1) | 303 (2.7) | 0.45 | <0.001 |
| Prothrombotic state | 67 (0.6) | <5 | 63 (0.6) | 0.51 | <0.001 |
| Current smoker (last 6 months) | 2208 (19.6) | 13 (14.6) | 2195 (19.6) | 0.13 | 0.24 |
| Preadmission medications | |||||
| Antiplatelet therapy | 4689 (41.5) | 26 (29.2) | 4663 (41.6) | 0.25 | 0.02 |
| Anticoagulant therapy | 1349 (12.0) | 14 (15.7) | 1335 (11.9) | 0.12 | 0.27 |
| Preadmission status—independent | 8906 (78.9) | 66 (74.2) | 8840 (78.9) | 0.12 | 0.27 |
| Stroke symptoms | |||||
| Aphasia | 3823 (33.9) | 39 (43.8) | 3784 (33.8) | 0.21 | 0.14 |
| Visual field defect | 1503 (13.3) | 12 (13.5) | 1491 (13.3) | 0.12 | 0.47 |
| Dysarthria | 4514 (40.0) | 29 (32.6) | 4485 (40.1) | 0.16 | 0.35 |
| Weakness | 9248 (81.9) | 73 (82.0) | 9175 (81.9) | 0.16 | 0.18 |
| Stroke subtype* | 0.45 | 0.08 | |||
| Lacunar | 1657 (22.1) | 5 (8.3) | 1652 (22.2) | ||
| Cardioembolic | 2889 (38.6) | 30 (50) | 2859 (38.5) | ||
| Large artery atherosclerosis | 1812 (24.2) | 12 (20) | 1800 (24.2) | ||
| Other | 630 (8.4) | 8 (13.3) | 622 (8.4) | ||
| Undetermined | 502 (6.7) | 5 (8.3) | 497 (6.7) | ||
| Stroke severity on admission | 0.42 | <0.001 | |||
| Mild | 6279 (55.6) | 34 (38.2) | 6245 (55.8) | ||
| Moderate | 2439 (21.6) | 19 (21.3) | 2420 (21.6) | ||
| Severe | 1995 (17.7) | 27 (30.3) | 1968 (17.6) | ||
| Coma | 348 (3.1) | 5 (5.6) | 343 (3.1) | ||
| Missing | 226 (2.0) | <5 | 222 (2.0) | ||
| Laboratory on arrival, mean±SD | |||||
| INR | 1.14±0.52 | 1.24±1.00 | 1.14±0.52 | 0.19 | 0.07 |
| Glucose | 7.76±3.41 | 7.90±3.22 | 7.76±3.41 | 0.04 | 0.7 |
| Thrombolysis therapy | |||||
| rtPA intravenous | 1737 (15.4) | 18 (20.2) | 1719 (15.4) | 0.14 | 0.45 |
Number between brackets indicate percentages, unless otherwise indicated. To preserve patients' identity, cell with number (%) of patients <5 are not reported. P value <0.05 significant. COPD indicates chronic obstructive pulmonary disease; DVT, deep venous thrombosis; PE, pulmonary embolism; rtPA, recombinant tissue plasminogen activator; SD, standardized difference.
Information available: total ischemic stroke, 7490; stroke with PE, 60; stroke without PE, 7430.
Outcome Measures
Overall, 1446 (12.8%) ischemic stroke patients died during the hospitalization. Higher in‐hospital mortality was found in stroke patients with PE (31.5% versus 12.7%, P<0.001). Total death and disability at discharge (mRS≥3) was significantly higher among AIS with PE compared to those without PE (85.4% versus 63.6%, P<0.001) (Table 2, Figure 1). While the mean LOS was 16 days, patients with PE group had longer LOS (36 versus 16 days, P<0.001). AIS patients with PE were also more often discharged to long‐term care facilities (21.3% versus 10%, P<0.001) and rehabilitation institutions (37.7% versus 35.1%, P<0.001). Approximately, only 20% of AIS patients with PE were discharged home compared to 43.9% of those without PE (P<0.001). In‐hospital complications and recurrent stroke within 30 days of admission, including ischemic and hemorrhagic stroke, were significantly more frequent in the PE group (Table 2). Survival curves at 30 days and 1 year after the stroke case index are shown in Figures 2 and 3. In the multivariable analysis, PE (OR 3.02, 95% CI 1.56 to 5.83) was a significant predictor of poor outcome (mRS 3 to 6) (Table 3) and lower survival at 30 days and 1 year (Figures 2 and 3).
Table 2.
Outcome Measures
| Total (n=11 287), % | Pulmonary Embolism Group (n=89), % | Non‐Pulmonary Embolism Group (n=11 198), % | P Value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Death or disability at discharge (mRS≥3) | 7196 (63.8) | 76 (85.4) | 7120 (63.6) | <0.001 |
| Secondary outcomes | ||||
| In‐hospital complication (within 30 days of admission) | ||||
| Cardiac or respiratory arrest | 469 (4.2) | 16 (18.0) | 453 (4.0) | <0.001 |
| Deep vein thrombosis | 106 (0.9) | 32 (36.0) | 74 (0.7) | <0.001 |
| GI hemorrhage | 185 (1.6) | 7 (7.9) | 178 (1.6) | <0.001 |
| Pneumonia | 818 (7.2) | 22 (24.7) | 796 (7.1) | <0.001 |
| Recurrent stroke (within 30 days of admission) | ||||
| Hemorrhage | 132 (1.2) | <5 | 129 (1.2) | 0.01 |
| Ischemic | 313 (2.8) | 6 (6.7) | 307 (2.7) | 0.01 |
| Total | 445 (3.9) | 9 (10.1) | 436 (3.9) | <0.003 |
| Disposition (alive patients only) | ||||
| Acute care facility | 746 (7.6) | 5 (5.6) | 741 (7.6) | <0.001 |
| Home | 4302 (43.7) | 12 (19.7) | 4290 (43.9) | |
| Long‐term care facility | 991 (10.1) | 13 (21.3) | 978 (10.0) | |
| Rehabilitation facility | 3454 (35.1) | 23 (37.7) | 3431 (35.1) | |
| Other | 346 (3.5) | 8 (13.1) | 338 (3.5) | |
| Length of hospital stay (days) | ||||
| Mean±SD | 15.81±23.5 | 35.82±42.0 | 15.65±23.2 | <0.001 |
| Stroke mortality | ||||
| At discharge | 1446 (12.8) | 28 (31.5) | 1418 (12.7) | <0.001 |
| 30 days | 1549 (13.7) | 23 (25.8) | 1526 (13.6) | <0.001 |
| 1 year | 2798 (24.8) | 42 (47.2) | 2756 (24.6) | <0.001 |
To preserve patients' identity, cell with number (%) of patients <5 are not reported. P value <0.05 significant. mRS indicates modified‐Rankin scale; SD, standard deviation.
Figure 1.

Functional outcomes based on mRS at discharge in IS with and without PE. Note: Functional status was not known in 5 (1.1%) patients with PE and 45 (0.4%) patients without PE. IS indicates ischemic stroke; mRS, modified Rankin Scale; PE, pulmonary embolism.
Figure 2.
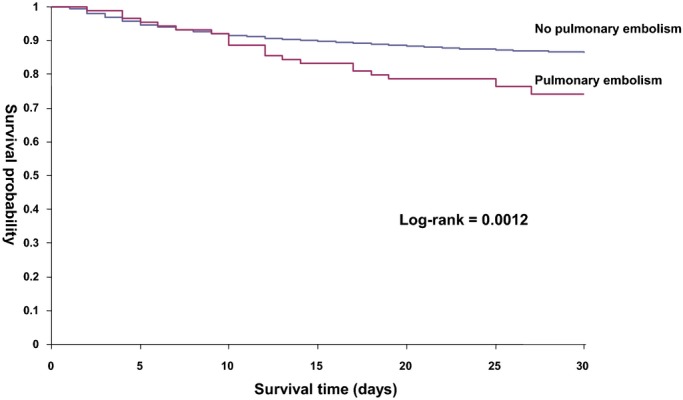
Kaplan–Meier curves for 30‐day survival in patients with and without PE. Log‐rank test <0.0012. PE indicates pulmonary embolism.
Figure 3.
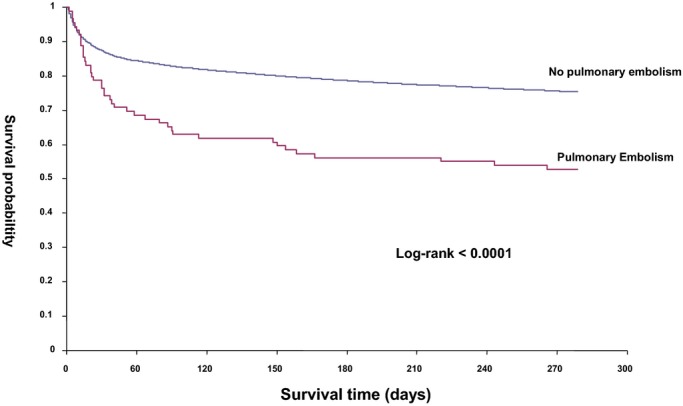
Kaplan–Meier curves for 1‐year survival in patients with and without PE. Log‐rank test <0.0001. PE indicates pulmonary embolism.
Table 3.
Multivariate Analysis: Variables Associated With Poor Outcome (mRS 3 to 6) Among Ischemic Stroke Patients
| OR (95% CI) | P Value | |
|---|---|---|
| Age group, y | ||
| Ref <60 | 1.0 | |
| 60 to 79 | 1.62 (1.45 to 1.82) | <0.0001 |
| ≥80 | 2.55 (2.25 to 2.90) | <0.0001 |
| Stroke severity on admission | ||
| Mild | 1.0 | |
| Moderate | 4.37 (3.91 to 4.90) | <0.0001 |
| Severe | 12.93 (10.99 to 15.22) | <0.0001 |
| Pulmonary embolism | 3.02 (1.56 to 5.83) | 0.001 |
Adjusted for age, sex, stroke severity, history of VTE, and cancer. Only significant variables are presented. P value <0.05 significant. CI indicates confidence interval; mRS, modified‐Rankin scale; OR, odds ratio.
Discussion
In this large cohort study including 11 287 patients with AIS, we found PE in nearly 1% of cases. Patients with PE had higher rates of in‐hospital death (almost one‐third of patients with PE after AIS died in the hospital) and disability, higher case fatality at 30 days and 1 year, higher prevalence of in‐hospital complications, and longer LOS. One‐third of stroke patients with PE died during the hospitalization and only 1 in 5 patients were discharged home. PE was a predictor of poor outcomes after adjusting for age, stroke severity, and other comorbid conditions. Risk factors for PE in stroke patients included age, stroke severity, history of cancer, previous DVT/PE, or development of in‐hospital DVT.
The frequency of PE in AIS in our cohort is consistent with previous studies (0.2% to 0.8%).2–3,2–19 Kelly et al20 studied venous thromboembolism in AIS patients receiving aspirin and graded compression stockings for thromboprophylaxis and PE in 11.8% of patients. In other studies including cryptogenic stroke and patent foramen ovale, PE was found in up 20% to 37% of patients, though the great majority of these cases were asymptomatic and only recognized by dedicated testing required by the research design.21–22
Risk factors of PE in the general population are well known, including age, obesity, immobility, cigarette smoking, hormonal replacement, pregnancy, previous medical illness (particularly PE or DVT, cancer, COPD, hypertension, amd congestive heart failure), stroke with limb paresis, thrombophilia, and recent surgery.14 In our AIS cohort we found higher risk of PE among patients with history of cancer or DVT/PE. In addition, DVT during the acute hospitalization had the highest risk of PE. This is not surprising as stroke patients with an acute DVT during hospitalization are the ones at the highest risk. We found no difference in the distribution of stroke subtypes between patients with and without PE. Similar to other reports, we found AIS patients with PE had more severe strokes.20
In the International Cooperative Pulmonary Embolism Registry (ICOPER)9 study of clinical outcomes in 2110 patients with acute PE in a general population, the poor prognostic factors included > 70 years, cancer, congestive heart failure, COPD, systolic arterial hypotension, tachypnea, and right‐ventricular hypokinesis on echocardiography. Laporte et al10 reported that the risk of death in patients with acute PE was associated with age >75 years, immobilization because of a neurologic disease, cardiac or respiratory disease, and cancer. Our study found that in patients with AIS complicated by PE, age of > 60 years and moderate‐to‐severe stroke severity (CNS<7.5) were associated with poor outcomes. In addition, PE was a predictor of poor outcomes after adjusting from relevant covariates.
The in‐hospital mortality rate in AIS patients with PE was 31.5% in our study, comparable with previous findings from Taiwan (35.7%)23 and Germany (46.8%)2; both including a substantially lower absolute number of stroke patients with PE. The 30‐day mortality rate of PE in AIS in our cohort was 25.8%, thus higher than the rates reported in association with PE in the general population (8.6% and 17%).9–10 Long‐term mortality of PE in AIS patients (47.2%) was also higher than previous rates reported in cohorts of PE patients from the general population (24%).11 It is noteworthy that barely 1 in 2 stroke patients with PE were alive at 1 year in our cohort.
PE is a potentially preventable complication after stroke. A review article suggests the coexistence of PE and pneumonia or PE misdiagnosed as pneumonia due to common risk factors and clinical presentations. PE accounts for the largest proportion of early death, highlighting the importance of thromboprophylaxis.24 More recently, thromboprophylaxis has become one of the quality measures in acute stroke care.25 Unfractionated heparin (UFH) and low‐molecular‐weight heparin (LMWH) reduce VTE after AIS.26–27 The Clots in Legs Or sTockings after Storke (CLOT) 3 study reported the intermittent pneumatic compression reducing the risk of DVT in AIS with immobility.28 The American Heart Association/American Stroke Association,29 Canadian best practice recommendations for stroke care,30 and the American College of Chest Physicians31 clinical guideline for ischemic stroke management recommend prophylactic LMWH or UFH be administered to all immobilized patients with an absence of contraindications. In patients who cannot receive anticoagulants for DVT prophylaxis, the use of aspirin and intermittent external compression devices are reasonable.29
Our study has some strengths and limitations that deserve comment. First, we had no information on factors that may have affected the risk of PE (eg, time to first mobilization, time of antithrombotics use). Second, we had limited information about the performance status of patients with cancer. Third, it is possible that the lack of association between some variables (eg, stroke subtype) could be due to the limited statistical power. Finally, information on the treatment during hospitalization for stroke patients with PE was not available. Despite these limitations, our study provides relevant information about specific risk factors for PE and the impact of PE on stroke outcomes. Other strengths include a large sample size, the adjustment for relevant covariates including stroke severity, and the near complete follow‐up of the patients.
In summary, PE is an uncommon but serious medical complication after an AIS. PE after stroke is associated with higher risk of death, worse disability, higher in‐hospital complications, and longer LOS. Our findings highlight the importance of ensuring compliance with thromboprophylaxis measures and guideline recommendations for patients with AIS.29–31 The risk of PE is higher in older patients with more severe strokes, history of cancer or DVT/PE, or DVT during admission. Early recognition of predisposing conditions may help implement strategies for its early detection and the appropriate preventative measures aimed at improving the quality of stroke care.
Sources of Funding
This study was supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care. The opinions, results and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by Institute for Clinical Evaluative Sciences or the Ontario Ministry of Health and Long‐Term Care is intended or should be inferred.
Disclosures
Dr Saposnik is supported in part by the Distinguished Clinician‐Scientist Award from the Heart and Stroke Foundation of Canada (HSFC).
Acknowledgments
These data sets were held securely in a linked, de‐identified form and analyzed at the Institute for Clinical Evaluative Sciences.
References
- 1.Johnston KC, Li JY, Lyden PD, Hanson SK, Feasby TE, Adams RJ, Faught RE, Jr, Haley EC., Jr Medical and neurological complications of ischemic stroke: experience from the RANTTAS trial. RANTTAS Investigators. Stroke. 1998; 29:447-453 [DOI] [PubMed] [Google Scholar]
- 2.Heuschmann PU, Kolominsky‐Rabas PL, Misselwitz B, Hermanek P, Leffmann C, Janzen RW, Rother J, Buecker‐Nott HJ, Berger KGerman Stroke Registers Study Group Predictors of in‐hospital mortality and attributable risks of death after ischemic stroke: the German Stroke Registers Study Group. Arch Intern Med. 2004; 164:1761-1768 [DOI] [PubMed] [Google Scholar]
- 3.Tong X, Kuklina EV, Gillespie C, George MG. Medical complications among hospitalizations for ischemic stroke in the United States from 1998 to 2007. Stroke. 2010; 41:980-986 [DOI] [PubMed] [Google Scholar]
- 4.Hong KS, Kang DW, Koo JS, Yu KH, Han MK, Cho YJ, Park JM, Bae HJ, Lee BC. Impact of neurological and medical complications on 3‐month outcomes in acute ischaemic stroke. Eur J Neurol. 2008; 15:1324-1331 [DOI] [PubMed] [Google Scholar]
- 5.Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik GCanadian Stroke Network; Stroke Outcome Research Canada (SORCan) Working Group Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011; 77:1338-1345 [DOI] [PubMed] [Google Scholar]
- 6.Bembenek J, Karlinski M, Kobayashi A, Czlonkowska AJ. Early stroke‐related deep venous thrombosis: risk factors and influence on outcome. J Thromb Thrombolysis. 2011; 32:96-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., III Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998; 158:585-593 [DOI] [PubMed] [Google Scholar]
- 8.Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population‐based study. J Thromb Haemost. 2007; 5:692-699 [DOI] [PubMed] [Google Scholar]
- 9.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999; 353:1386-1389 [DOI] [PubMed] [Google Scholar]
- 10.Laporte S, Mismetti P, Décousus H, Uresandi F, Otero R, Lobo JL, Monreal MRIETE Investigators Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008; 117:1711-1716 [DOI] [PubMed] [Google Scholar]
- 11.Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, Schwartz JS, Thompson BT, Popovich J, Jr, Hobbins TE. The clinical course of pulmonary embolism. N Engl J Med. 1992; 326:1240-1245 [DOI] [PubMed] [Google Scholar]
- 12.Skaf E, Stein PD, Beemath A, Sanchez J, Olson RE. Fatal pulmonary embolism and stroke. Am J Cardiol. 2006; 97:1776-1777 [DOI] [PubMed] [Google Scholar]
- 13.Ingeman A, Andersen G, Hundborg HH, Svendsen ML, Johnsen SP. In‐hospital medical complications, length of stay, and mortality among stroke unit patients. Stroke. 2011; 42:3214-3218 [DOI] [PubMed] [Google Scholar]
- 14.Goldhaber SZ. Pulmonary embolism. Lancet. 2004; 363:1295-1305 [DOI] [PubMed] [Google Scholar]
- 15.Saposnik G, Kapral MK, Liu Y, Hall R, O'Donnell M, Raptis S, Tu JV, Mamdani M, Austin PCInvestigators of the Registry of the Canadian Stroke Network; Stroke Outcomes Research Canada (SORCan) Working Group IScore: a risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation. 2011; 123:739-749 [DOI] [PubMed] [Google Scholar]
- 16.Saposnik G, Raptis S, Kapral MK, Liu Y, Tu JV, Mamdani M, Austin PCInvestigators of the Registry of the Canadian Stroke Network and the Stroke Outcome Research Canada Working Group The iScore predicts poor functional outcomes early after hospitalization for an acute ischemic stroke. Stroke. 2011; 42:3421-3428 [DOI] [PubMed] [Google Scholar]
- 17.Mamdani M, Sykora K, Li P, Normand SL, Streiner DL, Austin PC, Rochon PA, Anderson GM. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005; 330:960-962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaf E, Stein PD, Beemath A, Sanchez J, Bustamante MA, Olson RE. Venous thromboembolism in patients with ischemic and hemorrhagic stroke. Am J Cardiol. 2005; 96:1731-1733 [DOI] [PubMed] [Google Scholar]
- 19.Tirschwell DL, Kukull WA, Longstreth WT., Jr Medical complications of ischemic stroke and length of hospital stay: experience in Seattle, Washington. J Stroke Cerebrovasc Dis. 1999; 8:336-343 [DOI] [PubMed] [Google Scholar]
- 20.Kelly J, Rudd A, Lewis RR, Coshall C, Moody A, Hunt BJ. Venous thromboembolism after acute ischemic stroke: a prospective study using magnetic resonance direct thrombus imaging. Stroke. 2004; 35:2320-2325 [DOI] [PubMed] [Google Scholar]
- 21.Allendörfer J, Tanislav C, Puille M, Grebe M, Stolz E, Jauss M. Risk factors for pulmonary embolism in patients with stroke and patent foramen ovale. Cerebrovasc Dis. 2007; 24:138-139 [DOI] [PubMed] [Google Scholar]
- 22.Tanislav C, Puille M, Pabst W, Reichenberger F, Grebe M, Nedelmann M, Kaps M, Allendörfer J. High frequency of silent pulmonary embolism in patients with cryptogenic stroke and patent foramen ovale. Stroke. 2011; 42:822-824 [DOI] [PubMed] [Google Scholar]
- 23.Chen CC, Lee TH, Chung CY, Chang WH, Hong JP, Huang LT, Tang SF, Chen CK. Symptomatic pulmonary embolism among stroke patients in Taiwan: a retrospective cohort study. Top Stroke Rehabil. 2012; 19:361-368 [DOI] [PubMed] [Google Scholar]
- 24.Kelly J, Hunt BJ, Rudd A, Lewis RR. Pulmonary embolism and pneumonia may be confounded after acute stroke and may co‐exist. Age Ageing. 2002; 31:235-239 [DOI] [PubMed] [Google Scholar]
- 25.Schwamm LH, Fonarow GC, Reeves MJ, Pan W, Frankel MR, Smith EE, Ellrodt G, Cannon CP, Liang L, Peterson E, Labresh KA. Get With the Guidelines‐Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009; 119:107-115 [DOI] [PubMed] [Google Scholar]
- 26.Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011; 155:602-615 [DOI] [PubMed] [Google Scholar]
- 27.Kamphuisen PW, Agnelli G. What is the optimal pharmacological prophylaxis for the prevention of deep‐vein thrombosis and pulmonary embolism in patients with acute ischemic stroke? Thromb Res. 2007; 119:265-274 [DOI] [PubMed] [Google Scholar]
- 28.CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013; 382:516-524http://dx.doi.org/10.1016/S0140-6736(13)61050-8 [DOI] [PubMed] [Google Scholar]
- 29.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas HAmerican Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013; 44:870-947 [DOI] [PubMed] [Google Scholar]
- 30.Lindsay MP, Gubitz G, Bayfey M, Philips S. Canadian Best Practice Recommendations for Stroke Care Available at: http://www.strokebestpractices.ca. Accessed May 27, 2013.
- 31.Lansberg MG, O'Donnell MJ, Khatri P, Lang ES, Nguyen‐Huynh MN, Schwartz NE, Sonnenberg FA, Schulman S, Vandvik PO, Spencer FA, Alonso‐Coello P, Guyatt GH, Akl EAAmerican College of Chest Physicians Antithrombotic and thrombolytic therapy for ischemic stroke: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012; 1412 suppl:e601S-e636S10.1378/chest.11‐2302 [DOI] [PMC free article] [PubMed] [Google Scholar]


